Abstract
Purpose
The development of metastases is the most aggressive attribute of breast cancer. In this retrospective multicenter study, we evaluated if and how the different pathological breast cancer subtypes influence the spreading of tumor cells, the development of metastasis and the survival of breast cancer patients.
Methods
This retrospective German multicenter study is based on the BRENDA collective including 9625 breast cancer patients treated in the adjuvant setting. We used the χ 2 tests for the analysis of the categorical variables between groups of patients with different sites of metastasis. Survival distributions and median survival times were estimated using the Kaplan–Meier product-limit method. The log-rank test was applied to compare survival rates. The Cox proportional hazards model was used to estimate the hazard ratio and confidence intervals.
Results
886 women developed metastases during a time interval of 53 months after primary diagnosis. Luminal A tumor patients were more likely to get bone metastases than lung, liver or CNS metastases. Patients with a triple-negative subtype were, however, the least affected by metastasis in the skeleton. They were most likely to develop visceral metastases. Location, numbers of metastases herein and the subtype influenced the overall survival (OAS). Altogether, the best OAS was found in patients with the luminal A subtype, the worst in patients with the triple-negative subtype.
Conclusions
Knowledge of the typical metastatic pattern of the subtypes of breast cancer will help to personalize therapeutic options and follow-up examinations of cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, breast cancer (BC) is not regarded as a single entity but as a heterogeneous disease with different biological subtypes. Classic histopathologic variables have been combined with rankings based on multi-gene expression signatures, an important step towards personalizing BC treatment. Amongst the most popular platforms are the genomic grade index (GGI; MapQuant Dx®), MammaPrint® [1], Oncotype DX® [2], the wound-response model [3], Veridex 76-gene signature [4], 92-gene signature THEROS [5] and the intrinsic subtype model [6]. The latter provides the most extensive biological information and allows the classification of BC into five intrinsic subtypes: Luminal A, luminal B HER2 negative, luminal B HER2 positive, HER2-overexpressing and basal-like subtypes. Of course, clinico-pathological surrogate definitions exist and the basal-like subtype is usually treated equivalent to the triple-negative subtype (TNT) [7–11]. The BC subtypes show different biological behavior in terms of survival and recurrence and have typical patterns of metastatic spread [12–14]. For example, patients with hormone receptor-positive BC are more likely to develop bone metastases, whereas visceral recurrence is associated with a lack of the hormone receptors [15, 16]. TNT seems to metastasize more likely viscerally [13, 17, 18], predominantly into the lungs [19]. The HER2-overexpressing subtype is more likely to metastasize to lung, liver and brain than HER2-negative subtypes. Moreover, HER2-positive BC tends to metastasize less often to the skeleton [20, 21]. In contrast, Kenneke and coworkers evaluated that all subtypes, except basal-like tumors, metastasize most commonly to the bone [22].
Regarding the pattern of metastasis, the different molecular subtypes of BC demonstrate variable prognostic behavior. Metastases were found less frequently in patients with the luminal A subtype and more in the HER2-overexpressing and TNT subtypes [13]. The advanced TNT BC has a poor prognosis and fewer targeted therapies [23–26]. In view of the poor prognosis of advanced BC, the aim of this study was to analyze the typical metastatic pattern of the subtypes of BC in Germany, the sites of metastasis, and the outcome to get a better understanding of the biology and prognosis of the different BC subtypes.
Methods
The BRENDA (breast cancer care under evidence-based guidelines) database used for this retrospective study included 9625 patients treated in the adjuvant setting without metastases at the Department of Gynecology and Obstetrics at the University of Ulm and 16 partner clinics in Germany from 1992 to 2008. It has already been described in various publications with exact inclusion and exclusion criteria [27–30]. Metastatic disease was defined by X-ray imaging, computed tomography, magnetic resonance imaging, ultrasound or bone scan and/or by histological detection. Data were received from the physicians responsible for follow-up care. Moreover, the statistical registers were contacted.
