Abstract
Objective
Nuclear protein in testis (NUT) carcinoma is characterized by NUT gene rearrangement on chromosome 15. The objective of this study was to investigate the clinical features, immunohistochemistry, treatment, diagnosis and prognosis of sinonasal NUT carcinoma specifically.
Methods
Clinical data for 10 cases of NUT cancer confirmed by pathology were retrospectively analyzed, and the relevant literature was reviewed.
Results
Among the 10 patients, 6 were males, and 4 were females. The median age was 34 years (15–69 years). Nine patients presented with locally advanced cT4a stage. The most common treatment was complete resection combined with radiotherapy, chemotherapy, and targeted therapy. All 10 patients had pathologically poorly differentiated or undifferentiated carcinoma. Furthermore, immunohistochemical staining showed that NUT protein was positive in all 10 patients, and most cases expressed p63, p40 and CK. The Ki-67 positive index of 8 patients ranged from 40 to 80%, with a median of 50%, and NUTM1 gene disruption was detected in both of the remaining cases by FISH. As of April, 2023, all patients were followed up with for 1–51 months, with a median follow-up time of 14 months. Three patients died due to widespread systemic metastasis, 3 relapsed, and 4 had no recurrence or metastasis.
Conclusion
Sinonasal NCs (NUT carcinomas) is a rare and highly aggressive malignant tumor with rapid progression and a poor prognosis. Correct histopathological diagnosis is the primary prerequisite for determining appropriate treatment. There are currently no effective treatment options for NCs. Targeted therapy may become an effective method to treat NCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
NUT (nuclear protein in testis) carcinomas (NCs) is a poorly differentiated or undifferentiated, rare, and highly aggressive malignancy defined by rearrangement of the NUTM1 (aka NUT) gene [1, 2]. In 1991, Kubonishi reported the first case of translocated thymus chromosome t (15; 19) (q15; p13) NUT cancer [3]. Since most cases (about 70%) occur in the midline of the body, such as the chest or head and neck, this type of cancer was originally called “midline cancer” [4, 5]. However, more cases where the primary site occurs outside the midline have since been discovered, including at the parotid gland [6], ilium [7], kidney [8], and pancreas [9], and 50% of NUT cancer patients have distant metastases at diagnosis [10].
No significant difference has been found in the incidence of NUT cancer between men and women, and NC can occur at any age (0.1–82) [11]. In the past, NUT cancer has been reported to occur primarily in children and young adults with a median age of 16–23.6 years [12, 13], but in recent years, the frequency of diagnosis in adults has increased [14]. In 2022, one review wrote that among 310 patients with NC, the median age at diagnosis was 30 years old [11]. Furthermore, according to the literature, this tumor has a poor prognosis, is highly aggressive, and has a median overall survival (OS) of only 6.5–9.7 months [12].
The histological manifestations of NUT carcinoma are nonspecific, presenting as a poorly differentiated or undifferentiated carcinoma with epithelial features, and it may be focally squamous. The poorly or undifferentiated nature of NUT cancers frequently leads to misdiagnosis as undifferentiated squamous cell carcinoma, poorly differentiated nonsmall cell carcinoma, small cell lung cancer, round cell sarcoma, or diffuse large B-cell lymphoma [15]. In 2003, French et al. discovered the fusion gene BRD-NUT in NUT cancer, which encodes a chimeric protein that blocks squamous cell differentiation and keeps the cells in a highly proliferative, poorly differentiated state [16]. Current diagnoses are based on the cytogenetics and/or molecular biology of the tumor, which manifests itself phenotypically as the NUT gene on chromosome 15 rearranging with the BRD4 gene on chromosome 19 (about 70%) [17, 18], or with the BRD3 gene on chromosome 9 (25%) [14], though there are also other rare variants, such as the histone methyltransferase NSD3 on chromosome 8[t(8;15) (p11.23;q14)] [19], ZNF532 on chromosome 18 [t(15; 18)(q14; q23)] [20]. In recent years, the application of NUT rabbit monoclonal antibodies (C52B1, Cell Signaling) has greatly improved the diagnosis rate, with a specificity of 100% and a sensitivity of 87% [21]. In addition to immunohistochemistry (IHC), fluorescence in situ hybridization (FISH) using NUT cleavage probes is another sensitive method for detecting NUT rearrangement, with a sensitivity of 93% [12].
