Abstract
Selectins and their ligands play an important role in atherosclerosis. The role of these adhesion molecules in the pathogenesis of obstructive sleep apnea (OSA) may be of clinical relevance. Therefore, the aim of this study was to assess the serum content of platelet P-selectin (P-SEL) and P-selectin glycoprotein ligand 1 (PSGL-1) in different OSA stages. The study was performed in nondiabetic patients, aged 32–71, in whom OSA was verified by polysomnography. The apnea/hypopnea index (AHI) was used to stratify OSA stages: AHI <5, no sleep pathology (OSA-0); AHI 5–15, (OSA-1); AHI 16–30, (OSA-2); and AHI >30, (OSA-3). There were 16 patients in each group. P-SEL and PSGL-1 were assessed by ELISA kits. There were no appreciable differences in the patients’ glucose or high-specificity C-reactive protein content. We found that P-SEL and PSGL-1 significantly increased from OSA-0 to OSA-3. There were the following positive associations in all OSA patients: P-SEL vs. AHI, PSGL-1 vs. AHI, and P-SEL vs. PSGL-1. In addition, the adhesion molecules are associated with the anthropometric parameters, oxygen saturation, and sleep architecture in the OSA-1 group. We conclude that the adhesion molecules consistently increase in the blood of nondiabetic OSA patients, along with progression of disorder severity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adhesion molecules
- Apnea-hypopnea index
- Atherosclerosis
- Nondiabetic patients
- Obstructive sleep apnea
- Selectins
- Sleep pathology
1 Introduction
The obstructive sleep apnea (OSA) is a common sleep disorder, with 9–38% the prevalence in the adult population (Senaratna et al. 2017). The essential pathophysiologic feature is a recurrent cessation of breathing during sleep caused by upper airways collapse. The severity of OSA is usually stratified on the basis of the apnea-hypopnea index (AHI) as follows: mild (AHI 5–15), moderate (AHI 15–30), and severe (AHI >30) (Kapur et al. 2017). The most common risk factors for OSA include obesity, male gender, age, and postmenopausal state (Laratta et al. 2017), all of which are akin to the typical cardiovascular risk factors. Recurrent episodes of apnea or hypopnea cause many a pathological process, foremost hypoxia–reoxygenation cycles and rapid alterations in the intrathoracic pressure, leading to endothelial dysfunction, oxidative stress, and general atherosclerosis.
Atherosclerosis is a multifactorial inflammatory disease which is expected to be the main cause of mortality in the Western countries (Hansson 2005) due to the development of a spate of cardiovascular and cerebrovascular pathologies. The earliest stage of atherosclerosis is endothelial dysfunction, with dysregulation of thrombosis, redox imbalance, and inflammatory reactions (Gimbrone Jr and Garcia-Cardena 2016), which is followed by migration and adhesion of leukocytes to the endothelium. These processes are mediated by adhesion molecules, notably by P-selectin (P-SEL) which is a transmembrane glycoprotein, stored in endothelial cells and platelets. P-SEL translocates to the cell surface after cell activation (Ley 2003), where the molecule is conducive to leukocyte–endothelium and platelet–endothelium interactions (Budhiraja et al. 2007). Expression of P-SEL is remarkably enhanced in the endothelium overlying active atherosclerotic plaques (Johnson-Tidey et al. 1994). Adhesion of leukocytes to endothelial cells is mediated by the P-selectin glycoprotein ligand-1 (PSGL-1), which is a leukocyte protein responsible for about 90% of P-SEL binding. PSGL-1 also binds to L-selectin and enables the interaction between leukocytes on inflamed endothelial cells (McEver 2001). PSGL-1 deficiency prevents endothelial activation and reduces the risk of atherosclerosis in mice (Luo et al. 2012). Both P-SEL and PSGL-1 play a key role in the early stages of atherosclerosis. Therefore, this study seeks to define the serum content of platelet P-SEL and PSGL-1 in OSA patients.
