Abstract
Purpose
Obstructive sleep apnea (OSA) patients are at increased risk for cardiovascular disease, stroke, atherosclerosis, hypertension, and venous thromboembolism. Elevated soluble P-selectin (sP-selectin) levels are also associated with increased risk of above diseases. But whether sP-selectin levels in OSA patients are higher than their counterparts remain unclear, since previous studies yielded inconsistent results. Therefore, a meta-analysis is warranted.
Methods
PubMed, Embase, Cochrane Library, and Web of Science databases were searched for eligible studies. Studies were included if they reported sP-selectin levels of both OSA patients and non-OSA controls. Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were calculated to determine the effect sizes.
Results
Nine eligible studies were finally evaluated. When all the studies were pooled, sP-selectin levels in OSA patients were significantly higher than that in controls (SMD = 0.54, 95% CI 0.29–0.78, I2 = 66%, p < 0.0001). In the subgroup analysis based on BMI matched groups, sP-selectin levels were significantly higher in OSA patients than that in controls (SMD = 0.52, 95% CI 0.27–0.76, I2 = 23%, p < 0.0001). In the subgroup analysis stratified by blood source, either serum sP-selectin levels or plasma sP-selectin levels in OSA patients were higher than that in controls. Moderate-to-severe OSA patients had significant higher sP-selectin levels (SMD = 0.80, 95% CI 0.45–1.15, I2 = 67%, p < 0.00001), while mild OSA patients showed no significant difference with controls.
Conclusion
The pooled results reveal that OSA patients have higher sP-selectin levels than non-OSA controls. This conclusion remains unaltered in all subgroups other than the subgroup of mild OSA patients. Additional studies are warranted to better identify the role of sP-selectin as a potential biomarker in OSA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by recurrent partial or complete collapse of the upper airway during sleep, resulting in chronic intermittent hypoxia, respiratory cessation, recurrent micro-arousal, and daytime sleepiness. Untreated OSA patients are at increased risk for cardiovascular disease, stroke, atherosclerosis, hypertension, and venous thromboembolism [1,2,3,4]. Although the pathophysiological mechanisms behind the association are still not thoroughly clarified, several studies have proposed that OSA predisposes to a multifactorial prothrombotic state, in which endothelial dysfunction, exaggerated platelet activity, impaired fibrinolysis, as well as chronic, low-grade systematic inflammation are known to play a critical role[5,6,7,8].
P-selectin, as a member of the selectin family of adhesion molecules, is localized in the membranes of alpha-granules of platelets and the Weibel–Palade bodies of endothelial cells [9]. It is expressed upon stimulation by thrombin, hypoxia, histamine, or cytokines by both platelets and endothelial cells [10,11,12]. It is a critical mediator of platelet–leukocyte interaction and plays an important part in leukocyte recruitment, attachment, rolling, and diapedesis into the vessel wall in early stages of atherosclerosis [13,14,15]. It is also a key molecule in hemostasis and thrombosis mediating platelet rolling, generating procoagulant microparticles, enhancing fibrin deposition, and initiating the extrinsic pathway of the coagulation cascade indirectly [9, 16,17,18]. Taken together, P-selectin should no longer be regarded only as a simple marker of platelet or endothelial activation, but also as a direct inducer of procoagulant activity associated with vascular and thrombotic diseases [9].
Soluble P-selectin (sP-selectin) is the soluble form of P-selectin, which is a circulating protein derived from the membrane form of P-selectin on both platelet and endothelium cell by proteolytic cleavage [9, 19]. It is elevated in pathologic processes involving activated platelets and endothelial cells. Elevated levels of sP-selectin have been demonstrated in a variety of cardiovascular diseases, including coronary artery disease, myocardial infarction, stroke, hypertension, atrial fibrillation, and venous thromboembolism, with some relationship to prognosis [14, 19]. In addition, sP-selectin levels have been found to be positively correlated with apnea hypopnea index (AHI) and respiratory arousal index, inversely related to minimum oxygen saturation during sleep in some studies [20]. Based on the above findings, a growing number of studies have tried to assess sP-selectin levels in patients with OSA. Unfortunately, whether sP-selectin levels were elevated in OSA patients remain controversial, since previous studies yielded inconsistent results. And most of these studies had relatively small sample sizes. Hence, we conducted a meta-analysis of published studies to determine the levels of sP-selectin in OSA, aiming to further the understanding of the potential role of sP-selectin in patients with OSA.
Methods
We carefully followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) in this paper [21].
