Abstract
Nuclear protein in testis (NUT) midline carcinoma (NMC) is a rare, aggressive, poorly differentiated form of squamous cell carcinoma caused by a chromosomal rearrangement of the NUT gene on chromosome 15. These tumors have a predilection for midline and paramidline structures of the upper aerodigestive tract and mediastinum and can affect patients across a broad age range, including children. In the current example, a 53 year old male presented with a mass originating in the left nasal cavity. The clinical, radiographic, and morphologic features of NMC are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
History
A 53 year old male presented with a 9 month history of left nasal congestion, rhinorrhea, and sinus tenderness. The patient also noted recurrent epistaxis lasting longer than 30 min 6 months prior to presentation. Additionally, patient reported recent onset of left facial numbness in the V2 distribution of the trigeminal nerve. He denied fevers and weight loss, but did note drenching night sweats.
Radiographic Features
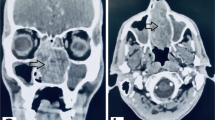
Sinus CT examination revealed a soft tissue mass which completely obstructed the left nasal cavity. Coronal views delineated osseous expansion with erosion and destruction of the nasal septum and medial wall of the left maxillary sinus (Fig. 1a). There was opacification of left ethmoid air cells and the left maxillary sinus. Corresponding MR images demonstrated an 8.4 × 3.3 × 2.3 cm mass centered in the left nasal cavity (Fig. 1b). The mass was heterogeneously enhancing on postcontrast T1-weighted images and displayed predominantly isointense signaling with T2-weighted studies. The mass measured 8.4 cm in anterior–posterior dimension, including a pedunculated component that extended past the choana into the nasopharynx (Fig. 2). No intracranial extension or erosion into the orbits or cribriform was present. Brain and neck MRI evaluation revealed no evidence of brain metastasis or cervical lymphadenopathy.
Treatment
Following an incisional biopsy, the patient underwent functional endoscopic sinus surgery including a left maxillary antrostomy, total ethmoidectomy, sphenoidotomy, left inferior turbinectomy and removal of the mass. Intraoperative findings revealed that the mass originated from the posterior inferior turbinate, which was subsequently removed. Mucosa was stripped from the lateral nasal wall, the posterior maxillary sinus wall, the area surrounding the sphenoid ostium, over the sphenopalatine foramen and laterally towards the infratemporal fossa to establish margins. The bone of the infratemporal fossa appeared thin and moth eaten. Despite a suggestion of circumscription, the surgical margins were widely involved by tumor and local recurrence was noted within one month. Continued treatment included paclitaxel/carboplatin based chemotherapy and RapidArc technique radiation therapy. The patient succumbed to his disease 3 months after surgery.
Diagnosis
The initial incisional biopsy of the mass was interpreted at an outside institution as a poorly differentiated, keratinizing squamous cell carcinoma. The resection specimen grossly consisted of multiple irregular portions of hemorrhagic, grey-tan soft tissue with a “rubbery” texture. The largest portion measured >3 cm in diameter. Microscopic examination revealed extensive involvement by a diffusely infiltrative malignancy composed of islands and nests of monotonous basaloid cells (Fig. 3). These cells were characterized by round to oval nuclei, granular chromatin, basophilic nucleoli and a narrow rim of slightly fibrillar, amphophilic cytoplasm. Scattered tumor islands exhibited focal “abrupt” squamous differentiation or keratinization (Fig. 4a). Conspicuous comedo and tumor necrosis were present along with prominent lymph-vascular and bone invasion. The tumor cells were immunoreactive for CK 5/6, p16, p40, p63 and NUT protein (Fig. 4b) with very focal CK20 and synaptophysin staining noted. Stains for CD34 and S100 were negative. The morphologic and immunophenotypic features were indicative of a NMC. Subsequent fluorescence in situ hybridization demonstrated the presence of a BRD4-NUT rearrangement, confirming this diagnosis.
Discussion
NMC is a rare, poorly differentiated squamous cell carcinoma variant that was first characterized in the early 1990s [1]. Initially regarded as a pediatric tumor, cases have now been identified in patients across a broad range of ages with males and females equally affected [1–3]. The most common locations include the mediastinum or thymus and the head and neck. In the head and neck sinonasal or nasopharyngeal involvement is most common [2]. This is a highly aggressive malignancy with a median survival of 6.7 months and an overall survival of 19 % at 2 years [4]. The tumor’s predilection for midline structure raises the possibility of primitive neural crest origin. This tends to correlate with the observation of high level NUT protein expression in the adult ciliary ganglion which is neural crest derived [5].