2 Methods
There were 64 consecutive patients, aged 32–71, with a suspicion of OSA, enrolled into the study. Each patient underwent a physical examination and was subjected to the Epworth Sleepiness Scale (ESS) to determine the subjective diurnal sleepiness. The patients were then referred to in-lab sleep polysomnographic (PSG) examination, performed with an EMBLA S4000-Remlogic setup equipped with Somnologica Studio v5.0 software (Embla Systems, Thornton, CO). The full-night recordings were analyzed manually to verify the data provided automatically. The apnea was defined as a cessation of airflow lasting for more than 10 s. The hypopnea was defined as at least 30% reduction of airflow with at least 4% desaturation, lasting for at least 10 s. On the basis of AHI (the number of apnea and hypopnea episodes per hour), we divided patients into four groups: OSA-0 (no disorder; AHI <5), OSA-1 (mild disorder; AHI 5–15), OSA-2 (moderate disorder; AHI 16–30), and OSA-3 (severe disorder; AHI >30); each group consisted of 16 subjects. Arterial blood pressure, complete blood count, high-sensitivity C-reactive protein (hsCRP), lipid profile, fasting (Glu-0), and 120-min (Glu-120) glycemia during oral glucose tolerance test (OGTT) were determined, using Dimension Xpand Plus Systems (Siemens Healthcare Diagnostics, Deerfield, IL). The content of P-SEL and PSGL-1 was measured by the ELISA method using a DRG Reagent Kit (DRG International Inc., Springfield, NJ) on microplate reader (Sunrise Tecan; Männedorf, Switzerland). The study participants with hsCRP <8.00 mg/L and no diabetes according to the OGTT results were enrolled. The exclusion criteria were as follows: chronic or acute heart diseases, stroke, respiratory failure, chronic kidney or liver disease, current smoking, neoplasm, diabetes mellitus, and current infection.
Data were expressed as medians and interquartile ranges (IQR: 25th–75th). The Shapiro-Wilk test was used to check data distribution. Since the distribution was skewed, nonparametric tests were used in further analysis. Differences among the studied groups were assessed with the Kruskal-Wallis test, followed by a post hoc Dunn’s test. The Spearman rank correlation coefficient (rho) was used to investigate correlations among parameters, with the following estimators of a rho strength: weak (0.10–0.29), average (0.30–0.49), strong (0.50–0.69), very strong (0.70–0.89), and almost full (0.90–1.00). Multiple regression analysis was conducted for relevant parameters. In all of the tests, p < 0.05 was taken as an indicator of statistical significance. The analysis was performed using a commercial statistical packet of Statistica v12.0 (StatSoft, Tulsa, OK).
3 Results
Clinical and laboratory characteristics of the study groups are shown in Table 1. The groups differed in the AHI and ESS results. There also were significant differences in the body mass index (BMI) and neck circumference, with the highest values in OSA-3 group. However, there were no intergroup differences in blood pressure, glucose levels during OGTT, total cholesterol, LDL-cholesterol, or triglycerides, nor were there any in the duration of sleep phases expressed as a percentage of the total sleep time neither.
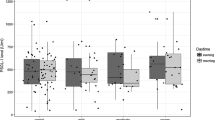
There were significant differences in P-SEL and PSGL-1 content among the OSA groups. P-SEL increased from OSA-0 to OSA-3 (Fig. 1), with the following statistical power of differences of the intergroup comparisons with Dunn’s test: OSA-0 vs. OSA-1 (p > 0.05), OSA-0 vs. OSA-2 (p < 0.002), OSA-0 vs. OSA-3 (p < 0.001), and OSA-1 vs. OSA-3 (p < 0.001). PSGL-1 also increased from OSA-0 to OSA-3 (Fig. 2), with the following intergroup significance: OSA-0 vs. OSA-1 (p = 1.0), OSA-0 vs. OSA-2 (p = 0.100), OSA-0 vs. OSA-3 (p < 0.001), and OSA-1 vs. OSA-3 (p < 0.001).