Surrogate definition
We used a surrogate definition that comprises the hormone receptors (HR) of estrogen (ER) and progesterone (PR), the HER2 receptor, and tumor grade (G). Low tumor grade is a grading of 1 and 2; 3 is high. Luminal A is HR positive, HER2 negative and low tumor grade. Luminal B HER2 negative (luminal B/HER−) is HR positive, HER2 negative and high tumor grade. Luminal B HER2 positive (luminal B/HER2+) represents HR positive/HER2 positive. The triple-negative subtype (TNT) is negative for HR and HER2. The HER2-overexpressing subtype is negative for HR and positive for HER2 [8, 9].
Statistical analysis
All categorical data were described using numbers and percentages. Comparisons of categorical variables between groups were made using χ 2 tests. Quantitative data were presented using median and range or mean and standard deviations. Overall survival (OAS) from the time of metastasis was defined as the interval between detection of the first distant metastasis and death. If the patient was lost to follow-up, data were censored at the date of the last known contact. When no information was available, the status was coded as missing data. Survival distributions and median survival times were estimated using the Kaplan–Meier product-limit method. The log-rank test was used to compare survival rates. The Cox proportional hazards model was used to estimate the hazard ratio and confidence intervals. The proportional hazards assumption was assessed by including the individual terms with time in the models. Multivariate Cox proportional hazards regression models were used to accommodate the differing risk factor distributions between groups. For classification, we used Exhausted CHAID (Chi-squared Automatic Interaction Detector), a type of decision tree technique, based upon adjusted significance testing (Bonferroni testing), to construct (non-binary) trees. p values less than 0.05 were considered statistically significant. Statistical analyses were two sided and carried out using R-3.20, NCSS 10 and SPSS 22.
Results
886 patients (9.2%) developed distant metastases during the observation period. Thereof, 61.3% had bone and 56.9% visceral metastases with the predominant locations: liver (37.0%), lung (30.6%), pleura (11.7%) and peritoneum (3.8%). The CNS was affected in 15.3% of the patients and 15.6% had other metastases (e.g., skin and soft tissues) (Table 1). The median observation time was 53 months (95% CI: 48–57 months) from date of primary diagnosis, and 27 months (95% CI: 24.5–29.5 months) from date of metastatic disease.
The pattern of metastasis and correlation to subtypes
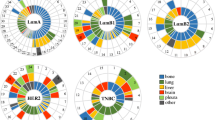
According to the subtypes of BC determined in St. Gallen in 2011 [7], visceral metastases were found most often in TNT BC (68.7%) and the least in luminal A BC (48.3%). The same applies to lung and CNS metastases (50.4 versus 21.0%, respectively, 25.2 versus 9.4%). Liver metastases were formed mostly by the luminal B/HER2+ subtype (46.6%) and the least by the luminal A subtype (33.0%). In each subtype, except TNT, liver metastases appeared more often than lung metastases. We investigated patients with the TNT subtype; 35.1% with liver metastases and 50.4% with lung metastases. Bone metastases occurred most often in patients with luminal A (66.8%) and the least in patients with TNT subtype (38.9%). Pleural metastases were discovered most often in TNT (13.0%), and the least in the luminal B/HER2+ subtype (4.9%). Peritoneal metastases occurred most often in the luminal B/HER2− (5%) and least of all in the luminal B/HER2+ subtype (1%) (Table 1).
Subtypes of breast cancer and numbers of sites of metastases
Three or more different sites of metastases were found most frequently in patients with TNT (26.7%) and the least in women with luminal A subtypes (14.5%). The luminal B/HER2− metastasized most often to two different sites, the luminal B/HER2+ subtype the least. Luminal A (58.8%) were most likely and luminal B/HER2− subtypes (42.5%; Pearson χ 2: p = 0.003) least likely to develop one site of metastasis (Fig. 1).
