Abstract
This study assessed brain structural alterations in two diverse clinical forms of functional (psychogenic) dystonia (FD) – the typical fixed dystonia (FixFD) phenotype and the “mobile” dystonia (MobFD) phenotype, which has been recently described in one study. Forty-four FD patients (13 FixFD and 31 MobFD) and 43 healthy controls were recruited. All subjects underwent 3D T1-weighted and diffusion tensor (DT) magnetic resonance imaging (MRI). Cortical thickness, volumes of gray matter (GM) structures, and white matter (WM) tract integrity were assessed. Normal cortical thickness in both FD patient groups compared with age-matched healthy controls were found. When compared with FixFD, MobFD patients showed cortical thinning of the left orbitofrontal cortex, and medial and lateral parietal and cingulate regions bilaterally. Additionally, compared with controls, MobFD patients showed reduced volumes of the left nucleus accumbens, putamen, thalamus, and bilateral caudate nuclei, whereas MobFD patients compared with FixFD demonstrated atrophy of the right hippocampus and globus pallidus. Compared with both controls and MobFD cases, FixFD patients showed a severe disruption of WM architecture along the corpus callous, corticospinal tract, anterior thalamic radiations, and major long-range tracts bilaterally. This study showed different MRI patterns in two variants of FD. MobFD had alterations in GM structures crucial for sensorimotor processing, emotional, and cognitive control. On the other hand, FixFD patients were characterized by a global WM disconnection affecting main sensorimotor and emotional control circuits. These findings may have important implications in understanding the neural substrates underlying different phenotypic FD expression levels.
Similar content being viewed by others
Introduction
Functional (psychogenic) movement disorders (FMDs), part of the spectrum of functional neurological disorders (FNDs) or conversion disorders – are defined as the occurrence of abnormal movements that do not result from a known general medical or neurologic cause. Functional dystonia (FD) is a controversial FMD, probably the most challenging in terms of diagnosis and treatment [1]. Patients with the typical FD phenotype present fixed dystonia (FixFD), characterized by sudden onset of fixed abnormal posture of the affected limb, usually precipitated by a minor peripheral trauma, and accompanied by early and severe pain [2]. Recently, new FD phenotypes, characterized predominantly by dystonia of different quality have been recognized [3,4,5]. In addition, besides well-defined FixFD cases, in our clinical experience we frequently observed another presentation of FD that we named “mobile” FD (MobFD), appearing mainly with axial distribution, absence of fixed dystonic posturing, and relapsing-remitting disease course [6].
The major question is whether underlying neurobiology of FD is common regardless of the distinct phenotypes or unique neurobiological mechanisms are responsible for different clinical presentations. The main neurobiological model of FMD suggests a key role for processes affecting attentional focus on movement, beliefs/expectations (“priors” within a predictive coding framework of brain function), and a resultant impairment of sense of agency [7]. Functional neuroimaging findings in FND suggest that the key pathways implicated in the pathophysiology are those involving prefrontal–insular–amygdalar regions, posterior parietal cortex/temporoparietal junction, and premotor areas [8].
The fact that the majority of studies in FND are based on functional neuroimaging [8], and that only a few cover FD solely [9], emphasizes the need for in vivo insight into the structural brain organization in FD. Interestingly, cortical thickness abnormalities in both motor and premotor regions were attributed to the type of motor conversion symptoms, that is, increased cortical thickness in patients with negative symptoms (functional paralysis) [10] and decreased in patients with positive (paroxysmal) symptoms (psychogenic non-epileptic seizures) [11]. Similarly, the opposite pattern of subcortical activity (basal ganglia and thalamus) was related to hypo- and hyper-kinetic symptoms in FND (decreased and increased activity, respectively) [9, 12]. Volumetric analysis of subcortical structures in FND demonstrated volume reductions of lentiform nuclei and thalamus [13]. Additionally, affection of white matter (WM) tracts that were supposed to be associated with emotion regulation and motor pathways (i.e., corona radiata, internal/external capsules, superior temporal WM, uncinate fasciculus), were described in patients with psychogenic non-epileptic seizures [14].