Data sources and search strategy
The authoritative databases including PubMed, Embase, Web of Science, and Cochrane Library were searched systematically by us to find out eligible studies. Each database was searched from inception through October, 2020. The search terms included: (“OSA” or “Obstructive Sleep Apnea” or “OSAS” or “Obstructive sleep apnea syndrome” or “sleep apnea”) and (“P selectin” or “CD62P”). Apart from the above databases, additional studies were also identified from the references cited in relevant articles.
Selection criteria
The inclusion criteria: (1) All participants were adults (age > 18 years). (2) All participants were examined by polysomnography (PSG). (3) The diagnosis of OSA was based on AHI ≥ 5 events/h. (4) The studies must have included at least two separate groups. Those with AHI ≥ 5 were allocated into OSA group and those with AHI < 5 were allocated into the control group. (5) All OSA patients were newly diagnosed and untreated. (6) Studies reported sP-selectin levels in both OSA group and control group quantitatively. (7) The number of participants for all groups must have been reported. (8) sP-selectin levels were expressed as mean ± standard deviation, mean ± standard error, median and range, or median and interquartile range. (9) Only English language studies were included.
The exclusion criteria: (1) conference abstracts, editorials, reviews, and case reports; (2) studies that did not provide available data for calculating effect estimate; (3) studies were not conducted in humans.
Quality assessment
The Newcastle–Ottawa quality assessment scale (NOS) was adopted to evaluate the quality of each study according to the following aspects: selection of participants, comparability of groups and exposure assessment. The quality score ranges from 0 to 9. Two authors (D.Z and Z.B.X) participated in the process of quality assessment independently. Any dispute was discussed with a third author (T.T.L) and resolved by consensus.
Data extraction
Two authors (D.Z and Z.B.X) identified articles that may be qualified by conducting a screening of titles and abstracts independently. After that, a further screening based on full-text review was undertaken. Only studies satisfied the above selection criteria were included. Data extraction was also carried out by above two authors independently. Then, another author (T.T.L) checked the extracted data for completeness and accuracy. Any discrepancy during data extraction between two independent authors was resolved by consensus with a third author (T.T.L) if necessary. The following data were extracted from the articles: name of first author, publication year, region, sample size, age, gender, body mass index (BMI), AHI of OSA group and control group, type of blood sample, time point of blood drawing, sP-selectin levels in OSA group and control group, and assay methods for sP-selectin levels. If the included studies provided data of median and range or median and interquartile range, the data were transformed to mean and standard deviation using an online computing tool (http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html) [22, 23].
Statistical analysis
Since the differences in assay methods or units, standardized mean difference (SMD) was chosen to calculate the effect size and results were presented as SMD and 95% confidence intervals (CI) for sP-selectin levels. We chose a random-effects model rather than a fixed-effects model because of this takes into account heterogeneity among multi-studies. We also performed subgroup analysis in this article. Heterogeneity across the studies was assessed using the I2 statistic, with a value of 25–49% representing low heterogeneity, 50–75% moderate heterogeneity, and > 75% high heterogeneity. Sensitivity analysis was conducted to assess the stability of pooled results. All analyses were performed with Review Manager (Version 5.2, The Cochrane Collaboration) and Stata (Version SE12.0, Stata Corporation, USA). A P value of less than 0.05 was judged as statistically significant.
Results
Identification of relevant studies
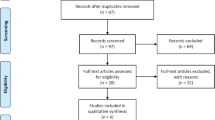
The detailed steps of the study selection process are shown in Fig. 1. A total of 513 records were initially obtained from electronic databases by the mentioned web-search strategies. After checked for duplicates, 167 articles remained. Among them, 143 articles were discarded on the basis of titles and abstracts, leaving 24 articles for further assessment in full texts. After reading full text thoroughly, 15 articles were excluded due to the following reasons: lack of basic information, OSA was not defined as AHI ≥ 5, controls were not non-OSA, without measuring soluble P-selectin level, and study population was children. Eventually, a total of nine eligible studies were included for analysis [15, 24,25,26,27,28,29,30,31].
Characteristics of the studies
Totally, nine studies involving 494 OSA patients and 446 controls were enrolled. The information such as the first author’s name, publication year, region, OSA severity, type of blood sample, time point of blood drawing, assay methods for sP-selectin levels, and NOS Score are summarized in Table 1. And the information of sample size, AHI, age, gender, BMI, and sP-selectin levels are presented in Table 2.
