Abstract
The resting state amplitude of low-frequency fluctuations (ALFF) in functional magnetic resonance imaging (fMRI) is believed to reflect spontaneous cerebral neural activity. The pathophysiology of blepharospasm (BSP), which is characterized by motor symptoms and also sensory symptoms, remains unclear. The present study aims to localize possible cerebral functional abnormalities in BSP patients using resting state fMRI, and explore their possible associations with clinical variables. Voxel-based analysis was used to characterize the difference of ALFF between eighteen BSP patients and eighteen matched healthy controls. Separate correlation analyses were conducted to explore the possible association between ALFF values of significantly different areas and clinical measures including onset age, disease duration, symptom severity evaluated by Jankovic rating scale (JRS), and presence of geste antagoniste. The whole-brain analysis indicated that the BSP group had significantly decreased ALFF in the left thalamus while increased ALFF in the left orbitofrontal areas extending from middle frontal gyrus to inferior frontal gyrus. The mean ALFF in the left thalamus was negatively correlated with the subscore of JRS-frequency (r = −0.484, p = 0.042) and JRS total score (r = −0.477, p = 0.045). A borderline positive correlation was detected between the mean ALFF in the left orbitofrontal area and disease duration(r = 0.485, p = 0.049). Our findings suggest sensorimotor integration is abnormal in BSP, and dysfunctional activity of thalamus may be used to measuring symptom severity in BSP patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Essential or idiopathic blepharospasm (BSP), a common adult-onset primary focal dystonia, is characterized by excessive involuntary closure of the eyelids caused by spasms of the orbicularis oculi muscles [1]. Although primary focal dystonia is classified as a movement disorder, various nonmotor symptoms have been observed in patients with primary focal dystonia. Photophobia, which frequently complained by BSP patients, is a sensory symptom preceding or occurring simultaneously with the development of eyelid spasm [2]. A characteristic feature with diagnostic clue is the response to tactile stimuli factoring relaxation of involved muscles, which may suggest the sensorimotor dysfunction in BSP [3]. Understanding the pathophysiology of BSP is important to achieving further advances in the therapy of BSP.
Functional magnetic resonance imaging (fMRI), a noninvasive tool to investigate functional brain reorganization, has identified a number of brain areas with altered activity in patients with BSP in recent years. However, these fMRI studies of BSP have involved the examination of brain activity during different motor tasks, and the results are not consistent. For example, BSP patients showed increased activation of visual cortex, anterior cingulate, primary motor cortex, thalamus and cerebellum than HC during spontaneous and voluntary blinking [4], while relative overactivity of the bilateral post-central gyrus and the caudal SMA with the task of whistling [5]. The complex patterns of these findings may be due to the different tasks, or the nature of the comparison groups used. Therefore, the different tasks make it difficult to explain the pathophysiology of BSP and reduce the cross-population comparability among various studies.
Different from the task-based fMRI, the recently developed resting-state fMRI (rfMRI) has provided a novel neuroimaging tool to investigate the brain activity in the resting state. As rfMRI avoids potential confound caused by misunderstanding or poor performance of the participants, as well as the effects of the tasks on the brain activity, it may be helpful to further the understanding of abnormalities in brain activity. Low-frequency fluctuations (LFF 0.01–0.08HZ) of blood oxygen level dependent (BOLD) signals in rfMRI data are thought to reflect spontaneous neural activity in human [6]. The quantitative measurement of LFF amplitude (ALFF) [7], which involves the spectral decomposition of the time series data with a focus on the relative amplitude that resides in low-frequency ranges, have been successfully employed to differentiate physiological state of the brain in HC, but also to assess pathological brain activity in various psychiatric and neurological disorders [8, 9].
The present study aims to investigate the alterations of cerebral activities of BSP patients in resting state using ALFF and their possible relationship with clinical features.