The aim of this study was to define patterns of cortical, basal ganglia, and WM tract alterations in two diverse clinical FD forms [6]. We hypothesized that FixFD, as clinically more severe and therapeutically resistant variant, would be characterized by a widespread damage of the main motor, cognitive, and behavioral brain networks, whereas MobFD would present more focal alterations in the key sensorimotor and emotional controlling brain nodes.
Methods
Participants
Forty-four patients with dystonia fulfilling criteria of “clinically documented” FMD proposed by Gupta and Lang [15] were included. Standard investigations for secondary dystonia were normal in all patients, including brain conventional magnetic resonance imaging (MRI) findings. Genetic tests for mutations in the DYT1, DYT6, and DYT11 genes were negative in all patients. FD patients were subdivided into two groups: 13 FixFD, characterized by fixed posture of extremities, pain at dystonia localization (often with the chronic regional pain syndrome, CRPS), static or progressive course with spreading of dystonia to other body regions, and no botulinum toxin treatment response; and 31 MobFD, characterized by cranial, cervical or trunkal localization, variable intensity of dystonia with phasic characteristics, relapsing-remitting course, good response to botulinum toxin treatment, and potential presence of additional FMD/FND. The whole group of FD patients was matched by age and gender with 43 healthy controls (HCs). For further analysis, HC group was divided into two groups: younger HC (yHC) to be age-matched with FixFD patients; and older HC (oHC) to be age-matched with MobFD patients (Table 1).
All patients underwent clinical examination and completed questionnaires regarding several demographic and clinical features (Table 1). Experienced movement disorders specialists (VSK, IP), blinded to MRI, examined all patients. Patients underwent semi-structured interview assessing triggers for dystonia, pain in dystonic regions and CRPS, presence of sensory tricks, botulinum toxin treatment. Botulinum toxin treatment response was defined as self-rated clinical improvement in percentage. Severity of dystonia was assessed using the Unified Dystonia Rating Scale (UDRS) [16], the Burke-Fahn-Marsden Dystonia Rating Scale (BFMS total score and disability score) [17], and the Psychogenic Movement Disorders Scale (PMD total, phenomenology score and functional scores) [18]. Cognitive and psychiatric evaluation was performed by expert psychiatrics, blinded to MRI, and consisted of Mini-Mental State Examination [19], Hamilton Depression Rating Scale [20], Hamilton Anxiety Rating Scale [21], Apathy Scale [22], Somatoform Dissociation Questionnaire [23], and Dissociative Experiences Scale II [24].
Approval was received from the ethics committee on human experimentation of the School of Medicine, University of Belgrade, and written informed consent was obtained from all subjects participating in the study
MRI study
Using a 1.5 Tesla Philips Medical System Achieva scanner, dual-echo turbo spin-echo, 3D sagittal T1-weighted Turbo Field Echo, and pulsed gradient spin-echo single shot echo-planar images for diffusion tensor (DT) MRI were acquired from all study participants. Cortical thickness analysis, gray matter (GM) volume measures, and voxel-wise DT MRI analysis were performed. The Supplementary data reports the complete MRI protocol, and details on MRI analysis.
Statistical analysis
Demographic and clinical variables were reported as mean ± standard deviation (SD) or absolute and relative frequencies (percentages) for continuous and categorical variables, respectively. Normal distribution assumption was checked by means of Q-Q plot and Shapiro–Wilk and Kolmogorov–Smirnov tests. Pairwise comparisons between groups were performed using the Mann–Whitney U-test and Fisher's exact test for continuous and categorical variables, respectively. All statistical analyses were performed using SAS Release 9.4 (SAS Institute, Cary, NC, USA).