Meta-analysis
By combining all included studies, the pooled results exhibited that sP-selectin levels in OSA patients were significantly higher than that in controls (SMD = 0.54, 95%CI 0.29–0.78, I2 = 66%, p < 0.0001). Due to the evidence of moderate heterogeneity (I2 = 66%), a random-effects model was adopted (Fig. 2).
Subgroup analysis
As is known to all, BMI is a main index to measure the degree of obesity. Several scholars have proposed that elevated sP-selectin levels observed in OSA group in their studies may be due to comorbid obesity rather than representing a direct effect of OSA itself [32,33,34]. Hence, it seems to be reasonable to select the studies which contain BMI matched OSA groups and control groups as a new subgroup for analysis. It showed that in this new subgroup, sP-selectin levels were still significantly higher in OSA patients than that in controls (SMD = 0.52, 95% CI: 0.27–0.76, I2 = 23%, p < 0.0001). Meanwhile, the heterogeneity (I2) decreased from 66 to 23% when we conducted subgroup analysis based on control groups with BMI matched, which indicated that BMI might be a potential source of heterogeneity. Besides, the remaining studies which contain BMI unmatched OSA groups and control groups were also combined into another subgroup. And in this subgroup, the average BMI of OSA group in each study was all higher than control group. The pooled result of this subgroup revealed that sP-selectin levels were higher in OSA patients than that in non-OSA controls with lower BMI (SMD = 0.55, 95% CI 0.15–0.95, I2 = 77%, p = 0.007) (Fig. 2).
Considering the impact of blood sample type on measuring sP-selectin levels, subgroup analyses based on different types of blood sample (serum or plasma) were performed. Four studies used serum as blood sample for measurement [24, 26, 28, 31]. The results demonstrated that OSA patients had significantly higher serum sP-selectin levels compared with their controls (SMD = 0.74, 95% CI: 0.28–1.19, I2 = 75%, p = 0.002). And another four studies used plasma as blood sample for measurement [15, 25, 27, 30]. Similarly, the pooled results of them indicated that plasma sP-selectin levels in OSA patients were also significantly higher than that in controls (SMD = 0.51, 95% CI 0.20–0.82, I2 = 56%, p = 0.001) (Fig. 3).
Subgroup analyses stratified by different OSA severity were also performed. It showed that compared with control group, the moderate-to-severe OSA (AHI ≥ 15 events/h) group had elevated sP-selectin levels (SMD = 0.80, 95% CI 0.45–1.15, I2 = 67%, p < 0.00001). However, the sP-selectin levels in mild OSA (5 ≤ AHI < 15 events/h) group were similar as that in control group (SMD = 0.14, 95% CI −0.29 to 0.57, I2 = 54%, p = 0.51) (Fig. 4).
Sensitivity analysis
The removal of each study did not alter the pooled results significantly, indicating that our results were reliable and stable (Fig. 5).
Publication bias
Owing to the limited number (below 10) of studies included in our meta-analysis, publication bias was not evaluated.
Discussion
The previous systematic review and meta-analysis performed by Nadeem et al. first reviewed and evaluated the selectins levels in OSA [35]. And their results were based on the pooled data of the whole selectins family including P-selectin, E-selectin, and L-selectin. Although significant elevation of the whole selectins levels was detected in OSA, the conclusions on P-selectin were still unknown, because Nadeem’s paper failed to analyze P-selectin independently. In addition, the number of selected studies at that time was too small to conduct subgroup analysis. So far, our paper is the first systematic review and meta-analysis to assess the association between sP-selectin levels and OSA. It provides convincing evidence that patients with OSA have significantly elevated levels of sP-selectin compared with controls. And after subgroup analysis, this conclusion remains unaltered in both BMI matched population and BMI unmatched population.
Most studies have concentrated primarily on obese subjects, because OSA is more common in obese/overweight populations. However, they ignored the fact that the subjects in control groups had normal weight or lower degree of obesity [15, 28, 30, 31]. While previous studies demonstrated that overweight and obese healthy subjects had higher plasma sP-selectin concentrations compared to normal-weight population [36, 37]. Ziccardi et al. also confirmed that weight reduction could result in a significant decrease of sP-selectin levels in obese subjects [37]. Robinson et al. detected significantly higher P-selectin levels in patients with OSA than their unmatched controls and found only BMI was correlated with levels of sP-selectin after multiple linear regression [32]. Even some scholars proposed that possibly obesity was a more potent factor maintaining high P-selectin concentrations than OSA. Therefore, the results of our included studies were probably confounded by the factor of obesity. To control the confounded factor, we performed a subgroup analysis based on BMI matched groups, which became a major merit of this meta-analysis. It showed that sP-selectin levels were still significantly higher in OSA patients than that in controls (SMD = 0.52, 95%CI 0.27–0.76, I2 = 23%, p < 0.0001). And the decrease of heterogeneity (I2) after subgroup analysis also indicated that BMI might be a potential source of heterogeneity.
