Abstract
Purpose
Previous studies have confirmed that patients with obstructive sleep apnea (OSA) have higher systemic inflammatory markers, including intercellular adhesion molecule-1(ICAM-1), vascular cell adhesion molecule-1(VCAM-1), and E-selectin compared to control subjects. However, the effects of continuous positive airway pressure (CPAP) therapy on circulating levels of ICAM-1, VCAM-1, and E-selectin in OSA patients remain inconsistent. Therefore, the primary purpose of the present meta-analysis is to estimate the effect of CPAP therapy on these cell adhesion molecules (CAMs) in patients with OSA.
Methods
The PubMed, Scopus, Embase, and Cochrane Library databases were searched. The overall effects were measured by the standardized mean difference (SMD) with a 95% confidence interval (CI). A random effects model or a fixed-effects model was used, depending on the heterogeneity of the studies.
Results
A total of 11 studies were included, comprising 650 OSA patients. The pooled results showed that CPAP therapy significantly decreased ICAM-1 (SMD = − 0.283, 95% CI − 0.464 to − 0.101, p = 0.002) and E-selectin levels (SMD = − 0.349, 95% CI − 0.566 to − 0.133, p = 0.002). In contrast, there was no significant improvement of VCAM-1 levels after CPAP treatment (SMD = − 0.160, 95% CI − 0.641 to 0.320, p = 0.513).
Conclusions
Our meta-analysis demonstrated that CPAP treatment significantly decreased the circulating levels of ICAM-1 and E-selectin in OSA patients. Thus, ICAM-1 and E-selectin may be effective markers to evaluate CPAP therapy for reducing OSA cardiovascular risk in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common sleep breathing disorder which is a significant health problem in society. OSA is characterized by intermittent hypoxia (IH). Increasingly, shreds of evidence showed close associations between the presence and severity of OSA and increased risk of hypertension, atherosclerosis, and cardiovascular disease (CVD) [1]. IH produces oxidative stress and inflammation, leading to endothelial dysfunction, all of which can contribute to the development of CVDs [2].
OSA patients have higher circulating levels of inflammatory biomarkers, including intercellular adhesion molecule-1(ICAM-1), vascular cell adhesion molecule-1(VCAM-1), E-selectin, interleukin-8, and C-reactive protein [3,4,5]. CAMs, such as ICAM-1, E-selectin, and VCAM-1, are involved in cell recognition, activation, and signal transduction and play an essential role in inflammatory response [6]. Circulating levels of soluble CAMs are potential markers of atherosclerosis development and increased risk of progression. Assessment of circulating inflammatory biomarkers, including CAMs, has been identified as a helpful tool for identifying patients at high risk of future cardiovascular events. CAMs play a role in plaque formation. An early stage of atherosclerosis involves the recruitment of inflammatory cells from the circulation and their migration. The process is mediated mainly by CAMs, which are expressed on vascular endothelium and circulating leukocytes in response to various inflammatory stimuli [7]. The elevation of various CAMs in OSA, including ICAM-1, VCAM-1, and E-selectin, may be involved in the pathological process of OSA-related CVD [8].
CPAP therapy is still considered the gold standard for OSA treatment. It has been shown to reduce the risk of cardiovascular events and the levels of circulating ICAM-1, VCAM-1, and E-selectin in OSA patients, but the results have been controversial. For example, the study by Pak et al. showed that in OSA patients, four months of CPAP treatment with good adherence did not reduce ICAM-1 levels [9]. However, another study showed a significant reduction in ICAM-1 levels during a 6–8-week period of CPAP treatment [10]. The same argument exists for VCAM-1 and E-selectin. Since a previous meta-analysis has shown that ICAM-1, VCAM-1, and E-selectin were significantly higher in OSA patients than in controls [11], we designed the present meta-analysis to quantitatively determine the difference between the CAMs (ICAM-1, VCAM-1, and E-selectin) levels in OSA patients before and after CPAP treatment.
Methods
PRISMA Statement
This meta-analysis was conducted under the guidance of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12].