Sinonasal NC is relatively rare, however, and the actual incidence is not well-known due to the lack of comprehensive analysis of large numbers of cases [1, 22]. According to relevant literature reports, nasal sinus NUT cancer accounts for more than 57%-70% [1, 18, 23] of all NUT cancers in the head and neck, and in 2017, NUT cancer was included for the first time in the Fourth Edition of the World Health Organization (WHO) Classification of Sinus Tumors [24]. Lee et al. [18] analyzed 362 cases of poorly differentiated and undifferentiated head and neck cancer, and found that 4 cases (1.1%) were NUT cancer, all of which originated in the nasal sinuses. Additionally, of the 151 primary Sinonasal cancers reported by Bishop, only three were NUT cancers [25]. Currently, there are no treatment guidelines for NCs. In this study, we retrospectively reported the clinical features, immunohistochemistry, treatment, diagnosis and prognosis of patients with sinonasal NUT carcinoma in order to raise clinicians’ awareness of NCs.
Materials and methods
General patient data collection
A retrospective analysis was conducted on patients with primary nasal and sinusoidal NUT cancer who were admitted to our hospital from January, 2019 to March, 2023, and clinical information and follow-up data for each patient were collected. Inclusion criteria were aggressive malignancies with NUT gene rearrangement, regardless of age and regardless of date of admission and being significantly positive as found by immunohistochemical nucleation for anti-NUT antibodies (>50%) and/or (15; 19) Translocation, and/or FISH assessment. Tumor staging was performed using the Eighth Edition of the American Joint Committee on Cancer (AJCC) TNM staging system.
Hematoxylin–eosin (HE) and IHC
Hematoxylin-eosin (HE) stained sections were used to observe cell morphology, growth patterns, squamous differentiation, and necrosis. Rabbit anti-NUT (Cell Signaling T technology, C52B1) monoclonal antibody was used on formalin-fixed paraffin-embedded tumor sections for NUT immunohistochemistry at 1:50 dilution. Diffuse (> 50%) concentrated staining and spot staining were all positive.
Fluorescence in situ hybridization (FISH)
A NUTM1 two-color separation probe was used for FISH detection. The probe was purchased from Wuhan HealthCare Biotechnology Co., Ltd., and the operation steps were carried out in strict accordance with the instructions. Red fluorescence (R) labels the 5'NUT (15q14) probe, and green fluorescence (G) labels the 3'NUT probe. The normal signal pattern is shown as a fusion of two red-green fluorescences (2F), and the typical positive signal pattern is 1G1R1F, where G is a green signal, R is a red signal, and F is a yellow signal or a green signal with a red superposition signal. If more than 20% contain the NUT splitting signals, they are considered FISH positive.
Analytical standard
Criteria for analysis included a description of the patient’s clinical features, radiolrogical manifestations, pathological features based on histology and pathology, modalities of treatment used, and patient prognosis.
Follow-up
Follow-up was conducted every 3 months in the first year and every 6 months thereafter. Both outpatient follow-up and telephone follow-up were used.
Statistical analysis
All data were expressed as median and range (minimum, maximum) and percentage. Duration of survival was calculated from the date of diagnosis to the date of death or at the last follow-up, and the date of the first biopsy was used to estimate the date of diagnosis.