Using SAS, cortical thickness measures, and GM regional volumes were reported as mean ± SD and were compared between FD groups and their age-matched HC using analysis of variance (ANOVA) models, which account for homogeneous or heterogeneous groups’ variances (on the basis of significance of Levene’s test) as appropriate, followed by two suitable post-hoc pairwise comparisons (i.e., FixFD vs yHC; MobFD vs oHC). The p-values were adjusted for multiple comparisons following the false discovery rate (FDR) method. Each p-value was corrected at a cluster level, by the number of investigated cortical thickness and GM regional volume variables, separately. The same ANOVA models were equivalently rerun including the following binary covariates: (i) presence of FD (yes vs no); (ii) belonging to a specific age class (old vs young); and (iii) FD-by-age class interaction term. To evaluate whether MRI findings between FD and HC were different according to FD variant (i.e., in order to identify patterns of MRI abnormalities specific to each FD variant), the significance of the interaction terms were considered (p interaction). Furthermore, to account for potential groups’ mismatch, ANOVA models were adjusted for subjects’ age.
DT MRI voxel-wise statistics were performed using a permutation-based inference tool for nonparametric statistical thresholding (“randomize”, part of FSL) [25]. The number of permutations was set at 5000. Mean diffusivity (MD), fractional anisotropy (FA), axial (axD), and radial (radD) diffusivity values within the skeleton were tested between FD groups and age-matched HC using permutation-based two-sample t-tests. To investigate regions of WM alterations specific to each FD variant, an interaction analysis was performed adjusting for age (see above). The resulting statistical maps were thresholded at p < 0.05 adjusting for multiple comparisons at a cluster level using the threshold-free cluster enhancement (TFCE) option [26].
In the two FD groups, correlations between cortical thickness measures and GM regional volumes and clinical characteristics were assessed by Spearman correlation coefficients (p < 0.05 FDR corrected; SAS). Correlations between DT MRI findings and clinical variables were assessed using general linear models in FSL (5000 permutations, p < 0.05 TFCE corrected for multiple comparisons).
Results
Clinical and demographic features
Each FD group was similar to age-matched HC in terms of age and sex (Table 1). Compared with MobFD, patients with FixFD were younger, with younger age at disease onset, more pronounced dystonia severity (BFMS, UDRS), as well as disability due to dystonia (BFMS disability), and less effective botulinum toxin treatment (Table 1). Additionally, pain was more frequent in FixFD relative to MobFD patients, whereas CRPS was exclusively present in FixFD (Table 1). The two groups of patients did not differ in cognitive and psychiatric features (Table 1).
Cortical thickness
FD patients relative to all HC did not show cortical thickness alterations (Supplementary table). When compared with age-matched HC groups, neither FixFD nor MobFD patients showed abnormalities in mean cortical thickness measurements (Supplementary table). However, in the interaction model, MobFD compared with FixFD patients showed cortical thinning of the left orbitofrontal cortex, and medial and lateral parietal and cingulate regions bilaterally (Supplementary table, Fig. 1).
Cortical thinning in MobFD compared with FixFD patients. Distribution of the cortical thinning on the pial surface in patients with mobile functional dystonia (MobFD) compared with those with fixed functional dystonia (FixFD). The p-values refer to interaction model, corrected for multiple comparisons using false discovery rate method (see text for further details). Colors represent p-values: orange = 0.001 ≥ p < 0.01; red = 0.01 ≥ p ≤ 0.02; dark red = 0.02 > p < 0.05. L left, R right
GM volumes
FD patients relative to all HC and FixFD patients relative to yHC did not show GM volume alterations (Table 2). Compared with oHC, MobFD patients showed reduced volumes of the left nucleus accumbens, putamen, thalamus, and bilateral caudate nuclei (Table 2). In the interaction analysis, MobFD compared with FixFD patients demonstrated atrophy of the right hippocampus and globus pallidus (Table 2).
WM tracts
FD patients relative to all HC showed decreased FA and increased MD and radD values in splenium of the corpus callosum, brainstem, and corticospinal tract, anterior thalamic radiations and major long-range WM tracts predominantly on the right side (superior longitudinal fasciculus [SLF] and inferior longitudinal fasciculus, inferior fronto-occipital fasciculus [IFOF], uncinate fasciculus, cingulum bundle) (p < 0.05, TFCE corrected; Supplementary figure). No axD alterations were detected.