Literature Search
We searched the PubMed, Embase, Cochrane Library, and Scopus databases up to August 22, 2021. We used the search terms that included free-text and subject-based terms related to “obstructive sleep apnea,” “intercellular adhesion molecule-1,” “E-selectin,” “vascular cell adhesion molecule-1,” and “continuous positive airway pressure”. Supplementary Table 1 describes the complete search strategy. Additionally, we supplemented the computerized search with a manual search of references from the related articles.
Inclusion and Exclusion Criteria of the Literature
The inclusion criteria were as follows: (1) Population: the diagnosis of OSA was made by polysomnography (apnea hypopnea index [AHI] ≥ 5); (2) Intervention: treatment with CPAP; (3) Comparator: patients with OSA before CPAP treatment; and (4) Outcome: the circulating ICAM-1, E-selectin, and VCAM-1 values before and after CPAP therapy.
The exclusion criteria were as follows: (1) repetition of previous publications; (2) reviews, letters, case reports, or editorials; (3) conference abstract; (4) no full text; (5) no relevant data; (6) non-English articles. We included the largest population of the studies if multiple studies reported outcomes on the same patient group. Two researchers independently screened the relevant articles and reviewed their full text. For articles with insufficient data, the corresponding author was contacted via email.
Data Extraction and Quality Assessment
Data extracted from each study included the first author’s name, year of publication, study country/region, sample type, study design, age, sex, mean body mass index (BMI), sample size, OSA severity (AHI), and the ICAM-1, E-selectin, and VCAM-1 levels. Two researchers independently extracted the data simultaneously. A third investigator resolved any disagreement.
The methodological quality of all included observational studies was assessed according to the risk of a bias assessment tool for non-randomized studies (RoBANS) [13]. We used the risk-of-bias assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions for randomized studies [14].
Statistical Analysis
The meta-analysis was conducted using Stata 15.1 (StataCorp, College Station, Texas, USA). We used the standard mean difference (SMD) to evaluate the difference between the ICAM-1, E-selectin, and VCAM-1 levels before and after CPAP treatment because the inflammatory markers were measured and reported differently. Results reported continuous data as medians and quartiles were converted to the mean and standard deviation (SD) using the methods of Wan et al. [15]. If there was evidence of statistical heterogeneity indicated by p < 0.10 or I2 > 50%, the data were analyzed using a random-effects model. Otherwise, a fixed-effects model was used for the analysis. Planned subgroup analyses and meta-regression analyses were performed to identify the possible sources of heterogeneity. A funnel plot and Egger’s test were used to detect any publication bias. For all the results, p < 0.05 was considered statistically significant for the overall effect size. A sensitivity analysis was conducted to assess the stability of the results by sequentially removing one study each time.
Results
Characteristics of Included Studies
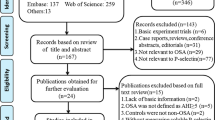
According to the search strategy, 86 pieces of literature were screened out. After performing exclusion based on the flow chart for literature screening (Fig. 1), 11 articles were finally included. Each study investigated one or more CAMs markers: 10 studies on ICAM-1 [5, 9, 10, 16,17,18,19,20,21,22], 5 on E-selectin [5, 17, 19, 21, 23], and 5 on VCAM-1 [5, 16, 19, 21, 23]. The characteristics of included studies for different CAMs markers are provided in Table 1. The quality assessments are shown in Table 2. These studies comprised 650 patients with OSA.
Pooled Analysis
The pooled results showed that CPAP therapy significantly decreased ICAM-1 levels (SMD = − 0.283, 95% confidence interval (CI) − 0.464 to − 0.101, p = 0.002; Fig. 2a). The random-effects model was used for meta-analysis because of significant heterogeneity among the eligible studies (I2 = 53.8%). The E-selectin levels also showed significant decrease after CPAP treatment (SMD = − 0.349, 95% CI − 0.566 to − 0.133, p = 0.002; Fig. 2b). The fixed-effects model was used for meta-analysis because of small heterogeneity among the eligible studies (I2 = 28.2%). In contrast, VCAM-1 showed no improvement after CPAP (SMD = − 0.160, 95% CI − 0.641 to 0.320, p = 0.513; Fig. 2c). The random-effects model was used for meta-analysis due to significant heterogeneity among the eligible studies (I2 = 83.8%).