Results
Population and clinical characteristics
There were 10 patients, comprising 6 males and 4 females, with a median age of 34 years (range: 15–69 years). Nasal congestion, epistaxis, and headache were the most common symptoms when patients saw a physician, as shown in Table 1. The tumors originated from the sinuses of the nose, including 6 cases of primary ethmoid sinus cancer, 3 cases of primary maxillary sinus cancer, and 1 case of primary nasal cavity cancer. Under nasal endoscopy most lesions showed dark red neoplasm with ulceration on the surface and easy bleeding when touched, and most of the neoplasm showed no necrosis or ulceration on the surface. Almost all patients had local advanced stages at the time of treatment: there were 0, 1, 0, 6 and 3 cases in stage 1, T2, T3, T4a and T4b, respectively, and 0, 1, 0, 6 and 3 cases in stage I, II, III, IVa, and IVb, respectively (the Eighth Edition of the American joint committee on cancer (AJCC) TNM stage).
HE and IHC
Histologically, all 10 patients showed poor differentiation. Most of the tumors were monoform, small to medium size, round or oval, and distributed in flakes or nests, with a small to medium amount of pink or transparent cytoplasm, round nuclei, deep nuclear staining, common mitotic images, and prominent nucleoli often accompanied by necrosis and apoptosis. Some tumor cells contained more transparent cytoplasm and resembled “fried eggs” (Fig. 1).
Histological features of sinonasal NUT carcinoma. A. Architecturally, the tumor consists of diffuse sheets and solid nests of tumor cells (H&E × 40) B. Abrupt squamous differentiation can be focally seen (H&E × 100) C&D. Sheets of monomorphous cells with pale pink or transparent cytoplasm and prominent nucleoli, resembling a “fried egg” shape(D) (H&E × 400)
Immunohistochemistry of NUT was also performed, and all patients showed positive expression of NUT antibodies in tumor cells (Fig. 2A and B), and spot nuclear staining with NUT fusion characteristics. Nine patients expressed squamous cell p63/p40 (Fig. 2D and E), 8 expressed CK (Fig. 2C), 7 expressed CK5/6, 4 expressed CK7, 2 patients expressed CK8/18, 7patients expressed p53, and 4 patients had positive p16 expression. S-100, SMA, CD5, CD117, CD56, CgA and Syn were negative for all patients, but for 8 patients, the Ki-67 positive index ranged from 40% to 80% with a median of 50%. The results of other immunohistochemical parameters are shown in Table 2.
Immunohistochemical staining of sinonasal NUT carcinoma. A (× 200) & B (× 400). Diffuse speckled nuclear immunoreactivity for NUT protein. C strong cytoplasmic expression of CK (× 100). D p63 were diffusely positive (× 200). E. p40 were diffusely positive (× 200). F FISH with NUT break-apart probes revealed splitting signals
FISH
The NUT (15q14) gene break probe was used to detect NUT gene rearrangement by FISH. As shown in Fig. 2, the cells showed separated green and red signals indicative of NUT gene rearrangement (Fig. 2F).
Radiologic characteristics
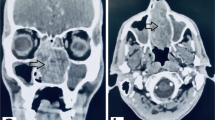
In patient 2, preoperative enhanced Computed tomography (CT) (Fig. 3A and B) showed space-occupying lesions in the left maxillary sinus and nasal cavity involving surrounding bone, soft tissue shadow in the left maxillary sinus and left nasal cavity, and indistinct boundary for each turbinate. Local bone discontinuity and thickened hyperplasia in the anterior wall of the left maxillary sinus and the suborbital neural tube were observed in the left maxillary sinus, the bone discontinuity in the upper wall of the left maxillary sinus (inferior orbital wall) was observed in the outer space of the inferior orbital quadrant, the local boundary with the inferior rectus muscle was unclear, the oral region of the left maxillary sinus was enlarged, and there was local bone discontinuity and thinning in the inner wall of the maxillary sinus. Local bone thinning in the left atrial septum was present as well.