And according to another subgroup analysis, moderate-to-severe OSA patients with AHI ≥ 15 events/h had significant higher sP-selectin levels, while mild OSA patients with 5 ≤ AHI < 15 events/h showed no significant difference with controls. In view of these results, we inferred a possible explanation that mild OSA patients probably suffered from relative lower degree of hypoxia and the mild injury caused by intermittent hypoxia also could be timely compensated or repaired by human body itself. It might not be sufficient to form an obvious prothrombotic state in vivo. As a result, it was no surprise that sP-selectin levels were similar in mild OSA group and non-OSA control group. Nevertheless, this was just a speculation. Reaching a convincing conclusion was difficult because of too small number of studies in the subgroup analysis. More studies are needed regarding the population of mild OSA.
Clinical implication
As is known to all, PSG is a gold standard modality for diagnosis and screening of OSA. The parameters of PSG (such as AHI, minimum oxygen saturation, mean oxygen saturation, and oxygen desaturation index) were also widely used in estimating curative effect of OSA patients before and after receiving treatments. However, the time-consuming process and expensive cost of PSG greatly restricted its wide application. And PSG required subjects to sleep in hospital wearing devices at least one night, which may affect sleep quality and comfort level resulting in failure of monitoring. Besides, limited by the space and the devices, PSG failed to be implemented in most primary hospitals, especially in developing countries [38]. Therefore, it is urgent to find readily measurable, comparatively cheap biomarkers to be helpful for screening OSA and evaluating the curative effect.
According to our findings in this meta-analysis, sP-selectin levels were significantly higher in OSA patients than non-OSA controls on the whole. We infer that if we try to put sP-selectin measurement before PSG, it may help clinician to identify people who are more likely to suffer from OSA. Then, the clinician can apply PSG more targetedly and avoid unnecessary PSG. However, this is only a preliminary hypothesis. Whether sP-selectin level can be served as a biomarker for screening or therapeutic monitoring of OSA is unknown, which needs further study. However, it was still a potential possible biomarker, because some single-armed studies with small sample size have found that OSA patients showed a fall in sP-selectin level after treatment of continuous positive airway pressure (CPAP), although the evidence grade was not strong [26, 39]. Besides, compared with measurement of P-selectin expression on membrane surface using flow cytometry, measurement of sP-selectin in blood using ELISA is easier, cheaper, and more reliable [40]. Therefore, from the medical economic point of view, measuring sP-selectin before PSG may be benefit.
Possible mechanisms
Although the results of this meta-analysis have important clinical significance, the potential mechanism needs to be further explored. It is well known that P-selectin does indeed reflect some aspect of platelet function or activity. And P-selectin was increasingly used as an important clinical marker of platelet activation [9]. The important pathophysiological consequences of OSA were intermittent hypoxemia and sleep fragmentation, which could result in an increased sympathetic activity shown by increased epinephrine levels in OSA patients [41]. And even when OSA patients were awake, they still exhibited high levels of sympathetic nerve activity [42]. Some scholars have found evidence that increased levels of P-selectin expression were observed in the situation of sympathetic activation [43, 44]. Although this sympathetic activation in their studies was induced by hypoglycemia, depression, or anxiety, we inferred that the increased sympathetic activity caused by OSA also might promote an increase in platelet activation and P-selectin level. In addition, animal experiments showed that a marked overexpression of P-selectin was observed in the larynx tissue and soft palate tissue in sleep apnea rat model [45]. Another study also found the expression of P-selectin on the endothelium in colonic venules was up-regulated only in the rat model of apnea group [46]. A vitro experiment conducted on human umbilical vein endothelial cells showed that P-selectin expression could be enhanced in both hypoxic condition and the stage of hypoxia reoxygenation [11]. Besides, hypoxia led to the inflammation and oxidative stress in endothelial cells, which triggered the release of P-selectin [47, 48]. In summary, elevation of P-selectin in OSA patients may be due to multiple mechanisms.