Methods
Participants
A total of 36 participants were recruited in this study: 18 BSP patients and 18 age- and sex-matched HCs from Neurology Department, West China Hospital of Sichuan University. All subjects were right-handed according to the Edinburgh Inventory [10]. Diagnosis of BSP was established by a neurologist with long-standing experience in movement disorders (HF Shang) based on the published criteria [1, 11]. Known causes of secondary dystonia were excluded on the basis of medical and drug histories, neurological examination, laboratory investigation and conventional MRI. All patients have neither other neurological abnormalities nor family history of movement disorders. The severity of BSP in all patients at the time just before MRI scanning was accessed according to the 0–4 scale Jankovic Rating Scale (JRS), which includes both severity and frequency of the involuntary orbicularis oculi muscle spasm [12]. Disease durations were calculated from symptom onset to scan date in months. The number of patients who manifested with geste antagoniste that can temporarily relieve the symptoms by touching or pulling the eyelids slightly or yawning, was documented in patients group. None of these patients were receiving medications for about 24 h prior to MRI scanning.
We state that this study was approved by the ethical committee of West China Hospital of Sichuan University and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all the participants prior to the inclusion in this study, and details that might disclose the identity of the subjects under study have been omitted.
MRI data acquisition
All of the images were acquired with 3.0-T scanner (Excite; GE, Milwaukee, WI) at the Huaxi MR Research Center (HMRRC), in West China Hospital of Sichuan University with an eight-channel phased-array head coil. Ear plugs were used, and movement was minimized by stabilizing the head with cushions. The fMRI scanning was performed in darkness and participants were told not to concentrate on any particular object, but to relax with eyes closed and stay awake and motionless. High-resolution T1-weighted images were acquired via a volumetric three-dimensional spoiled gradient recall sequence (TR = 8.5 ms, echo time = 3.4 ms, flip angle = 12°, slice thickness = 1 mm). Field of view (240 × 240 mm2) was used with an acquisition matrix comprising 256 readings of 128 phase encoding steps that produced 156 contiguous coronal slices, with a slice thickness of 1.0 mm. The final matrix size of T1-weighted images was automatically interpolated in-plane to 512 × 512, which yielded an in-plane resolution of 0.47 × 0.47 mm2. MR images sensitized to changes in BOLD signal levels (TR = 2,000 ms, echo time = 30 ms, flip angle = 90°) were obtained via a gradient-echo echo-planar imaging sequence (EPI). The slice thickness was 5 mm (no slice gap) with a matrix size of 64 × 64 and a field of view of 240 × 240 mm2, resulting in a voxel size of 3.75 × 3.75 × 5 mm3. Each brain volume comprised 30 axial slices and each functional run contained 200 image volumes.
Functional image preprocessing
fMRI data preprocessing and statistical analysis was performed using statistical parametric mapping software (SPM8, http://www.fli.ion.ucl.ac.uk/sm). The first ten volumes of functional images were removed for the signal equilibrium and participants’ adaptation to scanning environment. The remaining EPI images were preprocessed using the following steps: slice timing, motion correction, spatial normalization to the standard Montreal Neurological Institute (MNI) EPI template in SPM8 and resample to 3 × 3 × 3 mm3, followed by spatial smoothing with 8-mm full-width at half-maximum (FWHM) Gaussian kernel. According to the record of head motions within each fMRI run, no participant had more than 1.5 mm maximum displacement in any direction of x, y and z or more than 1° of angular rotation about each axis.
ALFF analysis
ALFF was calculated using REST (http://restfmri.net/forum/rest_v17) with a voxel-based approach similar to that used in our earlier study [8]. After preprocessing, the time series for each voxel was filtered (band pass, 0.01–0.08 Hz) to remove the effects of very low frequency drift and high-frequency noise, e.g., respiratory and heart rhythms. The filtered time series was transformed to a frequency domain using a fast Fourier transform (FFT) (parameters: taper percent = 0, FFT length = shortest) and the power spectrum was then obtained by square-rooted FFT and averaged across 0.01–0.08 Hz at each voxel. This averaged square root was taken as the ALFF. The ALFF of each voxel was divided by the global mean ALFF value to standardize data across subjects.
VBM analysis
Recent fMRI studies have suggested that gray matter (GM) abnormalities may produce partial effects on functional images [13]. To address the potential influence, a voxel-based morphometry (VBM) analysis for structural images was performed to explore whether GM abnormalities occur in our samples. In brief, scanner artifacts and gross anatomical abnormalities were checked for each subject; and the image origin was set to the anterior commissure. Next, MR images were segmented into GM, white matter, and cerebrospinal fluid using the unified segmentation model in SPM8. Afterwards, GM segmented images were normalized to a GM population template generated from the complete image set using the Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) registration method, which is a nonlinear warping technique minimizing between-subject structural variations [14]. Finally, spatially normalized images were modulated to ensure that the overall amount of each tissue class was not altered by the spatial normalization procedure, and smoothed with an 8-mm full-width at half-maximum Gaussian kernel.