Meta-analysis and forest plot. Comparison of a ICAM-1, b E-selectin, and c VCAM-1 levels before and after CPAP therapy. BMI body mass index, CI confidence interval, CPAP continuous positive airway pressure, ICAM-1 intercellular adhesion molecule-1, SMD standardized mean difference, VCAM-1 vascular cell adhesion molecule-1
Subgroup Analysis
To understand the causes of heterogeneity, we performed subgroup analyses based on BMI, therapy duration, and study region.
ICAM-1
When stratified by BMI (≥ 30 or < 30), CPAP treatment significantly decreased ICAM-1 levels both in OSA subjects with BMI < 30 (SMD = − 0.586, p < 0.001; I2 = 0) and BMI ≥ 30 (SMD = − 0.158, p = 0.049; I2 = 26.2%) (Table 3). When therapy duration was ≥ 3 months, the total SMD = − 0.205, p = 0.042, I2 = 55.9%. When therapy duration was < 3 months, the total SMD = − 0.568, p < 0.001, I2 = 0 (Table 3). Data analysis according to the study region revealed that studies in East Asia showed a statistically significant difference after CPAP treatment (SMD = − 0.581, p < 0.001 I2 = 0). Contrastingly, studies in Europe and North America only showed a decreased trend (SMD = − 0.158, p = 0.05, I2 = 26.1%) (Table 3). Sources of heterogeneities may be the study region and BMI by subgroup analysis.
E-selectin
Data analyzed according to BMI < 30 (SMD = − 0.319, p = 0.008, I2 = 0) had significantly lower E-selectin levels after CPAP treatment; there was no significant difference when BMI ≥ 30 (SMD = − 0.991, p = 0.23, I2 = 73.6%) (Table 3). Both therapy durations ≥ 3 months (SMD = − 0.311, p = 0.017, I2 = 22.6%) and < 3 months (SMD = − 0.448, p = 0.033, I2 = 49.7%) (Table 3) showed significant differences after CPAP. Next, data analysis according to study region showed no difference in E-selectin level after CPAP treatment in Europe and North America (SMD = − 0.463, p = 0.246, I2 = 63.1%). In East Asia, there was a significance difference after CPAP treatment (SMD = − 0.374, p = 0.004, I2 = 0) (Table 3).
VCAM-1
In studies with BMI ≥ 30, the VCAM-1 levels increased significantly (SMD = 0.215, p = 0.042, I2 = 1.6%); no significant improvements were observed after CPAP application in BMI < 30 (SMD = − 0.433, p = 0.072, I2 = 56.6%) (Table 3). Both therapy durations ≥ 3 months (SMD = − 0.167, p = 0.706, I2 = 95.9%) or < 3 months (SMD = − 0.129, p = 0.529, I2 = 0) (Table 3) showed no difference after CPAP treatment. The subgroup analyses of VCAM-1 levels according to study region were similar with BMI (Table 3).
Sensitive Analysis
We evaluated the stability of the results through sensitivity analysis by using the sequential exclusion of each study. When single studies evaluating the ICAM-1 levels after CPAP treatment were sequentially removed, the pooled SMD did not alter substantially. The effect size ranged between − 0.20 and − 0.33, suggesting that the meta-analysis results were stable (Fig. 3a). Sensitivity analysis produced nonrobust results after omitting the study by Jin et al. [5] (SMD = − 0.306, 95% CI − 0.649 to 0.037, p = 0.08), which indicated that after excluding this study, the E-selectin levels were not significantly decreased with CPAP therapy (Fig. 3b). When single studies were sequentially removed among studies in the VCAM-1 group, after excluding the study by Pak et al. [16], the pooled results showed a significant decrease in VCAM-1 level after CPAP treatment (SMD = − 0.387, 95% CI − 0.698 to − 0.075, p = 0.015) (Fig. 3c).