The enhanced magnetic resonance imaging (MRI) (Fig. 3C and D) showed an irregular soft tissue mass in the left maxillary sinus and left nasal cavity, showing equal T1 and T2 signals, the size of which was about 3.8x3.2x4.4cm. After enhancement, the lesion was slightly unevenly enhanced, and obvious strip-like and linear enhanced shadow could be seen inside. The boundary between the lesion and the middle and lower turbinate was unclear, and the lesion broke through the anterior wall of the left maxillary sinus. The local maxillofacial soft tissue was significantly strengthened, involving the tissue of the infraorbital wall and upward, and the suborbital quadrant outer conical space was significantly strengthened as well. Finally, the local boundary with the inferior rectus muscle was unclear.
Treatment
The most common treatment was complete surgical resection combined with chemoradiotherapy and targeted therapy. As shown in Table 3, among the 10 patients with NC, 3 received chemotherapy alone, 1 received radiotherapy alone, and the remaining 6 received surgery combined with radiotherapy and chemotherapy. The chemotherapy itself was combined with platinum-containing chemotherapy, which included the chemotherapy drugs (oxaliplatin, nedaplatin, carboplatin, docetaxel, etoposide, gemcitabine, 5-fluorouracil, etc.), and the radiotherapy dose was 65 to 70 Gy.
Patient outcomes
The 10 patients were followed up with for 1-51 months, with a median follow-up time of 14 months. Among them, 3 died due to widespread systemic metastasis, 3 relapsed, and 4 had no recurrence or metastasis.
Discussion
In the head and neck, NCs usually occur in the nasal sinuses. According to the literature, NCs may involve the ethmoid sinuses more frequently in the sinuses, which is consistent with our findings [26]. In recent years, the number of diagnoses of NUT cancer in the head and neck has increased significantly [14], and this apparent increase is widely believed to be the result of previous misdiagnosis due to inadequate diagnostic methods in the past.
The predominant clinical manifestations of nasal sinus NCs typically include nasal obstruction and bloody nasal discharge. Pain, swelling, visual impairment, diplopia, facial numbness, dysphagia, and epistaxis may also ensue due to tumor infiltration into adjacent tissues. These clinical presentations bear resemblance to those of other malignant tumors originating in the nasal sinuses and lack specificity [27]. Early NCs often exhibit symptoms that overlap with those of benign conditions such as nasal sinusitis. Therefore, the diagnosis of NC is prone to delay, and the patients are mostly in the local advanced stage when diagnosed. In our study, 9 out of 10 patients presented with a locally advanced stage that had invaded and destroyed surrounding structural tissue.
According to the existing literature, the imaging features of NUT carcinoma lack specificity [28], and they usually have similar imaging features to other common malignant solid tumors in the same anatomical location [29], except for showing low-density lesions with uneven enhancement and aggressive invasion. CT showed [30] soft tissue mass with unclear boundary accompanied by wormlike bone destruction and invasive growth. And MRI showed [31, 32] soft tissue density shadow with unclear boundary, uneven enhancement after reinforcement, and invasion of surrounding soft tissue. For the correct clinical staging of NC, patients are usually recommended to undergo enhanced computed tomography in conjunction with magnetic resonance imaging, which facilitates a more thorough evaluation of soft tissue, especially in assessing possible bone and/or vascular invasion, as well as tumors in the head and neck area [33]. CT and MRI can accurately and comprehensively display the scope of tumor invasion, and can thus relatively accurately stage and guide clinical treatment plans, making them two of the most important examination methods for early detection and diagnosis.