Statistical analysis
Clinical data were analyzed using SPSS 17.0 (SPSS Inc, Chicago, IL, USA). The results of continuous data were presented as mean ± standard deviation. Differences of age at scan among patients and HCs were analyzed using two-sample t-tests. Statistical comparison of gender was conducted using χ 2 tests. A two-tailed p-value less than 0.05 indicated statistical significant.
The differences of voxel-based GM volume between BSP patients and HCs groups were analyzed using SPM8 with a two-sample t-test with total intracranial volume as covariates. The significance of group differences was set at the threshold of voxel-wise p < 0.005 and cluster-level of p < 0.05 corrected by family-wise error (FWE) correction.
Voxel-based comparisons of entire brain ALFF maps between two groups were analyzed with a two-sample t test in SPM8. Significance was set at the threshold of voxel-wise p < 0.005 and cluster-level of p < 0.05 corrected by FWE correction. The results were presented using the voxel of peak significance.
The average ALFF of all voxels in the abnormal areas revealed by voxel-based analysis was extracted separately using the volume of interest (VOI) in SPM. Correlation analyses were conducted to investigate the possible relationship between the mean ALFF within VOIs and clinical features including age at onset, disease duration, JRS severity score, JRS frequency score, JRS total score, presence of geste antagoniste test, with p-values less than 0.05 (two-tailed) deemed statistically significant.
Results
Demographic and clinical characteristics
Clinical and demographic characteristics of the samples and levels of significance of the clinical variables were shown in Table 1. There were no significant differences in gender and age between BSP patients and HCs. Most of the BSP patients have never received botulinum neurotoxin (BoNT) treatment before fMRI scanning, except two patients were treated with BoNT 1 and 2 years ago, respectively.
ALFF changes in the BSP group
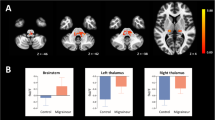
By comparison, the ALFF values of BSP patients and HCs, BSP patients showed significantly decreased ALFF in the left thalamus. The ALFF values were also lower in the right thalamus, but the difference was not significant. BSP patients had higher ALFF in the bilateral orbitofrontal area extending from middle frontal gyrus to inferior frontal gyrus, but the change in the right orbitofrontal area was not significant. The peak voxels within these significant different clusters were shown in Fig. 1; Table 2.
Sagittal, coronal and axial sections display ALFF differences between BSP patients and healthy controls. There were decreased ALFF in the bilateral thalamus, and increased ALFF in the bilateral orbitofrontal area. For visualization, data were shown at a voxel-wise threshold of p < 0.005 with cluster more than 40 voxels
Correlation between the ALFF and clinical data
In BSP patients, the mean ALFF in the left thalamus were negatively correlated with the sub-score of JRS-frequency (r = −0.484, p = 0.042, Fig. 2a) and JRS total score (r = −0.477, p = 0.045, Fig. 2a). A borderline positive correlation was detected between the mean ALFF in the left orbitofrontal area and disease duration (r = 0.485, p = 0.049, Fig. 2b).
Correlations between ALFF value of significantly different areas and clinical measurements. a Left thalamus shows increased ALFF and scatter plots show correlations between ALFF of this area and sub-score of JRS-frequency (r = −0.484, p = 0.042) and JRS total score (r = −0.477, p = 0.045) in the BSP group. b Left orbitofrontal area shows increased ALFF and scatter plots show correlations between ALFF of this area and disease duration in the BSP group (r = 0.485, p = 0.049)
VBM changes in the BSP group
The GM volume comparison didn’t show any significant difference between BSP patients and HCs.
Discussion
The present study investigates the regional brain activity in patients with BSP by ALFF approach at rest. Compared to HCs, BSP patients demonstrate altered neuronal activity in thalamus and orbitofrontal area. Furthermore, the ALFF value of the left thalamus negatively correlated with the sub-score of JRS-frequency and JRS total score, the ALFF value of the left orbitofrontal area positively correlated with disease duration.