Compared with both yHC and MobFD patients (interaction model), FixFD patients showed a widespread pattern of decreased FA and increased MD, axD, and radD values along the corpus callosum, corticospinal tract, anterior thalamic radiations, and major long-range WM tracts (p < 0.05, TFCE corrected; Figs. 2 and 3). All changes were distributed and bilateral on MD and radD maps, whereas they were less widespread with slightly more affected right side on FA and axD maps (p < 0.05, TFCE corrected; Figs. 2 and 3).
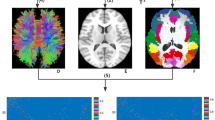
White matter diffusion tensor MRI findings in FixFD patients relative to healthy controls. Decreased fractional anisotropy (FA, red) and increased mean (MD, blue), axial (axD, pink), and radial diffusivities (raD, yellow) in patients with fixed functional dystonia (FixFD) relative to healthy controls. Results are overlaid on the axial sections of the Montreal Neurological Institute standard brain in neurological convention (right is right), and displayed at p < 0.05 corrected for multiple comparisons at the cluster level using the threshold-free cluster enhancement option. The white matter skeleton is green. L left, R right
White matter diffusion tensor MRI findings in FixFD patients relative to MobFD patients. Decreased fractional anisotropy (FA, red) and increased mean (MD, blue), axial (axD, pink), and radial diffusivities (raD, yellow) in patients with fixed functional dystonia (FixFD) relative to mobile functional dystonia (MobFD). Results are overlaid on the axial sections of the Montreal Neurological Institute standard brain in neurological convention (right is right), and displayed at p < 0.05 corrected for multiple comparisons at the cluster level using the threshold-free cluster enhancement option. The white matter skeleton is green. L left, R right
MobFD patients did not differ from oHC in any of DT MRI measurement.
Correlations
In FixFD and MobFD patients, no correlation was found between MRI and clinical variables.
Discussion
In this study, we showed different patterns of structural changes in two clinically distinct forms of FD – FixFD and MobFD. The main findings are: (1) normal cortical volumes in both FD patient groups compared with age-matched HC, but cortical thinning in the frontal, parietal, and cingulate regions in MobFD compared with FixFD patients; (2) atrophy of basal ganglia and thalamus in patients with MobFD; and (3) severe disruption of WM tract architecture (cognitive, emotional, and motor pathways) in patients with FixFD.
Brain cortical alterations in MobFD
The critical role of sensorimotor circuit and its connections in FND is well documented [11, 12, 27]. In line with previous reports [11], we confirmed decreased cortical thickness as a marker of positive motor conversion disorders, with predominant affection of extramotor regions.
Of particular interest is cortical thinning of parietal lobe areas we observed in MobFD compared with FixFD patients. Sense of involuntariness of functional symptoms/movements has been attributed to the loss of self-agency and impairment of intentional or prediction process due to hypoactivation of the right inferior parietal lobe, encompassing the right temporoparietal junction [27]. Further on, the left supramarginal gyrus, a critical region for transforming spatial information into code for action, could be involved in generating and planning motor action in conversion disorders [28]. It was suggested that functional palsy is a result of abnormal motor initiation [28], whereas FD might be due to an abnormal movement conceptualization process. The role of precuneus in FND is attributed to memory representations (including sensory or agency information) that could modulate motor or sensory processes [29]. We can speculate that altered morphology of parietal regions in MobFD patients could be driven by prior stress events, subsequently affecting motor behavior.
Cortical thinning of frontal areas such as inferior frontal gyrus (IFG) was also observed in MobFD subjects. Left IFG has been suggested to play a role in inhibitory processes, including the tendency to inhibit learning from undesirable information or inhibiting unwanted news from altering beliefs [30]. In line with this, left IFG pars orbitalis atrophy could be associated with abnormal beliefs and expectations, one of the main explanatory domains of the neurobiological theory of FMD [7].
In keeping with the role of cingulum in depression, memory, and pain processing [31], bilateral cortical thinning of the isthmus cingulate in MobFD patients is of quite relevance. However, although MobFD patients had higher scores on depression and apathy scales compared with FixFD, differences were not significant.