Differences in the location of metastasis and breast cancer subtypes
A highly significant difference in the visceral manifestations was found between the luminal B subtypes (59%), luminal A subtype (48.3%) and the HER2-overexpressing/TNT subtypes (68.1%; p < 0.001) (Fig. 2a). Furthermore, the frequency of bone metastases differed significantly between the luminal (67.5%) and the HER2-overexpressing/TNT subtypes (41.4%; p < 0.001) (Fig. 2b). In contrast, there was little difference in the frequency of liver metastases in the luminal B/HER2−/TNT/luminal A subtypes compared to the HER2 positive subtypes (p = 0.236); 34.9 and 45.1%, respectively (Fig. 2c). Examinations of the lung metastases showed again highly significant differences (p < 0.001): 32.5% of the luminal B and HER2-positive subtypes, 50.4% of the TNT subtype and 21.0% of the luminal A subtype (Fig. 2d) developed lung metastases. Metastases of the CNS occurred in 23.0% of HER2-overexpressing/TNT/luminal B/HER2+ subtypes, whereas only 11.2% of the luminal A and luminal B/HER2− subtypes developed CNS metastases (p < 0.001; Fig. 2e). In contrast, there was only a slight difference in pleural metastasis behavior between the luminal B/HER2+ (4.9%) and the other subtypes (p = 0.419; Fig. 2f).
Overall survival stratified by subtypes of breast cancer
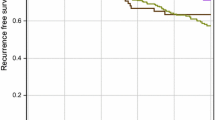
OAS of BC subtypes was analyzed in comparison to the luminal A subtype. Altogether, patients with the luminal A subtype have the best OAS from date of metastatic disease of all patients with metastases. Patients with luminal B/HER2+ subtype had a slightly lower OAS with a hazard ratio (HR) of 1.26 (95% CI: 0.96–1.65; p = 0.103). We observed significantly poorer OAS for patients with the luminal B/HER2− (HR 1.39; 95% CI: 1.13–1.72; p = 0.002), the HER2-overexpressing (HR 1.75; 95%: 1.30–2.36; p < 0.001) and the TNT subtype (HR 2.87; 95% CI: 2.27–3.64; p = 0.002) (Fig. 3). The number of months of OAS from date of metastatic disease is summarized in means and medians in Table 2.
Patients with the TNT subtype (HR 3.25; 95%: 2.29–4.61; p < 0.001) also had the worst OAS from date of metastatic disease of patients with only one site of metastasis. The luminal A subtype had the best OAS followed by not significantly reduced OAS of the HER2-overexpressing (HR 1.05; 95% CI: 0.51–2.15; p = 0.891), the luminal B/HER2− (HR 1.30; 95% CI: 0.94–1.79; p = 0.113) and the luminal B/HER2+ subtypes (HR 1.34; 95% CI: 0.89–2.01; p = 0.166) (Fig. 4a).
Interestingly, the OAS from date of metastatic disease of patients with three or more different sites of metastases is the best for patients with the luminal B/HER2+ subtype (HR 0.82; 95% CI: 0.51–1.33; p = 0.430). We observed no significant differences in the luminal A and the luminal B/HER2− subtype (HR 1.23; 95% CI: 0.83–1.82; p = 0.294). The HER2-overexpressing (HR 1.82; 95% CI: 1.00–3.32; p = 0.050) and the TNT subtypes (HR 1.90; 95% CI: 1.22–2.94; p = 0.004) had a significantly poorer OAS in this case (Fig. 4b).
Consecutively, we examined OAS from date of metastatic disease according to the different sites of metastases. Patients with the TNT subtype and liver metastases (HR 3.08; 95% CI: 2.11–4.49; p < 0.001) had the worst OAS compared to the luminal A subtype. An almost equal OAS existed for patients with the luminal B/HER2+ subtype (HR 0.98; 95% CI: 0.66–1.45; p = 0.919) with a slightly worse OAS for the luminal B/HER2− (HR 1.30; 95% CI: 0.93–1.77; p = 0.129) and the HER2-overexpressing subtype patients (HR 1.55; 95% CI: 0.98–2.45; p = 0.059) (Fig. 5a).