Our results demonstrated decreased ALFF in the thalamus, which has often been mentioned to be associated with BSP and other primary focal dystonia [15]. This result was supported by the abnormal brain activities in thalamus identified by position emission tomography (PET) studies and task-related fMRI studies [4, 15]. Gray matter atrophy in these areas also provided anatomical basis for the above findings [16]. The observation of secondary BSP in patients with thalamic infarcts further supported the deactivation of thalamus may be the primary defect in BSP [17]. As the thalamus is connected with a wide region of cortex including parietal cortex, extrastriate cortices, temporal lobe and somatosensory cortex [18], the dysfunction of thalamus-cortical loops may lead to various symptoms of BSP patients. Long latency of saccade reported in BSP patients may be contributed by the disturbance of thalamus, which is involved in integration of visual and ocluomotor signals [19, 20]. Most of our BSP patients suffered photophobia, a sensory symptom that ocular discomfort caused by bright light. This symptom may be mediated by the dysfunction of thalamus, which receives visual signals from lateral geniculate nucleus and mediates unconscious visual perception [21]. Although there is limited evidence on cognition of primary dystonia, relative poor performance on visuo-spatial tasks has been identified in patients with primary dystonia [22, 23]. Animal studies found the pulvinar of thalamus synchronized activity between interconnected cortical areas according to attention allocation, indicating its critical role in attention selection and more generally in regulating information transmission across the visual cortex [24]. The deficit of response competition in pulvinar-lesioned patients suggested pulvinar contributed to linking vision and action [25]. Taking together, the abnormality of thalamus is consistent with the hypothesis that primary dystonia is a manifestation of abnormal modification of cortical motor control and malfunction of sensorimotor integration [26]. In addition, we found the ALFF value of thalamus correlated with both the sub-score of JRS-frequency and JRS total score. The correlation of ALFF value and clinical symptom suggested the ALFF value of this region may be a quantitative marker in measuring the symptom of BSP.
In contrast to the decreased ALFF in the thalamus, we observed increased ALFF in the left orbitofrontal area extending from middle frontal gyrus to inferior frontal gyrus. Activation of the orbitofrontal cortex (OFC) during voluntary eye blinking was revealed by fMRI study and indicated that OFC is involved in eye blinking [27]. fMRI studies also found that this area is involved in sensorimotor output, and the activation in inferior and middle frontal is associated with motor inhibition [28, 29]. Hyperactivity in this area might suggest that BSP patients have to expend additional effort to compensate for lost sensorimotor control and malfunctioning sensorimotor integration. Another possible explanation for this finding is that the abnormal sensory input from repetitive movements or dystonic posture might alter cortical motor processing [30]. In addition, neuroimaging and neuropathologic studies have implicated the abnormality of the OFC in the pathophysiology of depression and obsessive–compulsive disorder (OCD) [31, 32], which have been associated with primary dystonia [33, 34]. The finding of OFC overactivity in this study might be a correlate of such an association. The trend towards correlation of increased ALFF in OFC with disease duration observed in present study, may support the hypothesis that repetitive involuntary movements cause cortical plasticity and sensorimotor disintegration in BSP patients as disease progression [30].
Interestingly, the present study have been unable to document morphometric changes in GM in our sample with relative short illness duration. This may suggest that the initial changes in the brain are functional and subsequently there are structural abnormalities that are detectable by morphometric imaging as the disease progresses.
Although previous task-related fMRI studies showed a wider range of abnormal brain activity in BSP patients, including visual cortex, cingulate cortex, cerebellum, supplementary motor area, somatosensory areas [15], the present study didn’t found significant deficit of brain activity in these areas in spite of using a relatively liberal threshold. We have to notice that few regions were consistently identified by these different task-related fMRI [15]. Relatively short disease duration of our patients may also contribute to the difference. Compared with the recently published rsfMRI study in BSP, our independent study with larger sample size and more stringent criteria for both controlling the head motion and setting threshold for significance of the results, gives a more convincing result and lower the possibility of false positive result. Furthermore, we have detected the association of thalamus with symptom severity, and the association of the orbitofrontal cortex with disease duration [35]. As ALFF measures reflect spontaneous synchronous neural activity at voxel level rather than the integration of functional brain activity at the network level, so we can’t observe the potential dysfunction of links among different brain regions in BSP patients. A combined analysis with functional network connectivity would further our understanding of the pathophysiology of BSP.