GM volume changes in MobFD
Abnormal subcortical activation, including the basal ganglia, thalamus, and cerebellum, was described in FND patients [9, 12], and could be related to altered fronto-subcortical circuits mediating motor intention or attention, with possible contribution from reciprocal connections with the amygdala and orbitofrontal cortex leading to disruption of movement, motivation and attention [9]. In keeping with this hypothesis, we found atrophy of the left nucleus accumbens, putamen and thalamus, and caudate nuclei bilaterally in MobFD patients compared with oHC. In addition, when compared with FixFD group, volume reductions of the right hippocampus and pallidum were observed in MobFD.
Thalamic structural [13] and functional changes [12] were previously reported in FND. Transient hypoactivation of contralateral thalamus and basal ganglia in functional sensory loss during symptomatic state was related to the role of striatothalamocortical premotor loops in voluntary movement generation and sensorimotor control [12]. Similarly, thalamic volume reductions were described in functional palsy [13], and following limb immobilization [32] suggesting that lack of motor activity or deafferentation might influence brain plasticity, and therefore induce changes of thalamic volume. Notably, decreased thalamic volume in our patients with MobFD did not correlate with clinical features. As in other forms of motor conversion disorders [13], this finding points toward primary nature of structural changes and crucial role of thalamic circuits in the pathogenesis of FND. Furthermore, structural thalamic alterations were common findings in different forms of isolated focal dystonia [33] making them an unspecific feature of various phenotypic disease expression levels.
The most significant volume reductions in MobFD patients were described in bilateral caudate nuclei, key relay subcortical structures that orchestrate cognitive and emotional processing with (aberrant) motor response [12]. Atrophy of the nucleus accumbens suggested the potential role of reward circuit in pathophysiology of conversion.
Right hippocampus atrophy, which we found in the comparison between FixFD and MobFD patients, is well documented in altered fear response [34]. Similarly, stress events preceding MobFD might contribute to hippocampal failure to process contextual cues, which continues even in the absence of real stressor exposition, leading to miscommunication among hippocampus, amygdala, and ventro-medial prefrontal cortex, and possibly to aberrant motor behavior.
Brain WM changes in FixFD
Compared with yHC, patients with FixFD had decreased FA and increased diffusivity values in the WM tracts of the cerebellum, brainstem, thalamus, and WM underlying frontal, parietal, temporal, and occipital cortices bilaterally. Global disruption of WM networks has not been previously described in FD or FND, whereas WM alterations are well known in various psychiatric disorders, including schizophrenia [35] and affective disorders [36]. Interestingly, in our patients with FixFD, changes in WM were not associated with pattern of cortical atrophy, as it would have been expected.
All associative WM pathways were affected in patients with FixFD. SLF is the largest fiber bundle that connects frontal lobes with parieto-temporal associative areas, and has roles in the regulation of higher aspects of motor behavior, spatial attention, ideomotor praxis, monitoring hand, and facial action [37]. Of particular interest is the part of the SLF connecting posterior parietal lobe with prefrontal cortex as it can be related to the concept of “body schema”, spatial neglect or various neglect syndromes [38]. Even though motor neglect (impaired motor intention generation despite the absence of corticospinal damage) was suggested to resemble to functional weakness [38], we suggest that also FixFD, with fixed posture, immobility, and loss-of-function of affected extremities could be seen as a kind of motor neglect. As previously suggested in conversion disorders [29], an increased recruitment of the prefrontal cortex can represent an altered self-related representation during movements that can inhibit the motor performance. Furthermore, the SLF links the inferior parietal and the lateral temporal cortices to the dorsolateral prefrontal cortex [37], region implicated in the attention network, and possibly plays a role in attentional focus related to movement [7].
IFOF, connecting the dorsolateral and inferolateral frontal cortex with the occipital lobe and posterior temporal cortex [37], contributes to salience and visual processing networks [36]. IFOF alterations may contribute to visual neglect manifestations by impairing the top–down modulation of visual areas from the frontal cortex [37].