NCs are generally poorly differentiated and usually appear as poorly differentiated or undifferentiated carcinomas with varying degrees of sudden squamous differentiation and extensive necrosis. NCs cells are monomorphic cells, small to medium in size, with round or oval nuclei, clear cytoplasm, and prominent nucleoli [34]. Immunohistochemically, almost all NCs are positive for NUT protein. And most NC cells are also detectable using markers of squamous differentiation, including positive expression of p63 and p40 [28]. Other markers of squamous epithelial differentiation that work for NCs include broad-spectrum keratin CKpan, epithelial cell membrane antigens EMA, CK5/6, CEA, CK7, and occasionally TTF-1, CD56, CgA, Syn, CD34, and CD99 [26, 35]. The immunoreactivity of several other antigens has also been reported, including syn [36], EGFR [37], and HER2 [38]. Ki-67 index can determine the proliferative activity of the tumor, and in some reports, Ki-67 expression has been very high, with an expression range between 80 and 100% [31, 39]. In our study, the immunohistochemical results were similar to those reported in other papers: 9 patients expressed p63 /p40 (Fig. 2D and E), a marker of squamous cell differentiation, cytokeratin CK (Fig. 2C), CK5/6, and CK7 was found in 7patients. The Ki-67 positive index ranged from 40 to 80%, with a median of 50%.
For patients with poorly differentiated/undifferentiated malignancies with relatively uniform morphology, NUT immunohistochemical testing is recommended for differential diagnosis from other tumors [26]. In addition, NUT rearrangement is also a basic requirement for a definitive diagnosis of NC. NUT gene rearrangement can be demonstrated either by fluorescence in situ hybridization (FISH) or by reverse transcription polymerase chain reaction (RT-PCR) for BRD-NUT fusion transcripts. Furthermore, a combination of immunohistochemistry and FISH can achieve 100% sensitivity and specificity [39], so FISH can be considered if the immunostain is negative but the diagnosis remains highly suspect [21]. For tumors, accurate diagnosis is crucial, and more accurate pathological diagnosis can better guide clinical treatment.
Due to the rarity and heterogeneity of NCs, there is currently no established standard treatment for patients diagnosed with this condition. Treatment guidelines for NCs generally align with those for other tumor types occurring in the same anatomical sites as NUT carcinoma. They have a poor prognosis, and chemotherapy or radiotherapy alone is often insufficient [1]. Moreover, there is no standardized chemotherapy regimen, and different systemic treatments have been reported in the literature, the most common being platinum-based chemotherapy regimens (used alone or in combination with other drugs). However, the value of this approach is usually limited, and the disease progresses rapidly [23]. In this case, a variety of chemotherapeutic compounds have been used, including cisplatin, carboplatin, cyclophosphamide, doxorubicin, radiosin D, etoposide, vinorelbine, vincristine, paclitaxel, docetaxel, bleomycin, ifosfamide, 5-fluorouracil, and gemcitabine [32, 40]. For head and neck NCs, such as sinusoidal NCs, aggressive primary surgical resection significantly improves overall survival (OS) and progression-free survival (PFS) [1, 23]. Depending on the size and extent of the tumor, various surgical procedures are chosen, such as ESS (endoscopic sinus surgery) and LR (lateral rhinotomy). Once diagnosed, patients are usually treated with a combination of surgery, radiation and chemotherapy.
The potential for targeted therapy for NCs is gradually emerging with the study of the molecular pathogenesis of NCs. One approach is BET inhibitors (BETis), which work by mimicking acetylated histones and competitively inhibiting the binding of BET proteins (such as BRD4) to acetylated chromatin, blocking its ability to activate oncogenic target genes [41]. In a Phase Ib clinical study, three patients (33%) with NCs treated with BETi Birabresib (MK-8628/OTX015) [NCT02698176] had a partial response (duration: 1.4–8.4 months). There are also a number of phase I/II clinical trials of BETi (RO6870810/TEN-010 [NCT01987362], GSK525762 [NCT01587703], INCB057643 [NCT02711137], BAY1238097 [NCT02369029], and BMS-986158 [NCT03936465]) that have been completed or are ongoing, and given the poor prognosis of these tumors, these inhibitors may become first-line therapies alone or in combination with other therapies.
Another approach is to use histone deacetylase inhibitors (HDACis) to reduce hyperacetylated chromatin in BRD4 BRD4 mega-domains, thereby restoring cellular transcription to normal levels [14]. One such case [42] was reported at MD Anderson Cancer Center for HDACis, in which patients experienced rapid disease progression after three cycles of cisplatin, docetaxel, and 5-fluorouracil, and responded well to Vorinostat (HDACi).