OAS of patients with pulmonary metastasis was the best for the luminal A subtype, followed by the luminal B/HER2− (HR 1.23; 95% CI: 0.81–1.86; p = 0.338), the luminal B/HER2+ (HR 1.34; 95% CI: 0.80–1.86; p = 0.226) and the HER2-overexpressing subtypes (HR 1.92; 95% CI: 1.09–3.38; p = 0.025); the TNT subtype had the worst OAS (HR 3.07; 95% CI: 0.94–0.79; p = 0.113) (Fig. 5b). We also demonstrated that the TNT subtype with bone metastases always had the worst OAS (HR 3.77; 95% CI: 2.66–5.350; p < 0.001). OAS is the best for patients with the luminal A subtype followed by the luminal B/HER2+ (HR 1.40; 95% CI: 1.00–1.96; p = 0.048), the luminal B/HER2− (HR 1.57; 95% CI: 1.22–2.02; p = 0.001) and the HER2-overexpressing subtypes (HR 2.03; 95% CI: 1.33–3.11; p = 0.001) (Fig. 5c).
Our analysis of CNS metastases demonstrated a slightly better OAS for patients with the luminal B/HER2+ (HR 0.89; 95% CI: 0.49–1.60; p = 0.350) subtype than for patients with the luminal A subtype. Similar is the OAS of patients with the HER2-overexpressing subtype (HR 1.05; 95% CI: 0.51–2.15; p = 0.891) followed by a slightly worse OAS for the luminal B/HER2− subtype (HR 1.32; 95% CI: 0.74–2.35; p = 0.350) (Fig. 5d).
Figure 6 shows the OAS of patients with metastatic BC treated in the adjuvant setting. OAS from the date of primary diagnosis of breast cancer is the best for luminal A, followed by luminal B/HER2− (HR 1.43; 95% CI: 1.16–1.77; p = 0.001), luminal B/HER2+ (HR 1.50; 95% CI: 1.14–1.97; p = 0.001) and HER2-overexpressing subtypes (HR 1.97; 95% CI: 1.46–2.67; p < 0.001). The TNT subtype (HR 2.86; 95% CI: 2.26–3.62; p < 0.001) has a significantly lower OAS (Fig. 6).
Table 3 summarizes the OAS and the metastasis-free survival (MFS) of the patients with advanced BC from date of primary diagnosis. Here, the MFS was 23 months (95% CI: 19.94–26.06; SEM = 1.56) for patients with luminal A, followed by luminal B/HER2− subtype with 19 months (95% CI: 15.83–22.17; SEM = 1.62). Interestingly, the MFS (median) of the luminal B/HER2+ subtype was only 15 months (95% CI: 11.38–18.62; SEM = 1.85), only 14 months for the HER2-overexpressing (95% CI: 9.25–18.75; SEM = 2.42) and only 13 months for the TNT subtype (95% CI: 10.76–15.42; SEM = 1.14) (Table 3).
Discussion
Metastasis of BC occurs in 20–30% of BC patients [31, 32]. It turns the local tumor growth into a systemic disease for lifetime [33]. In recent years, at least since the 12th International Breast Cancer Conference in St. Gallen, BC is no longer seen as a uniform disease but is divided into different subgroups according to the gene expression patterns [7]. Therefore, many biological characteristics and tools of BC are a focus of research. Molecular aspects such as gene expression profiles [34], microRNAs [35], circulating tumor DNA [36], circulating tumor cells [37, 38], tumor stem cells [39, 40], different signaling pathways in tumor cells [41], and different aspects of the environment with immune cells like myeloid-derived suppressor cells and regulatory T cells [42, 43] are playing increasingly important roles.