Associations between P-SEL and PSGL-1 content and other parameters in the OSA groups are shown in Table 2. P-SEL is strongly or very strongly associated with the level of AHI, minimum oxygen saturation, white blood cell count, and total cholesterol. Likewise, PSGL-1 is associated with the magnitude of AHI. On the other hand, duration of non-REM-3 sleep is adversely associated with the content of both P-SEL and PSGL-1.
Multiple regression analysis was used to assess which investigated parameters could independently influence the content of either P-SEL or PSGL-1 (Table 3). Increasing AHI might explain about 95% of linear increase in PSGL-1 in OSA-3 and 83% of linear increase in PSGL-1 in OSA-2. Moreover, elevated PSGL-1 might explain 84% of linear increase in P-SEL in OSA-3.
4 Discussion
The major finding of the present study was that the blood content of the adhesion molecules P-SEL and PSLG-1 in the blood of OSA patients progressively increased from mild to severe OSA. Moreover, there was a significant inverse association between average arterial oxygen saturation and P-SEL content. On the other side, P-selectin is associated rather weakly with the anthropometrical parameters such as BMI or waist and neck circumference. These findings confirm some of the other studies on the issue, although differ in details with yet some other studies. A previous study in 80 male non-smoking Caucasians has also shown a progressive increase of P-SEL along the increase of OSA severity (Cofta et al. 2013). Another one has shown that P-SEL, and also the incidence of silent brain infarction, is significantly higher in moderate-to-severe OSA when compared to the control group of obese patients but not suffering from OSA. Moreover, CPAP treatment reverses the increased P-SEL content (Minoguchi et al. 2007). Dyugovskaya et al. (2008) have shown in a group of 68 patients that P-SEL content is increased in patients with severe OSA when compared to mild OSA. Rather unexpectedly, those authors find the difference in P-SEL loses significance after adjustment to BMI, which may point to a key role of obesity in the enhancement of the molecule content in OSA. A study by Jurado-Gamez et al. (2012) has used the oxygen desaturation index per hour (ODI), as opposed to AHI in the studies outlined above, as a measure of OSA severity. The authors have shown an increase in P-SEL in severe “desaturators” (ODI ≥30%) when compared to mild-to-moderate “desaturators” (ODI 5–30%). In another study, no difference in P-SEL has been noticed between “non-desaturators” (ODI <10%) when compared with “desaturators” (ODI ≥10%) (Priou et al. 2010). In the experimental rat model, blood P-SEL content increased in the apnea group (Nacher et al. 2007). There are, however, studies failing to show the dependence of blood P-SEL content on OSA severity, although it correlated with BMI (Robinson et al. 2004).
In this study, we also found an increase in the blood content of PSGL-1, a P-SEL glycoprotein ligand, depending on the OSA severity. The association of PSGL-1 and AHI was particularly strong in OSA-2 and OSA-3 groups. PSGL-1 is strongly associated with P-SEL in OSA-3 group. In contrast, PSGL-1 is weakly associated with BMI. It is rather hard to compare our results with the literature data as there is a paucity of studies on the content of PSGL-1 in the course of OSA. In a study by Horváth et al. (2018), the authors fail to report an appreciable difference in PSGL-1 content between OSA and non-OSA patients.
We did not find any appreciable differences in the duration of NREM-3 sleep stage, expressed as a percentage of total sleep time, across all of the OSA groups investigated. Interestingly, strong adverse P-SEL–NREM-3 and PSGL-1–NREM-3 associations were unravelled. Currently, there are no data linking disorders of sleep stages with cardiovascular risk. Further investigations are required to explore whether a reduction in NREM-3 sleep could be an early presage of the development of arteriosclerosis.