Limitation
Several limitations of our meta-analysis should be acknowledged. First, the number of included studies and their sample sizes were relatively small, which may lower the statistical significance. More studies with larger sample size would be needed. Second, although all the blood samples from subjects were detected timely, it could not be guaranteed that the processes were conducted with identical method among different studies. The difference of detection methods and the diversity of detection kits might influence the accuracy of sP-selectin level. Third, the uses of common drugs such as statin, aspirin, and clopidogrel have been found to reduce the levels of sP-selectin, which may interfere with our results [49,50,51]. We could not guarantee that all participants were free from the therapy of these drugs. Because, the concrete medication histories of participants were not provided in most of the included studies. Fourth, we could not read and understand the studies published in non-English language since the language barrier. Therefore, only studies published in English language were searched and included in our meta-analysis. Now, English has gone global. In non-English native speaking countries, those studies with positive results were relatively easier to be published in English language; while those studies with negative results were more likely to be published in their native language journals instead of English journals, which might introduce a language bias. Therefore, we should acknowledge that this bias was unavoidable in this meta-analysis.
Suggestions for further research
Since all included studies were preliminary studies, the consequence of this paper was regarded as a proposal. We make four suggestions for further research in this field.
First, oxygen desaturation index (ODI), as another measure of OSA severity, has been adopted to classify patients by some scholars in their studies [48, 52]. Jurado-Gamez et al. found an increase in sP-selectin in severe “desaturators” (ODI > 30%) when compared to mild-to-moderate “desaturators” (ODI 5–30%) [48]. Compared to AHI, ODI is more selective of hypoxemic events in OSA patients. And ODI provides a direct measure of intermittent hypoxemia. It has been proved that intermittent hypoxemia causes increased platelet activation. And a correlation between increased platelet activation and the severity of intermittent hypoxemia was also observed [52]. Hence, ODI-based grouping is worth exploring in future studies.
Second, not all the studies in this field used the unified cut-off values of AHI (5 events/h) as criterion to distinguish OSA from non-OSA. For example, Lo´pez-Cano et al. took 10 events/h of AHI as cut-off value [53]. O’Brien et al. took 1 event/h of AHI as cut-off value in the population of children [20]. And Harsch et al. even selected the OSA patients according to unknown criteria without giving a concrete cut-off value of AHI [54]. The above inconsistent criteria limited the persuasiveness of their studies, which became a reason for us to exclude them from our meta-analysis. Besides, the division criteria for OSA severity were also different among various studies. Consequently, the unified criteria of diagnosis and severity classification should be established in future studies.
Third, some of included studies surveyed the serum level of sP-selectin and others surveyed the plasma level of sP-selectin. Therefore, we conducted a subgroup analysis stratified by blood source (serum or plasma). Although the same conclusion was drawn both in serum subgroup and plasma subgroup, we still suggested that blood sample type should be unified in future studies to avoid confounding factor.
Fourth, whether sP-selectin could be served as a biomarker to evaluate the curative effect of CPAP in OSA patients was still controversial, since some studies show a fall in sP-selectin after CPAP treatment and some not [26, 32, 39]. However, the designs of most studies were single-armed. The absence of either a placebo-controlled arm or a matched control significantly weakened the credibility of their findings. In addition, the treatment duration of CPAP varied from studies to studies. For example, the CPAP treatment duration in Robinson’s study was only 1 month which might be too short to produce a detectable curative effect of CPAP [32]. Therefore, the randomized-controlled trials with longer treatment period, larger sample size are needed to confirm this issue in the future.
Conclusion
On the whole, the pooled results reveal that OSA patients have higher sP-selectin levels than non-OSA controls. And after subgroup analysis, this conclusion still remains unaltered in all subgroups other than the subgroup of mild OSA patients. Only sP-selectin levels in mild OSA subgroup do not exhibit a statistically significant increase when compared to controls. Additional studies are warranted to better identify the role of sP-selectin as a potential biomarker in OSA patients.