Therefore, it was a priority for us to examine closely the distinct pattern of metastasis of different BC subtypes to create a basis for understanding the biological characteristics. The surrogate definition [8, 9] was used because the KI-67 labeling, which differs between the luminal A and B subtype, was unavailable in the BRENDA specimen [7, 10] as in the previous literature [13]. Altogether, 9.2% of the patients developed distant metastases during the observation period. This is slightly less than that described in the literature [32]. This difference could be due to the variable time periods (1992–2008 vs. 1973–2003) and to the associated different adjuvant therapies.
Next, we analyzed the metastatic pattern depending on the subtypes. Pogoda et al. described metastasizing TNT BC: 15% in the CNS, 14% in the lungs, 11% in the skeleton, 8% in the liver and 14% loco-regional [44]. This corresponds to our data, which describe more visceral, lung and CNS metastases of the TNT than of the luminal A subtype. In a recent study, the metastatic pattern of the different subtypes in Germany was described with a somewhat different classification of distant metastases in “bone”, “visceral”, “bone and visceral” and “brain (with/without bone or visceral metastases)” metastases. This is comparable to our data [13]. The same applies to other literature [17, 19, 22, 45, 46]. Smid and coworkers observed slightly different data which may be due to some modifications in the subtype classification [47].
Already known are the following results of several actual trials: with respect to the first site of metastasis, the HER2-overexpressing subtype had a highly significant rate of liver metastases and the TNT subtype a greater risk of developing lung metastases than the luminal A subtype. CNS metastases seem to be associated with the HER2-overexpressing subtype; bone metastases are more frequent in luminal A subtypes [12]. In contrast to our data, Kast et al. showed bone-only metastases to be more likely in luminal A and luminal B/HER2− subtypes. Visceral metastases were associated with HER2-overexpressing, TNT, luminal B/HER2+ subtypes, while CNS metastases were associated with luminal B/HER2+ and TNT subtypes [13]. In this case, the results of Dent et al. match well with our data because they observed that visceral metastases were four times more likely to develop in the TNT subtype [18]. On the other side, Kennecke et al. described that HER2-positive subtypes were more likely to metastasize in the brain, liver and lungs [22]. These literature data are congruent to our results.
Kast and coworkers described the metastatic pattern of the different subtypes of BC for the first time in 304 metastasized patients in Germany between 2006 and 2011 [13]. In our study, the number of patients was higher with 886 patients from 1992 to 2008. Kast and coworkers analyzed data of BC patients treated in four different clinics in Dresden and Radebeul. Our study involved a greater overall area, the Department of Gynecology and Obstetrics at the University of Ulm and 16 partner clinics (all certified breast cancer centers) in Baden-Wuerttemberg in Germany. Therefore, our data could well complement the results from Dresden [13].
In the RegistHER2 trial, they found one site of metastasis in 51.3% of patients with the HER2-positive subtypes, two sites in 25.8%, three sites in 14.5% and four or more sites of metastasis in 8.3% [48]. The number of metastasis sites has already been analyzed for patients with brain metastases: 60.9% of the luminal subtype, 73.7% of the luminal B subtype, 64.9% of the HER2-positive subtype, but only 6.81% of the TNT subtype with brain metastasis had three or more sites of metastasis [49]. These data were supplemented by our investigations.
Lobbezoo and coworkers described the best survival with metastases in the HR-positive/HER2-positive subtype and the worst in the TNT subtype, independent of the site of metastasis, from 2007 to 2009 [46]. In contrast, Lee et al. analyzed a not significantly better survival of HR-positive subtypes compared to TNT or HER2-positive subtypes [50]. Significant differences in the median survival from time of first metastasis were described by Kenneke et al.: the longest survival was seen for the luminal A subtype, followed by the luminal B, the luminal/HER2, the HER2-overexpressing, the basal-like and the TNT non-basal subtype [22]. This coincides well with previous results [12] and our analyzed data. An equal median best survival was shown by Kast and coworkers for HER2-positive and luminal A subtypes [13]. TNT subtypes had the worst survival. The described differences in the HER2-positive subtypes could be due to the availability of specific HER2-positive-directed therapies [51–53].