Atherosclerosis is a chronic inflammatory disease, resulting from the interplay of dysfunctional endothelium with systemic hemostatic and inflammatory mechanisms (Lui and Sau-Man 2012). It is characterized by the accumulation of lipids and fibrous elements especially in large arteries (Lusis 2000). This process is the main cause of cardiovascular disorders (CVD), including stroke, myocardial infract, and heart failure, the irreversible effects of advanced atherosclerosis. The early stage of atherosclerosis is a challenge for clinical practice, especially since patients could benefit from therapeutic modifications of CVD risk factors. One of the earliest stages of atherosclerosis is leukocyte rolling on activated endothelium, which is promoted by the adhesion molecules P-SEL and its glycoprotein ligand PSGL-1. Enhanced plasma content of P-SEL reflects both endothelial dysfunction and platelet activation (Blann et al. 2003). Burger and Wagner (2003) have shown that P-SEL contributes to atherosclerosis through mediating the monocyte rolling, recruitment of leukocytes to atherosclerotic lesions, and facilitation of thrombosis.
P-SEL is elevated in the blood of patients with acute myocardial infraction (Chiu et al. 2005; Ikeda et al. 1994). Moreover, an inhibitor of P-SEL suppresses plaque formation, rupture, and the intimal bleeding (Guo et al. 2015). Chung et al. (2009) and O’Connor et al. (1999) have reported the elevation of P-SEL in congestive heart failure, although it failed to associate with the ejection fraction. Interestingly, the authors find no difference in P-SEL depending on the ischemic and nonischemic background of heart failure, nor does the content of P-SEL depend on the use or not of acetylsalicylic acid by patients. P-SEL also is elevated in line with the progressing vascular dysfunction of hypertension (Sanada et al. 2005; Verhaar et al. 1998) and in the early stage of progressing ischemic stroke (Wang et al. 2013).
PSGL-1 is constitutively expressed on the cell surface (Kappelmayer and Nagy Jr 2017; Frenette et al. 2000). It recruits leukocytes and platelets into the atherosclerotic lesions (Huo and Xia 2009; Frenette et al. 2000). It also adversely affects lipid metabolism as reduced content of total cholesterol, LDL-C, and triglycerides and elevated HDL-C are noticed in PSGL-1-deficient mice (Li et al. 2018). Ozaki et al. (2014) have shown that PSGL-1 expression is higher in acute than in stable coronary insufficiency, possibly due to plaque instability (Kitamura et al. 2018). These findings are supported by the experimental studies in the mouse showing that PSGL-1 deficiency is associated with reduced atherosclerosis burden and less endothelial damage (Luo et al. 2012).
Elevated risk of atherosclerosis in OSA patients has been widely discussed. Untreated OSA leads to increased morbidity and mortality. Cardiovascular disorders and their sequelae are the most frequent complications (Lavie et al. 1995). The Sleep Health Heart Study has shown the odds ratio for patients with AHI >11 of 1.27 for coronary artery disease and 1.58 for stoke (Lui and Sau-Man 2012). McNicholas and Bonsigore (2007) consider OSA as an independent risk factor of cardiovascular events and show that CPAP therapy reduces this risk. There also are a lot of data concerning the impact of OSA on stroke. Redline et al. (2010) in a study in about 5,500 patients have shown an association between OSA and ischemic stroke, with the adjusted hazard ratio of up to 2.86 in men. Those authors have also observed a risk of stroke in women with AHI >25, the risk increasing by 6% with each unit of AHI increase from 5 to 25. Similar findings have been reported in other studies (Sharma and Culebras 2016; Yaggi et al. 2005).
We conclude that in nondiabetic obstructive sleep apnea patients, moderate and severe stage of the disease enhances the blood content of the adhesion molecules P-selectin and P-selectin glycoprotein ligand-1 in the disease severity-dependent manner as assessed from the magnitude of the apnea-hypopnea index. The increase in the content of adhesion molecules in OSA is liable to be conducive to enhance propensity for arteriosclerosis and consequently cardiovascular morbidity and mortality of the disease.