The defining molecular feature of NMC is a rearrangement of the NUT gene, most commonly in a t(15;19) translocation with the BRD4 gene. A minority of NMC cases harbor BRD3-NUT or NUT-variant fusions [5, 6]. The fusion gene product is thought to be responsible for pathogenesis by suppressing transcription, which leads to differentiation arrest. The result is unregulated growth of undifferentiated neoplastic cells [6].
The radiographic features of NMC are highly suspicious for an aggressive malignant process. CT images are most frequently characterized by hypoattenuation and heterogenous enhancement of an infiltrative or destructive appearing mass. Occasional intralesional calcifications have been reported [7]. Despite the frequent evidence of disregard for normal tissue boundaries, the current case displayed some evidence circumscription and osseous remodeling. Microscopically, however; it was extensively invasive. MR images also characteristically demonstrate a heterogenous mass with hypointense T1 and hyperintense T2 signalling, although the current case was only mildly hyperintense on both. MRI is the preferred modality with regards to delineation of tumor margins and for treatment planning [7]. The non-specific radiographic features of NMC necessitate the development of a broad differential diagnosis with primary consideration given to aggressive malignancies, including sinonasal undifferentiated carcinoma, neuroendocrine carcinoma, melanoma, rhabdomyosarcoma, Ewing sarcoma family tumors and hematologic malignancies. The majority of NMC cases are recognizable as carcinomas with conventional histologic examination with the frequent presence of focal abrupt keratinization suggesting squamous differentiation. In the absence of keratinization, other forms of undifferentiated carcinoma may warrant consideration.
Because NMC is associated with an aggressive clinical course, rapid and accurate diagnosis is imperative. Inclusion of immunostaining for NUT expression should be a consideration for all poorly differentiated carcinomas arising in the head and neck or chest or that lack glandular differentiation. NMCs are generally immunoreactive for p63 or p40, consistent with squamous differentiation and the majority of tumors are cytokeratin positive [8]. The current case demonstrated positive CK 5/6, CK 7, p40, p63 and p16 staining. Confirmatory FISH testing was performed, revealing the BRD4-NUT translocation, however; the diagnosis can be made based on immunohistochemical demonstration of nuclear reactivity to NUT antibody. Specificity and sensitivity are 100 and 87 %, respectively [6].
Treatment by traditional means results in only modest gains in survival. In a retrospective study of 54 patients complete gross resection and radiation were independent predictors of improved progression-free and overall survival. There is no clear evidence that a particular chemotherapeutic regimen is effective as all but a few tumors were eventually unresponsive to chemotherapies of all types [4]. However, targeted therapy is under investigation. Clinical trials evaluating the efficacy of either histone deacetylase inhibitors (HDACi) or Bromodomain and Extra-Terminal motif (BET) inhibitors may eventually show promise in treating this aggressive malignancy [5].
References
Stelow EB, French C. Carcinomas of the upper aerodigestive tract with rearrangement of the nuclear protein of the testis (NUT) gene (NUT midline carcinomas). Adv Anat Pathol. 2009;16:92–6.
Stelow EB. A review of NUT midline carcinoma. Head Neck Pathol. 2011;5:31–5.
Shah AA, Jeffus SK, Stelow EB. Squamous cell carcinoma variants of the upper aerodigestive tract: a comprehensive review with a focus on genetic alterations. Arch Pathol Lab Med. 2014;138:731–44.
Bauer D, Mitchell C, Strait K, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773–9.
French C. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. 2010;63:492–6.
French C. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol. 2013;7:11–6.
Bair RJ, Chick JF, Chauhan NR, French C, Madan R. Demystifying NUT midline carcinoma: radiologic and pathologic correlations of an aggressive malignancy. AJR Am J Roentgenol. 2014;203:W391–9.
Fang W, French CA, Cameron MJ, Han Y, Liu H. Clinicopathological significance of NUT rearrangements in poorly differentiated malignant tumors of the upper respiratory tract. Int J Surg Pathol. 2013;21:102–10.
Acknowledgments
The authors would like to thank Dr. Chris French and Mr. Adlai Grayson (Brigham and Women’s Hospital/Harvard Medical School) for performing FISH testing on this case.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Disclaimer The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Army, Air Force, Department of Defense nor the U.S. Government.
Rights and permissions
About this article
Cite this article
Edgar, M., Caruso, A.M., Kim, E. et al. NUT Midline Carcinoma of the Nasal Cavity. Head and Neck Pathol 11, 389–392 (2017). https://doi.org/10.1007/s12105-016-0763-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-016-0763-0