References
Devulapally K, Pongonis R Jr, Khayat R (2009) OSA: the new cardiovascular disease: part II: overview of cardiovascular diseases associated with obstructive sleep apnea. Heart Fail Rev 14(3):155–164. https://doi.org/10.1007/s10741-008-9101-2
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353(19):2034–2041. https://doi.org/10.1056/NEJMoa043104
Drager LF, Polotsky VY, Lorenzi-Filho G (2011) Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest 140(2):534–542. https://doi.org/10.1378/chest.10-2223
Lippi G, Mattiuzzi C, Franchini M (2015) Sleep apnea and venous thromboembolism. A systematic review. Thromb Haemost 114(5):958–963. https://doi.org/10.1160/th15-03-0188
Geiser T, Buck F, Meyer BJ, Bassetti C, Haeberli A, Gugger M (2002) In vivo platelet activation is increased during sleep in patients with obstructive sleep apnea syndrome. Respiration 69(3):229–234. https://doi.org/10.1159/000063625
Salman LA, Shulman R, Cohen JB (2020) Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep 22(2):6. https://doi.org/10.1007/s11886-020-1257-y
Liak C, Fitzpatrick M (2011) Coagulability in obstructive sleep apnea. Can Respir J 18(6):338–348. https://doi.org/10.1155/2011/924629
von Känel R, Dimsdale JE (2003) Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest 124(5):1956–1967. https://doi.org/10.1378/chest.124.5.1956
André P, Hartwell D, Hrachovinová I, Saffaripour S, Wagner DD (2000) Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA 97(25):13835–13840. https://doi.org/10.1073/pnas.250475997
Kameda H, Morita I, Handa M, Kaburaki J, Yoshida T, Mimori T, Murota S, Ikeda Y (1997) Re-expression of functional P-selectin molecules on the endothelial cell surface by repeated stimulation with thrombin. Br J Haematol 97(2):348–355. https://doi.org/10.1046/j.1365-2141.1997.522700.x
Closse C, Seigneur M, Renard M, Pruvost A, Dumain P, Belloc F, Boisseau MR (1996) Influence of hypoxia and hypoxia-reoxygenation on endothelial P-selectin expression. Haemostasis 26(Suppl 4):177–181. https://doi.org/10.1159/000217296
Sun WY, Abeynaike LD, Escarbe S, Smith CD, Pitson SM, Hickey MJ, Bonder CS (2012) Rapid histamine-induced neutrophil recruitment is sphingosine kinase-1 dependent. Am J Pathol 180(4):1740–1750. https://doi.org/10.1016/j.ajpath.2011.12.024
Dong ZM, Brown AA, Wagner DD (2000) Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation 101(19):2290–2295. https://doi.org/10.1161/01.cir.101.19.2290
Ridker PM, Buring JE, Rifai N (2001) Soluble P-selectin and the risk of future cardiovascular events. Circulation 103(4):491–495. https://doi.org/10.1161/01.cir.103.4.491
Horváth P, Lázár Z, Gálffy G, Puskás R, Kunos L, Losonczy G, Mészáros M, Tárnoki ÁD, Tárnoki DL, Bikov A (2020) Circulating P-selectin glycoprotein ligand 1 and P-selectin levels in obstructive sleep apnea patients. Lung 198(1):173–179. https://doi.org/10.1007/s00408-019-00299-0
Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, Furie B (1994) P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci USA 91(19):8767–8771. https://doi.org/10.1073/pnas.91.19.8767
Polgar J, Matuskova J, Wagner DD (2005) The P-selectin, tissue factor, coagulation triad. J Thromb Haemost 3(8):1590–1596. https://doi.org/10.1111/j.1538-7836.2005.01373.x
del Conde I, Nabi F, Tonda R, Thiagarajan P, López JA, Kleiman NS (2005) Effect of P-selectin on phosphatidylserine exposure and surface-dependent thrombin generation on monocytes. Arterioscler Thromb Vasc Biol 25(5):1065–1070. https://doi.org/10.1161/01.ATV.0000159094.17235.9b
Blann AD, Nadar SK, Lip GY (2003) The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J 24(24):2166–2179. https://doi.org/10.1016/j.ehj.2003.08.021
O’Brien LM, Serpero LD, Tauman R, Gozal D (2006) Plasma adhesion molecules in children with sleep-disordered breathing. Chest 129(4):947–953. https://doi.org/10.1378/chest.129.4.947
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 27(6):1785–1805. https://doi.org/10.1177/0962280216669183