Uncinate damage in FixFD might be relevant for disrupted emotional control and impaired amygdala habituation to negative emotional stimuli in motor conversion disorder. The uncinate joins the orbital and polar frontal cortex to the anterior temporal lobe, reaching the amygdala, hippocampal gyrus, uncus, and temporal pole [38]. As being considered as a part of limbic system, and a possible pathway for prefrontal cortex control over limbic regions, uncinate could be involved in decreased top–down control over emotion processing [36]. The motor control can be widely affected by self-relevant emotional information; indeed, the limbic system can induce a significant inhibition on the motor network, which can overcome the normal control of movement mediated by the cortical and subcortical motor structures [29]. Furthermore, reduced FA in the uncinate was consistently shown in the major depression disorder [36], often comorbidity in FD [2, 39].
Damage to the cingulum bundle has been associated with a number of higher order abnormalities, including attention, memory, and emotional processing, as it connects the cingulate gyrus with several cerebral regions, including the premotor, prefrontal and parietal cortices, thalamus, and hippocampus. Of special interest in the pathophysiology of FND are the interconnections of the dorsal anterior cingulate cortex and lateral prefrontal cortex and premotor regions, which are related to attention network, motor preparation, selection of action, cognitive control, emotional appraisal, and expression [8]. Even more, Perez et al. [38] have suggested “neural functional unawareness” construct as a pathophysiology model for FND, with cingular abnormalities, together with posterior parietal cortex, dorsolateral prefrontal cortex and premotor regions, being one of main structures mediating in impaired emotional and interoceptive awareness. Corpus callosum has a central role in interhemispheric connectivity and coordination of cognitive, sensory, and motor functions. Damage of the anterior callosal sections and forceps minor disconnecting frontal areas, present in FixFD, could be related to disruption of motor control [40]. Of special interest might be alterations of callosal fibers connecting parietal regions, because of their role in the perceptual spatial deficits of neglect [38].
The role of the cerebello–thalamo–cortical and basal ganglia–thalamo–cortical circuits are well known in the pathophysiology of “organic” dystonia. Some alterations in anterior thalamo–cortical circuits were described in cervical dystonia [41] suggesting disruption of motor planning and control, which might be relevant for FixFD too. Additionally, changes in the anterior thalamic radiations, together with changes in the medial lemniscus in the pontine brainstem, can be linked to the damage of discriminative and affective pain perception [42], a very important clinical feature of FixFD. The cerebellar output disruption might be important for determining the occurrence of motor symptoms. Finally, damage of the main motor tracts confirms the breakdown of the motor controlling system in FixFD.
FixFD group had more prominent alterations of FA and axD on the right side. It has been suggested that motor and spatial unawareness could be driven by lateralized right-hemisphere dysfunction [38], irrespective to the lateralization of affected body part. Functional interhemispheric disconnection, with the relative preservation of a narrative, interpretative left hemisphere may result in delusional disorders [43], explaining persistent false beliefs in FND patients regarding their disease [38].
Limitations and conclusions
Some limitations of this study should be considered. First, when divided into two groups, MobFD sample was almost three times as large as FixFD group, which, together with different age, challenged the direct comparison between patient groups and requires caution in discussion. Second, the lack of clinical-imaging correlations limits the interpretation of clinical significance of morphological alterations. Potential reasons for the absence of MRI–clinical correlations could be: relatively small groups, heterogeneity of dystonia distribution, and age adjusting, which may have reduced the power of the analysis. Thus, we were not able to suggest a clear relation between dystonia characteristics and specific morphological findings. Third, a thorough neuropsychological and behavioral testing is necessary in interpretation of complex changes affecting cognitive and emotional networks.
Despite these shortcomings, to our knowledge, this is the first description of a specific, cortico–subcortical structural model for clinically different forms of FD. MobFD had morphological changes in GM structures implicated in the pathophysiology of conversion disorders, that is, regions important for sensorimotor processing, emotional, and cognitive control. Therefore, MobFD resembles other FMD/FND, both clinically and morphologically [8]. On the other hand, we found that FixFD, neurologically more severe and complex form of FD, therapeutically resistant, with quite poor prognosis, had a massive and distributed WM damage but without cortical or subcortical GM alterations. We can speculate that FixFD is a disconnection syndrome comparable to other major psychiatric or neurodegenerative diseases where the breakdown of WM networks connecting crucial nodes of motor and emotional control circuits is a primary trait [35, 36]. Clearly, longitudinal studies are warranted to elucidate potential dynamic WM changes or consequent GM damage in this unique pattern of FND. Finally, one may speculate that different structural patterns of the two distinct forms of FD represent two ends of the same tale, or complementary changes of a complex pathophysiology, with FixFD being more similar to major psychiatric/neurodegenerative disorders, and MobFD more close to conversion disorders. All these questions require further research.