Meta-Regression
The outcome variable was the SMD of ICAM-1, E-selectin, and VCAM-1 levels. Meta-regression included the following variables as covariates: sample size, study region (Europe and North America or East Asia), mean age, male proportion, mean BMI, mean AHI, therapy duration. Meta-regression showed that the study region might be the possible source of heterogeneity of ICAM-1; meta-regression results are shown in Table 4.
Publication Bias
Funnel plots showed the potential existence of asymmetry in the meta-analysis (Fig. 4). However, Egger’s test indicated an absence of publication bias ICAM-1 (p = 0.319), E-selectin (p = 0.498), and VCAM-1 (p = 0.631).
Discussion
Our results demonstrated that CPAP was an effective intervention for reducing ICAM-1 and circulating E-selectin levels, but not VCAM-1.
OSA is an independent risk factor for CVD, and the IH-induced chronic inflammatory response may be associated with various CVDs. CAMs are related to inflammation, which plays a crucial role in atherosclerosis [7] and is associated with chronic kidney disease (CKD) [24], ischemic cerebrovascular disease [25], and CVD [26]. Renal function may be a factor affecting CAMs. Inflammation and oxidative stress increase in patients with moderate to severe CKD [27, 28]. These increased levels of inflammation and oxidative stress may accelerate the process of atherosclerosis, exacerbating the risk of CVD. The influence of renal function on CAMs in OSA patients, especially after CPAP therapy, remains unclear. More studies are needed to confirm whether CPAP therapy is effective in reducing CAMs levels in patients with CKD and OSA; this could help in understanding the causes of the increased risk of CVD in patients with end-stage renal disease. A previous study had demonstrated that chronic IH activated nuclear factor-kappa B (NF-κB) and induced its expression in cardiovascular tissues in mice. CPAP treatment in OSA patients could reverse increased monocyte NF-κB activity [29]. NF-κB can further activate the CAMs and mediate inflammatory response [30]. This IH-induced NF-κB activation could increase the expression of proinflammatory NF-κB-dependent genes, including the genes of adhesion molecules—ICAM-1, VCAM-1, and E-selectin [31]. Notably, CPAP therapy may improve cardiovascular outcomes by significantly reducing the levels of these markers, but the results are controversial. ICAM-1, E-selectin, and VCAM-1 decreased after CPAP treatment in several studies [5, 10, 23]. However, interestingly, some studies reported an increase in CAMs levels [9, 16]. In our meta-analysis, the combined results confirmed that the circulating ICAM-1 and E-selectin levels were decreased in patients with OSA after CPAP treatment, but this was not the case for VCAM-1 levels, indicating that CPAP therapy could reduce inflammatory responses in OSA patients.
Subgroup analyses suggested that BMI might be a source of heterogeneities for ICAM-1. CPAP therapy was likely to produce a better response in decreasing ICAM-1 levels in OSA patients with lower BMI than in those with higher BMI. It is speculated that once the thinner OSA patients are relieved of repeated airway obstruction after CPAP treatment, their levels of circulating CAMs decrease more rapidly than that of fatter patients. Obesity is regarded as a chronic low-grade inflammatory state, which may be related to an increase in adhesion molecule levels. In a recent study of OSA patients, ICAM-1, E-selectin, and VCAM-1 were significantly associated with BMI [32]. The results from one meta-analysis also showed a significant correlation between BMI and ICAM-1 or VCAM-1 [11]. This may explain why the ICAM-1 and E-selectin levels of OSA patients in East Asia responded better to CPAP treatment—probably because of a relatively low BMI. The increased levels of CAMs by obesity may not be alleviated effectively by CPAP therapy. Subgroup analyses indicated that the treatment duration of < 3 months appears to be more effective in decreasing the circulating ICAM-1 and E-selectin levels. This may be related to patient adherence, as one study showed that a longer therapy duration might influence CPAP adherence [33]. Therefore, further long-term RCTs, involving weight-matched patients with good adherence, are needed to verify the effects of CPAP treatment on circulating ICAM-1, E-selectin, and VCAM-1 in OSA patients, after controlling for other influencing factors.