The limitations of our study were as follows. First, the sample size is relatively small, which limits the statistical power and the generalization of the results. Second, similarly to other rfMRI studies, we could not completely eliminate the effects of physiological noise, such as cardiac and respiratory fluctuations. Third, lack of objective assessment of neuropsychological measures, such as affective processing, cognitive function, hinders our interpretations of our results.
Conclusions
In conclusion, we adopted ALFF to investigate the difference in resting state brain activity between BSP patients and HCs. Our findings suggest sensorimotor integration is abnormal in BSP, and dysfunctional activity of thalamus may be used to measuring symptom severity in BSP patients.
References
Hallett M, Evinger C, Jankovic J, Stacy M (2008) Update on blepharospasm: report from the BEBRF international workshop. Neurology 71(16):1275–1282. doi:10.1212/01.wnl.0000327601.46315.85
Herz NL, Yen MT (2005) Modulation of sensory photophobia in essential blepharospasm with chromatic lenses. Ophthalmology 112(12):2208–2211. doi:10.1016/j.ophtha.2005.06.030
Hallett M (1995) Is dystonia a sensory disorder? Ann Neurol 38(2):139–140. doi:10.1002/ana.410380203
Baker RS, Andersen AH, Morecraft RJ, Smith CD (2003) A functional magnetic resonance imaging study in patients with benign essential blepharospasm. J Neuroophthalmol 23(1):11–15
Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, Ceballos-Baumann AO (2006) Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain 129(Pt 1):36–46. doi:10.1093/brain/awh665
Goncalves SI, de Munck JC, Pouwels PJ, Schoonhoven R, Kuijer JP, Maurits NM, Hoogduin JM, Van Someren EJ, Heethaar RM, Lopes da Silva FH (2006) Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variability. Neuroimage 30(1):203–213. doi:10.1016/j.neuroimage.2005.09.062
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29(2):83–91. doi:10.1016/j.braindev.2006.07.002
Luo C, Chen Q, Huang R, Chen X, Chen K, Huang X, Tang H, Gong Q, Shang HF (2012) Patterns of spontaneous brain activity in amyotrophic lateral sclerosis: a resting-state FMRI study. PLoS One 7(9):e45470. doi:10.1371/journal.pone.0045470
Guo WB, Liu F, Xun GL, Hu MR, Guo XF, Xiao CQ, Chen HF, Wooderson SC, Chen JD, Zhao JP (2013) Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog Neuropsychopharmacol Biol Psychia 40:153–159. doi:10.1016/j.pnpbp.2012.08.014
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Fahn S, Marsden CD, Calne DB (1987) Classification and investigation of dystonia. In: Marsden CD, Fahn S (eds) Movement disorders, vol 2. Butterworth, London, pp 332–358
Jankovic J, Orman J (1987) Botulinum A toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology 37(4):616–623
Oakes TR, Fox AS, Johnstone T, Chung MK, Kalin N, Davidson RJ (2007) Integrating VBM into the general linear model with voxel wise anatomical covariates. Neuroimage 34(2):500–508. doi:10.1016/j.neuroimage.2006.10.007
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1):95–113. doi:10.1016/j.neuroimage.2007.07.007
Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MAJ (2011) Structural, functional and molecular imaging of the brain in primary focal dystonia—a review. Neuroimage 56(3):1011–1020. doi:10.1016/j.neuroimage.2011.02.045
Obermann M, Yaldizli O, De Greiff A, Lachenmayer ML, Buhl AR, Tumczak F, Gizewski ER, Diener HC, Maschke M (2007) Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord 22(8):1117–1123. doi:10.1002/mds.21495
Miranda M, Millar A (1998) Blepharospasm associated with bilateral infarcts confined to the thalamus: case report. Mov Disord 13(3):616–617. doi:10.1002/mds.870130347
Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6(7):750–757. doi:10.1038/nn1075
Bollen E, Van Exel E, van der Velde EA, Buytels P, Bastiaanse J, van Dijk JG (1996) Saccadic eye movements in idiopathic blepharospasm. Mov Disord 11(6):678–682. doi:10.1002/mds.870110613