References
Espay AJ, Lang AE. Phenotype-specific diagnosis of functional (psychogenic) movement disorders. Curr Neurol Neurosci Rep. 2015;15:32.
Schrag A, Trimble M, Quinn N, Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain. 2004;127(Pt 10):2360–72.
Batla A, Stamelou M, Edwards MJ, Parees I, Saifee TA, Fox Z, et al. Functional movement disorders are not uncommon in the elderly. Mov Disord. 2013;28:540–3.
Fasano A, Valadas A, Bhatia KP, Prashanth LK, Lang AE, Munhoz RP, et al. Psychogenic facial movement disorders: clinical features and associated conditions. Mov Disord. 2012;27:1544–51.
Ganos C, Aguirregomozcorta M, Batla A, Stamelou M, Schwingenschuh P, Munchau A, et al. Psychogenic paroxysmal movement disorders--clinical features and diagnostic clues. Park Relat Disord. 2014;20:41–46.
Petrovic IN, Tomic A, Voncina MM, Pesic D, Kostic VS. Characteristics of two distinct clinical phenotypes of functional (psychogenic) dystonia: follow-up study. J Neurol. 2018;265:82–88.
Edwards MJ. Neurobiologic theories of functional neurologic disorders. Handb Clin Neurol. 2017;139:131–7.
Perez DL, Dworetzky BA, Dickerson BC, Leung L, Cohn R, Baslet G, et al. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci. 2015;46:4–15.
Schrag AE, Mehta AR, Bhatia KP, Brown RJ, Frackowiak RS, Trimble MR, et al. The functional neuroimaging correlates of psychogenic versus organic dystonia. Brain. 2013;136(Pt 3):770–81.
Aybek S, Nicholson TR, Draganski B, Daly E, Murphy DG, David AS, et al. Grey matter changes in motor conversion disorder. J Neurol Neurosurg Psychiatry. 2014;85:236–8.
Ristic AJ, Dakovic M, Kerr M, Kovacevic M, Parojcic A, Sokic D. Cortical thickness, surface area and folding in patients with psychogenic nonepileptic seizures. Epilepsy Res. 2015;112:84–91.
Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124(Pt 6):1077–90.
Nicholson TR, Aybek S, Kempton MJ, Daly EM, Murphy DG, David AS, et al. A structural MRI study of motor conversion disorder: evidence of reduction in thalamic volume. J Neurol Neurosurg Psychiatry. 2014;85:227–9.
Lee S, Allendorfer JB, Gaston TE, Griffis JC, Hernando KA, Knowlton RC, et al. White matter diffusion abnormalities in patients with psychogenic non-epileptic seizures. Brain Res. 2015;1620:169–76.
Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol. 2009;22:430–6.
Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Dystonia Study G. Rating scales for dystonia: a multicenter assessment. Mov Disord. 2003;18:303–12.
Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73–77.
Hinson VK, Cubo E, Comella CL, Goetz CG, Leurgans S. Rating scale for psychogenic movement disorders: scale development and clinimetric testing. Mov Disord. 2005;20:1592–7.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55.
Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–62.
Nijenhuis ER, Spinhoven P, Van Dyck R, Van der Hart O, Vanderlinden J. The development and psychometric characteristics of the Somatoform Dissociation Questionnaire (SDQ-20). J Nerv Ment Dis. 1996;184:688–94.
Carlson EB, Putnam FW, Ross CA, Torem M, Coons P, Dill DL, et al. Validity of the Dissociative Experiences Scale in screening for multiple personality disorder: a multicenter study. Am J Psychiatry. 1993;150:1030–6.
Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25.
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98.
Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–8.
de Lange FP, Toni I, Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia. 2010;48:1782–8.
Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323–37.
Sharot T, Kanai R, Marston D, Korn CW, Rees G, Dolan RJ. Selectively altering belief formation in the human brain. Proc Natl Acad Sci USA. 2012;109:17058–62.
Peng D, Shi F, Li G, Fralick D, Shen T, Qiu M, et al. Surface vulnerability of cerebral cortex to major depressive disorder. PLoS ONE. 2015;10:e0120704.
Draganski B, Moser T, Lummel N, Ganssbauer S, Bogdahn U, Haas F, et al. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006;31:951–7.
Waugh JL, Kuster JK, Levenstein JM, Makris N, Multhaupt-Buell TJ, Sudarsky LR, et al. Thalamic volume is reduced in cervical and laryngeal dystonias. PLoS ONE. 2016;11:e0155302.
Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B, et al. The association of PTSD symptom severity with localized hippocampus and amygdala abnormalities. Chronic Stress (Thousand Oaks) 2017;1:1–18.
Canu E, Agosta F, Filippi M. A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res. 2015;161:19–28.
Jenkins LM, Barba A, Campbell M, Lamar M, Shankman SA, Leow AD, et al. Shared white matter alterations across emotional disorders: a voxel-based meta-analysis of fractional anisotropy. Neuroimage Clin. 2016;12:1022–34.
Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94.
Perez DL, Barsky AJ, Daffner K, Silbersweig DA. Motor and somatosensory conversion disorder: a functional unawareness syndrome? J Neuropsychiatry Clin Neurosci. 2012;24:141–51.
Tomic A, Petrovic I, Pesic D, Voncina MM, Svetel M, Miskovic ND, et al. Is there a specific psychiatric background or personality profile in functional dystonia? J Psychosom Res. 2017;97:58–62.
Berlucchi G. Frontal callosal disconnection syndromes. Cortex. 2012;48:36–45.
Bonilha L, de Vries PM, Hurd MW, Rorden C, Morgan PS, Besenski N, et al. Disrupted thalamic prefrontal pathways in patients with idiopathic dystonia. Park Relat Disord. 2009;15:64–67.
Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415–8.
Devinsky O. Delusional misidentifications and duplications: right brain lesions, left brain delusions. Neurology. 2009;72:80–87.
Acknowledgements
The study was partially supported by the Ministry of Education and Science Republic of Serbia (grant #175090).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AT, ES, SB, DP, MK, and AF declare that they have no conflict of interest. FA is Section Editor of NeuroImage: Clinical; has received speaker honoraria from Biogen Idec and Novartis; and receives or has received research supports from the Italian Ministry of Health, AriSLA (Fondazione Italiana di Ricerca per la SLA), and the European Research Council. IP has received speaker honoraria from Boehringer Ingelheim, GSK, El pharma, Roche and Actavis. VSK has received research grants from Ministry of Education and Science, Republic of Serbia and the Serbian Academy of Science and Arts; speaker honoraria from Novartis and Boehringer Ingelheim. MF is Editor-in-Chief of the Journal of Neurology; serves on a scientific advisory board for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Teva Pharmaceutical Industries, Novartis, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer’s Drug Discovery Foundation (ADDF), the Jacques and Gloria Gossweiler Foundation (Switzerland), and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tomic, A., Agosta, F., Sarasso, E. et al. Are there two different forms of functional dystonia? A multimodal brain structural MRI study. Mol Psychiatry 25, 3350–3359 (2020). https://doi.org/10.1038/s41380-018-0222-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0222-2
- Springer Nature Limited
This article is cited by
-
Structural brain heterogeneity underlying symptomatic and asymptomatic genetic dystonia: a multimodal MRI study
Journal of Neurology (2024)
-
Functional MRI connectivity of the primary motor cortex in functional dystonia patients
Journal of Neurology (2022)
-
Early-life trauma endophenotypes and brain circuit–gene expression relationships in functional neurological (conversion) disorder
Molecular Psychiatry (2021)