Meta-regression suggested that the study region might be a source of heterogeneities for ICAM-1. However, we did not find the source of heterogeneity for VCAM-1 by subgroup and meta-regression. Sensitive analyses showed that the results of pooled analyses of E-selectin levels after CPAP therapy were not stable upon omission of Jin et al.’s results [5]. Possible reasons may include the following: (i) the number of patients included in this study is larger than that in other studies (Fig. 2b) and/or (ii) the number of articles included was small (n = 5). When excluding the data from Pak et al. [16], the pooled results of VCAM-1 significantly decreased after CPAP treatment. Unexpectedly, in Pak et al.’s study, the VCAM-1 levels increased after CPAP treatment. Due to uncertain therapeutic effects and significant heterogeneity in the analysis, more studies are needed to evaluate the effects of CPAP treatment on VCAM-1.
To the best of our knowledge, this is the first meta-analysis to study the effects of CPAP on CAMs in patients with OSA. The statistical analysis supports the effectiveness of CPAP treatment. Thus, ICAM-1 and E-selectin may be effective markers to evaluate CPAP therapy for reducing cardiovascular risk in OSA patients. Nonetheless, this meta-analysis has several limitations. Firstly, the included studies had relatively small sample sizes. The small sample sizes of patients clearly reduced the reliability of the results. Secondly, although we studied the changes in CAMs in each patient before and after CPAP treatment, we still cannot exclude the possibility that our results were subject to various confounding factors, such as obesity [32], tumors [34], infections [35], and diabetes [36], which could affect circulating CAMs levels. This possibility should receive significant attention in future studies. Thirdly, in this meta-analysis, the exclusion of non-English articles may cause potential publication bias.
Conclusions
Our meta-analysis demonstrated that CPAP treatment significantly improved the circulating ICAM-1 and E-selectin levels in OSA patients. Thus, ICAM-1 and E-selectin may be effective markers to evaluate CPAP therapy for reducing OSA cardiovascular risk in clinical practice.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Collen J, Lettieri C, Wickwire E, Holley A (2020) Obstructive sleep apnea and cardiovascular disease, a story of confounders! Sleep Breath 24(4):1299–1313. https://doi.org/10.1007/s11325-019-01945-w
Ma L, Zhang J, Liu Y (2016) Roles and mechanisms of obstructive sleep apnea-hypopnea syndrome and chronic intermittent hypoxia in atherosclerosis: evidence and prospective. Oxid Med Cell Longev 2016:8215082. https://doi.org/10.1155/2016/8215082
Huang T, Goodman M, Li X, Sands SA, Li J, Stampfer MJ, Saxena R, Tworoger SS, Redline S (2021) C-reactive protein and risk of OSA in four US cohorts. Chest. https://doi.org/10.1016/j.chest.2021.01.060
Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y (2003) Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol 94(1):179–84. https://doi.org/10.1152/japplphysiol.00177.2002
Jin F, Liu J, Zhang X, Cai W, Zhang Y, Zhang W, Yang J, Lu G, Zhang X (2017) Effect of continuous positive airway pressure therapy on inflammatory cytokines and atherosclerosis in patients with obstructive sleep apnea syndrome. Mol Med Rep 16(5):6334–6339. https://doi.org/10.3892/mmr.2017.7399
Harjunpaa H, Llort Asens M, Guenther C, Fagerholm SC (2019) Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front Immunol 10:1078. https://doi.org/10.3389/fimmu.2019.01078
Blankenberg S, Barbaux S, Tiret L (2003) Adhesion molecules and atherosclerosis. Atherosclerosis 170(2):191–203. https://doi.org/10.1016/s0021-9150(03)00097-2