Arend I, Machado L, Ward R, McGrath M, Ro T, Rafal RD (2008) The role of the human pulvinar in visual attention and action: evidence from temporal-order judgment, saccade decision, and anti saccade tasks. Prog Brain Res 171:475–483. doi:10.1016/s0079-6123(08)00669-9
Emoto H, Suzuki Y, Wakakura M, Horie C, Kiyosawa M, Mochizuki M, Kawasaki K, Oda K, Ishiwata K, Ishii K (2010) Photophobia in essential blepharospasm––a positron emission tomographic study. Mov Disord 25(4):433–439. doi:10.1002/mds.22916
Scott RB, Gregory R, Wilson J, Banks S, Turner A, Parkin S, Giladi N, Joint C, Aziz T (2003) Executive cognitive deficits in primary dystonia. Mov Disord 18(5):539–550. doi:10.1002/mds.10399
Hinse P, Leplow B, Humbert T, Lamparter U, Junge A, Emskotter T (1996) Impairment of visuospatial function in idiopathic spasmodic torticollis. J Neurol 243(1):29–33
Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S (2012) The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337(6095):753–756. doi:10.1126/science.1223082
Danziger S, Ward R, Owen V, Rafal R (2004) Contributions of the human pulvinar to linking vision and action. Cogn Affect Behav Neurosci 4(1):89–99
Kerrison JB, Lancaster JL, Zamarripa FE, Richardson LA, Morrison JC, Holck DE, Andreason KW, Blaydon SM, Fox PT (2003) Positron emission tomography scanning in essential blepharospasm. Am J Ophthalmol 136(5):846–852
Tsubota K, Kwong KK, Lee TY, Nakamura J, Cheng HM (1999) Functional MRI of brain activation by eye blinking. Exp Eye Res 69(1):1–7. doi:10.1006/exer.1999.0660
Swick D, Ashley V, Turken AU (2008) Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 9(1):102. doi:10.1186/1471-2202-9-102
Simonyan K, Ludlow CL (2010) Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex 20(11):2749–2759. doi:10.1093/cercor/bhq023
Egger K, Mueller J, Schocke M, Brenneis C, Rinnerthaler M, Seppi K, Trieb T, Wenning GK, Hallett M, Poewe W (2007) Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord 22(11):1538–1542. doi:10.1002/mds.21619
Drevets WC (2007) Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci 1121:499–527. doi:10.1196/annals.1401.029
Lagemann T, Rentzsch J, Montag C, Gallinat J, Jockers-Scherubl M, Winter C, Reischies FM (2012) Early orbitofrontal hyper activation in obsessive-compulsive disorder. Psychiatry Res 202(3):257–263. doi:10.1016/j.pscychresns.2011.10.002
Broocks A, Thiel A, Angerstein D, Dressler D (1998) Higher prevalence of obsessive-compulsive symptoms in patients with blepharospasm than in patients with hemifacial spasm. Am J Psychia 155(4):555–557
Lencer R, Steinlechner S, Stahlberg J, Rehling H, Orth M, Baeumer T, Rumpf HJ, Meyer C, Klein C, Muenchau A, Hagenah J (2009) Primary focal dystonia: evidence for distinct neuropsychiatric and personality profiles. J Neurol Neurosurg Psychiatry 80(10):1176–1179. doi:10.1136/jnnp.2008.170191
Zhou B, Wang J, Huang Y, Yang Y, Gong Q, Zhou D (2013) A resting state functional magnetic resonance imaging study of patients with benign essential blepharospasm. J Neuroophthalmol. doi:10.1097/WNO.0b013e31828f69e5
Acknowledgments
This study was supported by the National Natural Science Foundation (Grant Nos. 30973149, 81030027, 81227002 and 81220108013) and National Key Technologies R&D Program (Program No. 2012BAI01B03) of China. We thank Ms. XiaoYan Guo for statistical assistance, and thank the technical staff of the Department of Radiology for their collaboration and assistance.
Conflicts of interest
No conflict of interest concerning the research related to the manuscript and no founding support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, J., Luo, C., Song, W. et al. Altered regional spontaneous neuronal activity in blepharospasm: a resting state fMRI study. J Neurol 260, 2754–2760 (2013). https://doi.org/10.1007/s00415-013-7042-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7042-8