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Chang YT, Lin HC, Chang WN, Tsai NW, Huang CC, Wang HC, Kung CT, Su YJ, Lin WC, Cheng BC, Su CM, Chen TY, Chiang YF, Lu CH (2017) Impact of inflammation and oxidative stress on carotid intima-media thickness in obstructive sleep apnea patients without metabolic syndrome. J Sleep Res 26(2):151–158. https://doi.org/10.1111/jsr.12477
Cofta S, Wysocka E, Dziegielewska-Gesiak S, Michalak S, Piorunek T, Batura-Gabryel H, Torlinski L (2013) Plasma selectins in patients with obstructive sleep apnea. Adv Exp Med Biol 756:113–119. https://doi.org/10.1007/978-94-007-4549-0_15
Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Oda N, Tanaka A, Yamamoto M, Ohta S, O’Donnell CP, Adachi M (2007) Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med 175(6):612–617. https://doi.org/10.1164/rccm.200608-1141OC
Niżankowska-Jędrzejczyk A, Almeida FR, Lowe AA, Kania A, Nastałek P, Mejza F, Foley JH, Niżankowska-Mogilnicka E, Undas A (2014) Modulation of inflammatory and hemostatic markers in obstructive sleep apnea patients treated with mandibular advancement splints: a parallel, controlled trial. JCSM 10(3):255–262. https://doi.org/10.5664/jcsm.3522
Bravo Mde L, Serpero LD, Barceló A, Barbé F, Agustí A, Gozal D (2007) Inflammatory proteins in patients with obstructive sleep apnea with and without daytime sleepiness. Sleep Breath 11(3):177–185. https://doi.org/10.1007/s11325-007-0100-7
Dyugovskaya L, Polyakov A, Lavie P, Lavie L (2008) Delayed neutrophil apoptosis in patients with sleep apnea. Am J Respir Crit Care Med 177(5):544–554. https://doi.org/10.1164/rccm.200705-675OC
García Suquia A, Alonso-Fernández A, de la Peña M, Romero D, Piérola J, Carrera M, Barceló A, Soriano JB, Arque M, Fernández-Capitán C, Lorenzo A, García-Río F (2015) High D-dimer levels after stopping anticoagulants in pulmonary embolism with sleep apnoea. Eur Respir J 46(6):1691–1700. https://doi.org/10.1183/13993003.02041-2014
Winiarska HM, Cofta S, Bielawska L, Płóciniczak A, Piorunek T, Wysocka E (2020) Circulating P-selectin and its glycoprotein ligand in nondiabetic obstructive sleep apnea patients. Adv Exp Med Biol 1279:61–69. https://doi.org/10.1007/5584_2020_501
Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR (2004) Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax 59(9):777–782. https://doi.org/10.1136/thx.2003.018739
Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK (2000) Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol 279(1):H234-237. https://doi.org/10.1152/ajpheart.2000.279.1.H234
Corsonello A, Malara A, Ientile R, Corica F (2002) Leptin enhances adenosine diphosphate-induced platelet aggregation in healthy subjects. Obes Res 10(4):306. https://doi.org/10.1038/oby.2002.42
Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Naseem J, Loomba R (2013) Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. JCSM 9(10):1003–1012. https://doi.org/10.5664/jcsm.3070
De Pergola G, Pannacciulli N, Coviello M, Scarangella A, Di Roma P, Caringella M, Venneri MT, Quaranta M, Giorgino R (2008) sP-selectin plasma levels in obesity: association with insulin resistance and related metabolic and prothrombotic factors. Nutr Metab Cardiovasc Dis 18(3):227–232. https://doi.org/10.1016/j.numecd.2006.09.010
Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D’Andrea F, Molinari AM, Giugliano D (2002) Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 105(7):804–809. https://doi.org/10.1161/hc0702.104279
Peng M, Chen R, Cheng J, Li J, Liu W, Hong C (2018) Application value of the NoSAS score for screening sleep-disordered breathing. J Thorac Dis 10(8):4774–4781. https://doi.org/10.21037/jtd.2018.07.46
Harsch IA, Wallaschofski H, Koebnick C, Pour Schahin S, Hahn EG, Ficker JH, Lohmann T (2004) Adiponectin in patients with obstructive sleep apnea syndrome: course and physiological relevance. Respiration 71(6):580–586. https://doi.org/10.1159/000081758
Csongrádi É, Káplár M, Nagy B Jr, Koch CA, Juhász A, Bajnok L, Varga Z, Seres I, Karányi Z, Magyar MT, Oláh L, Facskó A, Kappelmayer J, Paragh G (2017) Adipokines as atherothrombotic risk factors in obese subjects: Associations with haemostatic markers and common carotid wall thickness. Nutr Metab Cardiovasc Dis 27(6):571–580. https://doi.org/10.1016/j.numecd.2017.02.007