Pak VM, Grandner MA, Pack AL (2014) Circulating adhesion molecules in obstructive sleep apnea and cardiovascular disease. Sleep Med Rev 18(1):25–34. https://doi.org/10.1016/j.smrv.2013.01.002
Pak VM, Maislin DG, Keenan BT, Townsend RR, Benediktsdottir B, Dunbar SB, Pack AI, Gislason T, Kuna ST (2021) Changes in sleepiness and 24-h blood pressure following 4 months of CPAP treatment are not mediated by ICAM-1. Sleep Breath. https://doi.org/10.1007/s11325-020-02257-0
Nowicki M, Zawiasa-Bryszewska A, Taczykowska M, Białasiewicz P, Nowak D (2020) The pattern of overnight changes in novel markers of acute kidney injury in patients with obstructive sleep apnea. Adv Clin Exp Med 29(9):1065–1072. https://doi.org/10.17219/acem/123356
Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Naseem J, Loomba R (2013) Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 9(10):1003–1012. https://doi.org/10.5664/jcsm.3070
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341. https://doi.org/10.1016/j.ijsu.2010.02.007
Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, Jang BH, Son HJ (2013) Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 66(4):408–414. https://doi.org/10.1016/j.jclinepi.2012.09.016
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J (2019) Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 10:ED000142. https://doi.org/10.1002/14651858.ED000142
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Pak VM, Keenan BT, Jackson N, Grandner MA, Maislin G, Teff K, Schwab RJ, Arnardottir ES, Júlíusson S, Benediktsdottir B, Gislason T, Pack AL (2015) Adhesion molecule increases in sleep apnea: beneficial effect of positive airway pressure and moderation by obesity. Int J Obes 39(3):472–479. https://doi.org/10.1038/ijo.2014.123
Zamarrón C, Riveiro A, Gude F (2011) Circulating levels of vascular endothelial markers in obstructive sleep apnoea syndrome. Effects of nasal continuous positive airway pressure. Arch Med Sci. 7(6):1023–8. https://doi.org/10.5114/aoms.2011.26615
Campos-Rodriguez F, Asensio-Cruz MI, Cordero-Guevara J, Jurado-Gamez B, Carmona-Bernal C, Gonzalez-Martinez M, Troncoso MF, Sanchez-Lopez V, Arellano-Orden E, Garcia-Sanchez MI, Martinez-Garcia MA (2019) Effect of continuous positive airway pressure on inflammatory, antioxidant, and depression biomarkers in women with obstructive sleep apnea: a randomized controlled trial. Sleep. https://doi.org/10.1093/sleep/zsz145
Chin K, Nakamura T, Shimizu K, Mishima M, Nakamura T, Miyasaka M, Ohi M (2000) Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med 109(7):562–567. https://doi.org/10.1016/s0002-9343(00)00580-5
Yoshikawa M, Yamauchi M, Fujita Y, Koyama N, Fukuoka A, Tamaki S, Yamamoto Y, Tomoda K, Kimura H (2014) The impact of obstructive sleep apnea and nasal CPAP on circulating adiponectin levels. Lung 192(2):289–295. https://doi.org/10.1007/s00408-013-9550-9
Nikitidou O, Daskalopoulou E, Papagianni A, Vlachogiannis E, Dombros N, Liakopoulos V (2020) The impact of OSA and CPAP treatment on cell adhesion molecules’ night-morning variation. Sleep Breath. https://doi.org/10.1007/s11325-020-02232-9
Harańczyk M, Konieczyńska M, Płazak W (2021) Endothelial dysfunction in obstructive sleep apnea patients. Sleep Breath. https://doi.org/10.1007/s11325-021-02382-4
Htoo AK, Greenberg H, Tongia S, Chen G, Henderson T, Wilson D, Liu SF (2006) Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath 10(1):43–50. https://doi.org/10.1007/s11325-005-0046-6
Petreski T, Piko N, Ekart R, Hojs R, Bevc S (2021) Review on inflammation markers in chronic kidney disease. Biomedicines. https://doi.org/10.3390/biomedicines9020182