Conclusion
In conclusion, NUT cancer is a rare and highly aggressive malignant tumor with rapid progression, advanced stage when detected, poor prognosis that is characterized by NUT gene abnormalities. Correct histopathological diagnosis is the primary prerequisite for determining appropriate treatment. The disease tends to occur in young people, but in our study, patients have a higher average age. This may be due to the fact that the proportion of adolescent patients is lower than adult in our hospital. Accurate diagnosis of NC at an early stage is critical, and with more systematic detection tools and increased awareness among physicians, cases of NC are expected to increase in the coming years. There are currently no effective treatment options for NC, however, not even surgery or chemoradiotherapy. Although targeted therapy for NC has recently been applied in several different settings and targeted therapy may become an effective method to treat NC, this is still years away.
Data availability
The datasets generated and/or analyzed in this study are not publicly available due to protection of the privacy of patients, but are available from the corresponding author upon reasonable request.
References
Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, Hsi P, Bauer DE, Lathan CS, Rodriguez-Galindo C, Tishler RB, Haddad RI, Sallan SE, Bradner JE, French CA (2016) Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer 122(23):3632–3640
Stevens TM, Morlote D, Xiu J, Swensen J, Brandwein-Weber M, Miettinen MM, Gatalica Z, Bridge JA (2019) NUTM1-rearranged neoplasia: a multi-institution experience yields novel fusion partners and expands the histologic spectrum. Mod Pathol 32(6):764–773
Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T, Miyoshi I (1991) Novel t(15;19) (q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res 51(12):3327–3328
Polsani A, Braithwaite KA, Alazraki AL, Abramowsky C, Shehata BM (2012) NUT midline carcinoma: an imaging case series and review of literature. Pediatr Radiol 42(2):205–210
French CA, Kutok JL, Faquin WC et al (2004) Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol 22(20):4135–4139
Saik WN, Da Forno P, Thway K, Khurram SA (2021) NUT carcinoma arising from the parotid gland: a case report and review of the literature. Head Neck Pathol 15(3):1064–1068
Mertens F, Wiebe T, Adlercreutz C, Mandahl N, French CA (2007) Successful treatment of a child with t (15;19)-positive tumor. Pediatr Blood Cancer 49(7):1015–1017
Yang F, Shen D, Shi J (2021) Primary renal NUT carcinoma identified by next-generation sequencing: a case report and literature review. Int J Clin Exp Pathol 14(5):662–669
Shehata BM, Steelman CK, Abramowsky CR, Olson TA, French CA, Saxe DF, Ricketts RR, Katzenstein HM (2010) NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a 2-year-old with a pancreatic/hepatic primary. Pediatr Dev Pathol 13(6):481–485
Lemelle L, Pierron G, Fréneaux P, Huybrechts S, Spiegel A, Plantaz D, Julieron M, Dumoucel S, Italiano A, Millot F, Le Tourneau C, Leverger G, Chastagner P, Carton M, Orbach D (2017) NUT carcinoma in children and adults: a multicenter retrospective study. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.26693
Lauer UM, Hinterleitner M, Horger M, Ohnesorge PV, Zender L (2022) NUT Carcinoma-An Underdiagnosed Malignancy. Front Oncol 26(12):914031
Wang L, Zhu Z, Wang W, Zha Y, Wang X, Surita A, Liu Y, Lv W (2023) Sinonasal NUT carcinoma: a retrospective case series from a single institution. Front Surg 1(10):1098704
Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, Lathan CS, DuBois SG, Haddad RI, Shapiro GI, Sallan SE, Dhar A, Nelson JJ, French CA (2019) An Anatomical Site and Genetic-Based Prognostic Model for Patients With Nuclear Protein in Testis (NUT) Midline Carcinoma: Analysis of 124 Patients. JNCI Cancer Spectr 4(2):pkz094