References
Blann AD, Nadar SK, Lip GY (2003) The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J 24(24):2166–2179
Budhiraja R, Parthasarathy S, Quan SF (2007) Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med 3(4):409–415
Burger PC, Wagner DD (2003) Platelet P-selectin facilitates atherosclerotic lesion development. Blood 101(7):2661–2666
Chiu CA, Wu CJ, Yang CH, Fang CY, Hsieh YK, Hang CL, Hung WC, Chen CJ, Chen SM, Yu TH, Yeh KH, Fu M, Yip HK (2005) Levels and value of soluble P-selectin following acute myocardial infarction: evaluating the link between soluble P-selectin levels and recruitment of circulating white blood cells and the marker for the rapid diagnosis of chest pain. Chang Gung Med J 28(10):699–707
Chung I, Choudhury A, Patel J, Lip GY (2009) Soluble, platelet-bound, and total P-selectin as indices of platelet activation in congestive heart failure. Ann Med 41(1):45–51
Cofta S, Wysocka E, Dziegielewska-Gesiak S, Michalak S, Piorunek T, Batura-Gabryel H, Torlinski L (2013) Plasma selectins in patients with obstructive sleep apnea. Adv Exp Med Biol 756:113–119
Dyugovskaya L, Polyakov A, Lavie P, Lavie L (2008) Delayed neutrophil apoptosis in patients with sleep apnea. Am J Respir Crit Care Med 177(5):544–554
Frenette PS, Denis CV, Weiss L, Jurk K, Subbarao S, Kehrel B, Hartwig JH, Vestweber D, Wagner DD (2000) P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J Exp Med 191(8):1413–1422
Gimbrone MA Jr, Garcia-Cardena G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118(4):620–636
Guo L, Sun G, Wang G, Ning W, Zhao K (2015) Soluble P-selectin promotes acute myocardial infarction onset but not severity. Mol Med Rep 11(3):2027–2033
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352(16):1685–1695
Horváth P, Lázár Z, Mészáros M, Gálffy G, Puskás R, Losonczy G, Kunos L, Bikov A (2018) The role of P- selectin glycoprotein ligand-1 (PSGL-1) in the pathogenesis of obstructive sleep apnea. Eur Respir J 52(Suppl 62):PA2536
Huo Y, Xia L (2009) P-selectin glycoprotein ligand-1 plays a crucial role in the selective recruitment of leukocytes into the atherosclerotic arterial wall. Trends Cardiovasc Med 19(4):140–145
Ikeda H, Nakayama H, Oda T, Kuwano K, Muraishi A, Sugi K, Koga Y, Toshima H (1994) Soluble form of P-selectin in patients with acute myocardial infarction. Coron Artery Dis 5(6):515–518
Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN (1994) Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol 144(5):952–961
Jurado-Gamez B, Bujalance Cabrera C, Caballero Ballesteros L, Marin Hinojosa C, Munoz Cabrera L, Perez-Jimenez F, Lopez-Miranda J (2012) Association of cellular adhesion molecules and oxidative stress with endothelial function in obstructive sleep apnea. Intern Med 51(4):363–368
Kappelmayer J, Nagy B Jr (2017) The interaction of selectins and PSGL-1 as a key component in thrombus formation and cancer progression. Biomed Res Int 2017:6138145
Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG (2017) Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 13(3):479–504
Kitamura K, Sato K, Sawabe M, Yoshida M, Hagiwara N (2018) P-selectin glycoprotein ligand-1 (PSGL-1) expressing CD4 T cells contribute plaque instability in acute coronary syndrome. Circ J 82(8):2128–2135
Laratta CR, Ayas NT, Povitz M, Pendharkar SR (2017) Diagnosis and treatment of obstructive sleep apnea in adults. CMAJ 189(48):E1481–E1488
Lavie P, Herer P, Peled R, Berger I, Yoffe N, Zomer J, Rubin AH (1995) Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep 18(3):149–157
Ley K (2003) The role of selectins in inflammation and disease. Trends Mol Med 9(6):263–268