Frijns CJ, Kappalle LJ (2002) Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke 33(8):2115–2122. https://doi.org/10.1161/01.str.0000021902.33129.69
Marchini T, Mitre LS, Wolf D (2021) Inflammatory cell recruitment in cardiovascular disease. Front Cell Dev Biol 9:635527. https://doi.org/10.3389/fcell.2021.635527
Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J (2004) Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65(3):1009–1016. https://doi.org/10.1111/j.1523-1755.2004.00465.x
Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ, Ball S, Townend JN, Baigent C (2004) Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis 43(2):244–253. https://doi.org/10.1053/j.ajkd.2003.10.037
Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF (2006) Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun 343(2):591–596. https://doi.org/10.1016/j.bbrc.2006.03.015
Cheng HS, Njock MS, Khyzha N, Dang LT, Fish JE (2014) Noncoding RNAs regulate NF-kappaB signaling to modulate blood vessel inflammation. Front Genet 5:422. https://doi.org/10.3389/fgene.2014.00422
Yamauchi M, Kimura H (2008) Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antioxid Redox Signal 10(4):755–768. https://doi.org/10.1089/ars.2007.1946
Peres BU, Allen AJH, Kendzerska T, Shah A, Fox N, Laher I, Almeida F, Jen R, Sandford AJ, van Eeden SF, Ayas NT (2020) Obstructive sleep apnea severity, body mass index, and circulating levels of cellular adhesion molecules. Lung 198(6):939–945. https://doi.org/10.1007/s00408-020-00401-x
BaHammam AS, Alassiri SS, Al-Adab AH, Alsadhan IM, Altheyab AM, Alrayes AH, Alkhawajah MM, Olaish AH (2015) Long-term compliance with continuous positive airway pressure in Saudi patients with obstructive sleep apnea. A prospective cohort study. Saudi Med J 36(8):911–919. https://doi.org/10.15537/smj.2015.8.11716
Bui TM, Wiesolek HL, Sumagin R (2020) ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol 108(3):787–799. https://doi.org/10.1002/JLB.2MR0220-549R
Amalakuhan B, Habib SA, Mangat M, Reyes LF, Rodriguez AH, Hinojosa CA, Soni NJ, Gilley RP, Bustamante CA, Anzueto A, Levine SM, Peters JI, Aliberti S, Sibila O, Chalmers JD, Torres A, Waterer GW, Martin-Loeches I, Bordon J, Blanquer J, Sanz F, Marcos PJ, Rello J, Ramirez J, Sole-Violan J, Luna CM, Feldman C, Witzenrath M, Wunderink RG, Stolz D, Wiemken TL, Shindo Y, Dela Cruz CS, Orihuela CJ, Restrepo MI (2016) Endothelial adhesion molecules and multiple organ failure in patients with severe sepsis. Cytokine 88:267–273. https://doi.org/10.1016/j.cyto.2016.08.028
Pankow JS, Decker PA, Berardi C, Hanson NQ, Sale M, Tang W, Kanaya AM, Larson NB, Tsai MY, Wassel CL, Bielinski SJ (2016) Circulating cellular adhesion molecules and risk of diabetes: the multi-ethnic study of atherosclerosis (MESA). Diabet Med 33(7):985–991. https://doi.org/10.1111/dme.13108
Funding
This work was supported by the Chinese National Natural Science Foundation (Grant Number: 81670080).
Author information
Authors and Affiliations
Contributions
DT and MXL contributed to study concept and design and provided supervision. MXL contributed to revision and submission of the manuscript. ZST, JYX, and JK contributed to literature search. HYS and ZZM contributed to data extractions. ZST and JYX contributed to data analysis and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, Z., Xiao, J., Kang, J. et al. Effects of Continuous Positive Airway Pressure on Cell Adhesion Molecules in Patients with Obstructive Sleep Apnea: A Meta-Analysis. Lung 199, 639–651 (2021). https://doi.org/10.1007/s00408-021-00487-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-021-00487-x