Salati M, Baldessari C, Bonetti LR, Messina C, Merz V, Cerbelli B, Botticelli A (2019) NUT midline carcinoma: Current concepts and future perspectives of a novel tumour entity. Crit Rev Oncol Hematol 144:102826
Harms A, Herpel E, Pfarr N, Penzel R, Heussel CP, Herth FJ, Dienemann H, Weichert W, Warth A (2015) NUT carcinoma of the thorax: Case report and review of the literature. Lung Cancer 90(3):484–491
French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA (2003) BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res 63(2):304–307
Cho YA, Choi YL, Hwang I, Lee K, Cho JH, Han J (2020) Clinicopathological characteristics of primary lung nuclear protein in testis carcinoma: A single-institute experience of 10 cases. Thorac Cancer 11(11):3205–3212
Lee T, Cho J (2020) Baek CH et al Prevalence of NUT carcinoma in head and neck: Analysis of 362 cases with literature review. Head Neck 42(5):924–938
French CA, Rahman S, Walsh EM, Kühnle S, Grayson AR, Lemieux ME, Grunfeld N, Rubin BP, Antonescu CR, Zhang S, Venkatramani R, Dal Cin P, Howley PM (2014) NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 4(8):928–941
Alekseyenko AA, Walsh EM, Zee BM, Pakozdi T, Hsi P, Lemieux ME, Dal Cin P, Ince TA, Kharchenko PV, Kuroda MI, French CA (2017) Ectopic protein interactions within BRD4-chromatin complexes drive oncogenic megadomain formation in NUT midline carcinoma. Proc Natl Acad Sci U S A 114(21):E4184–E4192
Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, Hong SM, Schwartz BE, Cameron MJ, Rubin MA, Chang MC, Aster JC, French CA (2009) Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 33(7):984–991
Salles PG, Moura Rde D, Menezes LM, Bacchi CE (2014) Expression of P16 in NUT carcinomas with no association with human papillomavirus (HPV). Appl Immunohistochem Mol Morphol 22(4):262–265
Bauer DE, Mitchell CM, Strait KM et al (2012) Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 18(20):5773–5779
Thompson LDR, Franchi A (2018) New tumor entities in the 4th edition of the World Health Organization classification of head and neck tumors: Nasal cavity, paranasal sinuses and skull base. Virchows Arch 472(3):315–330
Bishop JA, Westra WH (2012) NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol 36(8):1216–1221
Minato H, Kobayashi E, Nakada S, Kurose N, Tanaka M, Tanaka Y, Suzuki S, Tanioka F, Saikawa Y, Miwa T, Nojima T (2018) Sinonasal NUT carcinoma: clinicopathological and cytogenetic analysis with autopsy findings. Hum Pathol 71:157–165
Lemelle L, Moya-Plana A, Dumont B, Fresneau B, Laprie A, Claude L, Deneuve S, Cordero C, Pierron G, Couloigner V, Bernard S, Cardoen L, Brisse HJ, Jehanno N, Metayer L, Fréneaux P, Helfre S, Kolb F, Thariat J, Réguerre Y, Orbach D (2022) NUT carcinoma in children, adolescents and young adults [J]. Bull Cancer 109(4):491–504
Edgar M, Caruso AM, Kim E, Foss RD (2017) NUT Midline Carcinoma of the Nasal Cavity. Head Neck Pathol 11(3):389–392
Orman G, Masand P, Hicks J, Huisman TAGM, Guillerman RP (2020) Pediatric thoracic mass lesions: Beyond the common. Eur J Radiol Open 7:100240
Jimenez C, Stanton E, Kondra K, Nickels EM, Jacob L, Shah R, Hammoudeh JA (2023) NUT carcinoma of the mandible in a child case report and systematic review. Int J Oral Maxillofac Surg 52(3):304–312
Sholl LM, Nishino M, Pokharel S, Mino-Kenudson M, French CA, Janne PA, Lathan C (2015) Primary pulmonary NUT midline carcinoma: clinical, radiographic, and pathologic characterizations. J Thorac Oncol 10(6):951–959
Giridhar P, Mallick S, Kashyap L, Rath GK (2018) Patterns of care and impact of prognostic factors in the outcome of NUT midline carcinoma: a systematic review and individual patient data analysis of 119 cases. Eur Arch Otorhinolaryngol 275(3):815–821