Li B, Lu X, Wang J, He X, Gu Q, Wang L, Yang Y (2018) The metabonomics study of P-selectin glycoprotein ligand-1 (PSGL-1) deficiency inhibiting the progression of atherosclerosis in LDLR(−/−) mice. Int J Biol Sci 14(1):36–46
Lui MM, Sau-Man M (2012) OSA and atherosclerosis. J Thorac Dis 4(2):164–172
Luo W, Wang H, Ohman MK, Guo C, Shi K, Wang J, Eitzman DT (2012) P-selectin glycoprotein ligand-1 deficiency leads to cytokine resistance and protection against atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis 220(1):110–117
Lusis AJ (2000) Atherosclerosis. Nature 407(6801):233–241
McEver RP (2001) Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost 86(3):746–756
McNicholas WT, Bonsigore MR (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29(1):156–178
Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Oda N, Tanaka A, Yamamoto M, Ohta S, O'Donnell CP, Adachi M (2007) Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med 175(6):612–617
Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM (2007) Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol 155(1):93–96
O’Connor CM, Gurbel PA, Serebruany VL (1999) Usefulness of soluble and surface-bound P-selectin in detecting heightened platelet activity in patients with congestive heart failure. Am J Cardiol 83(9):1345–1349
Ozaki Y, Imanishi T, Teraguchi I, Nishiguchi T, Orii M, Shiono Y, Shimamura K, Ishibashi K, Tanimoto T, Yamano T, Ino Y, Yamaguchi T, Kubo T, Akasaka T (2014) Association between P-selectin glycoprotein ligand-1 and pathogenesis in acute coronary syndrome assessed by optical coherence tomography. Atherosclerosis 233(2):697–703
Priou P, Gagnadoux F, Tesse A, Mastronardi ML, Agouni A, Meslier N, Racineux JL, Martinez MC, Trzepizur W, Andriantsitohaina R (2010) Endothelial dysfunction and circulating microparticles from patients with obstructive sleep apnea. Am J Pathol 177(2):974–983
Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM (2010) Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 182(2):269–277
Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR (2004) Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax 59(9):777–782
Sanada H, Midorikawa S, Yatabe J, Yatabe MS, Katoh T, Baba T, Hashimoto S, Watanabe T (2005) Elevation of serum soluble E- and P-selectin in patients with hypertension is reversed by benidipine, a long-acting calcium channel blocker. Hypertens Res 28(11):871–878
Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC (2017) Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 34:70–81
Sharma S, Culebras A (2016) Sleep apnoea and stroke. Stroke Vasc Neurol 1(4):185–191
Verhaar MC, Beutler JJ, Gaillard CA, Koomans HA, Fijnheer R, Rabelink TJ (1998) Progressive vascular damage in hypertension is associated with increased levels of circulating P-selectin. J Hypertens 16(1):45–50
Wang Q, Zhao W, Bai S (2013) Association between plasma soluble P-selectin elements and progressive ischemic stroke. Exp Ther Med 5(5):1427–1433
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353(19):2034–2041
Conflict of Interest
The authors declare no conflicts of interests in relation to this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of Poznan University of Medical Sciences in Poland.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Winiarska, H.M., Cofta, S., Bielawska, L., Płóciniczak, A., Piorunek, T., Wysocka, E. (2020). Circulating P-Selectin and Its Glycoprotein Ligand in Nondiabetic Obstructive Sleep Apnea Patients. In: Pokorski, M. (eds) Health and Medicine. Advances in Experimental Medicine and Biology(), vol 1279. Springer, Cham. https://doi.org/10.1007/5584_2020_501
Download citation
DOI: https://doi.org/10.1007/5584_2020_501
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51120-3
Online ISBN: 978-3-030-51121-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)