Seravalle G, Mancia G, Grassi G (2018) Sympathetic nervous system, sleep, and hypertension. Curr Hypertens Rep 20(9):74. https://doi.org/10.1007/s11906-018-0874-y
Abboud F, Kumar R (2014) Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Investig 124(4):1454–1457. https://doi.org/10.1172/jci70420
Joy NG, Mikeladze M, Younk LM, Tate DB, Davis SN (2016) Effects of equivalent sympathetic activation during hypoglycemia on endothelial function and pro-atherothrombotic balance in healthy individuals and obese standard treated type 2 diabetes. Metabolism 65(12):1695–1705. https://doi.org/10.1016/j.metabol.2016.09.001
Aschbacher K, Mills PJ, von Känel R, Hong S, Mausbach BT, Roepke SK, Dimsdale JE, Patterson TL, Ziegler MG, Ancoli-Israel S, Grant I (2008) Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun 22(4):493–502. https://doi.org/10.1016/j.bbi.2007.10.002
Almendros I, Carreras A, Ramírez J, Montserrat JM, Navajas D, Farré R (2008) Upper airway collapse and reopening induce inflammation in a sleep apnoea model. Eur Respir J 32(2):399–404. https://doi.org/10.1183/09031936.00161607
Nácher M, Serrano-Mollar A, Farré R, Panés J, Seguí J, Montserrat JM (2007) Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol 155(1):93–96. https://doi.org/10.1016/j.resp.2006.06.004
Jurado-Gámez B, Fernandez-Marin MC, Gómez-Chaparro JL, Muñoz-Cabrera L, Lopez-Barea J, Perez-Jimenez F, Lopez-Miranda J (2011) Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. Eur Respir J 37(4):873–879. https://doi.org/10.1183/09031936.00027910
Jurado-Gamez B, Bujalance Cabrera C, Caballero Ballesteros L, Marin Hinojosa C, Muñoz Cabrera L, Perez-Jimenez F, Lopez-Miranda J (2012) Association of cellular adhesion molecules and oxidative stress with endothelial function in obstructive sleep apnea. Intern Med 51(4):363–368. https://doi.org/10.2169/internalmedicine.51.6571
Seljeflot I, Tonstad S, Hjermann I, Arnesen H (2002) Reduced expression of endothelial cell markers after 1 year treatment with simvastatin and atorvastatin in patients with coronary heart disease. Atherosclerosis 162(1):179–185. https://doi.org/10.1016/s0021-9150(01)00696-7
Özsavcı D, Şener A, Oba R, Yanıkkaya Demirel G, Uras F, Yardımcı TK (2010) New in vitro effects of clopidogrel on platelets in hyperlipidemic and healthy subjects. Turk j haematol 27(2):99–108. https://doi.org/10.5152/tjh.2010.07
Ferroni P, Martini F, Riondino S, La Farina F, Magnapera A, Ciatti F, Guadagni F (2009) Soluble P-selectin as a marker of in vivo platelet activation. Clin Chim Acta 399(1–2):88–91. https://doi.org/10.1016/j.cca.2008.09.018
Krieger AC, Anand R, Hernandez-Rosa E, Maidman A, Milrad S, DeGrazia MQ, Choi AJ, Oromendia C, Marcus AJ, Drosopoulos JHF (2020) Increased platelet activation in sleep apnea subjects with intermittent hypoxemia. Sleep Breath. https://doi.org/10.1007/s11325-020-02021-4
López-Cano C, Rius F, Sánchez E, Gaeta AM, Betriu À, Fernández E, Yeramian A, Hernández M, Bueno M, Gutiérrez-Carrasquilla L, Dalmases M, Lecube A (2019) The influence of sleep apnea syndrome and intermittent hypoxia in carotid adventitial vasa vasorum. PLoS ONE 14(2):e0211742. https://doi.org/10.1371/journal.pone.0211742
Harsch IA, Bergmann T, Koebnick C, Wiedmann R, Ruderich F, Hahn EG, Konturek PC (2007) Adiponectin, resistin and subclinical inflammation–the metabolic burden in Launois Bensaude Syndrome, a rare form of obesity. J physiol pharmacol 58(Suppl 1):65–76
Funding
No Funding was received.
Author information
Authors and Affiliations
Contributions
Conceptualization: DZ; literature search: ZX; formal analysis: DZ and ZX; writing—original draft preparation: DZ; writing—review and editing: YL; supervision: TL
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent is needed for a systematic review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, D., Xu, Z., Liu, T. et al. Soluble P-selectin levels in patients with obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 278, 4633–4644 (2021). https://doi.org/10.1007/s00405-021-06831-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06831-4