Napolitano M, Venturelli M, Molinaro E, Toss A (2019) NUT midline carcinoma of the head and neck: current perspectives. Onco Targets Ther 30(12):3235–3244
Schwartz BE, Hofer MD, Lemieux ME, Bauer DE, Cameron MJ, West NH, Agoston ES, Reynoird N, Khochbin S, Ince TA, Christie A, Janeway KA, Vargas SO, Perez-Atayde AR, Aster JC, Sallan SE, Kung AL, Bradner JE, French CA (2011) Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res 71(7):2686–2696
Cho HJ, Lee HK (2020) Lung nuclear protein in testis carcinoma in an elderly Korean woman: a case report with cytohistological analysis. Thorac Cancer 11(6):1724–1727
Mao N, Liao Z, Wu J, Liang K, Wang S, Qin S, Dou Y, Lin H, Dong X (2019) Diagnosis of NUT carcinoma of lung origin by next-generation sequencing: case report and review of the literature. Cancer Biol Ther 20(2):150–156
Zhou L, Yong X, Zhou J, Xu J, Wang C (2020) Clinicopathological analysis of five cases of NUT midline carcinoma, including one with the gingiva. Biomed Res Int 13(2020):9791208
Jung M, Kim S, Lee JK, Yoon SO, Park HS, Hong SW, Park WS, Kim JE, Kim J, Keam B, Kim HJ, Kang HJ, Kim DW, Jung KC, Kim YT, Heo DS, Kim TM, Jeon YK (2019) Clinicopathological and preclinical findings of NUT carcinoma: a multicenter study. Oncologist 24(8):e740–e748
Chatzopoulos K, Boland JM (2021) Update on genetically defined lung neoplasms: NUT carcinoma and thoracic SMARCA4-deficient undifferentiated tumors. Virchows Arch 478(1):21–30
Lauer UM, Hinterleitner M, Horger M, Ohnesorge PV, Zender L (2022) NUT carcinoma-an underdiagnosed malignancy. Front Oncol 12:914031
Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE (2010) Selective inhibition of BET bromodomains. Nature 468(7327):1067–1073
Maher OM, Christensen AM, Yedururi S, Bell D, Tarek N (2015) Histone deacetylase inhibitor for NUT midline carcinoma. Pediatr Blood Cancer 62(4):715–717
Acknowledgements
The authors wish to thank the individuals who participated in this study. And the authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Funding
This work was supported by the Fund for Distinguished Young Scholars of Beijing Tongren Hospital.
Author information
Authors and Affiliations
Contributions
Zhigang Huang, Conception and design, Administrative support, Provision of study materials or patients, Final approval of manuscript; Yang Zhang, Conception and design, Administrative support, Provision of study materials or patients, Final approval of manuscript; Wen Gao, Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript; Lifei Feng, Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript; Zishi Huan, Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript; Gaofei Yin, Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript; Duoxuan Chen, Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript; Xinming Zhao, Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
All authors have read the manuscript and approved its submission for publication. All authors declare no conflicts of interest.
Ethical approval
The study design was approved by the Human Ethics Committee of Beijing Tongren Hospital, Capital Medical University. All methods were performed in accordance with the relevant guidelines and regulations of Helsinki declaration of 1964 and its amendments. The ethical approval statement and the need for informed consent were waived by the Human Ethics Committee of Beijing Tongren Hospital, Capital Medical University for this manuscript because the individual data was fully anonymized.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, W., Feng, L., Zhao, X. et al. Clinical analysis and treatment progress of NUT carcinoma in the nasal cavity and sinuses: a retrospective study from a single institution. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08898-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08898-1