Altogether, the best OAS in our study is for patients with the luminal A subtype, the worst is for patients with the TNT subtype. The survival of patients with liver metastases has been described to be the poorest for the TNT subtype [54]. A better prognosis for patients with HR-positive subtypes was described previously [55, 56] and matches our data. Although, we could only show the significant difference in OAS between the TNT and the luminal A subtype, Ge and coworkers failed to show any significant differences between the different subtypes [57]. The data of patients with lung metastases analyzed by Yhim et al. showed the best survival parameters for HR-positive subtypes and the worst for HER2-positive and TNT subtypes, corresponding to our data [58]. The survival of patients with bone metastases was observed by Lee et al. to be longer in HR-positive than in HER2-positive and TNT subtypes [50]. These results complement again our analysis. Regarding brain metastasis, a poorer OAS of the HER2-positive and the TNT subtypes was described previously [59, 60]. In contrast, Arslan et al. described a slightly better survival for the luminal B, followed by the HER2-positive, the luminal A and the TNT subtypes [61]. There were some differences to our data, maybe resulting from the fact that we observed the OAS from date of CNS, rather than brain metastases.
The analysis of the MFS of the different subtypes of BC showed very interesting results. The median MFS of the luminal B/HER2+ subtype, the HER2-overexpressing and the TNT subtypes is 13–15 months. This is a very short interval regarding the tumor doubling time. Recently, Coumans and coworkers analyzed a tumor doubling time of 1.7 ± 0.9 months in more than 38,000 BC patients. Furthermore, they summarized the previous literature without shorter tumor doubling times [62]. The tumor volume doubling time of primary BC tumors analyzed by mammograms or ultrasound was at least 100 days [63, 64]. Ryu and coworkers detected a 2.4-fold shorter tumor volume doubling time for TNT subtypes compared to ER-positive tumors [63]. The relatively short MFS of luminal B/HER2+, HER2-overexpressing and TNT subtypes leads to the question of whether there were really no metastases at the time of primary diagnosis. We recommend thorough staging examinations for patients with these subtypes always, and repeating the diagnostics immediately in case of doubt.
Conclusion
The different subtypes of BC had a typical pattern of metastasis: the subtypes of BC, the sites and the numbers of metastases were pivotal for prognosis. The better knowledge of the biology of BC subtypes will help to personalize therapeutic options and follow-up examinations.
References
Tian S, Roepman P, Van’t Veer LJ, Bernards R, de Snoo F, Glas AM (2010) Biological functions of the genes in the mammaprint breast cancer profile reflect the hallmarks of cancer. Biomark Insights 5:129–138. doi:10.4137/BMI.S6184
Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22. doi:10.1007/s10549-013-2666-z
Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van’t Veer LJ, Bartelink H, van de Rijn M, Brown PO, van de Vijver MJ (2005) Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA 102(10):3738–3743. doi:10.1073/pnas.0409462102
Zhang Y, Sieuwerts AM, McGreevy M, Casey G, Cufer T, Paradiso A, Harbeck N, Span PN, Hicks DG, Crowe J, Tubbs RR, Budd GT, Lyons J, Sweep FC, Schmitt M, Schittulli F, Golouh R, Talantov D, Wang Y, Foekens JA (2009) The 76-gene signature defines high-risk patients that benefit from adjuvant tamoxifen therapy. Breast Cancer Res Treat 116(2):303–309. doi:10.1007/s10549-008-0183-2
Ma XJ, Patel R, Wang X, Salunga R, Murage J, Desai R, Tuggle JT, Wang W, Chu S, Stecker K, Raja R, Robin H, Moore M, Baunoch D, Sgroi D, Erlander M (2006) Molecular classification of human cancers using a 92-gene real-time quantitative polymerase chain reaction assay. Arch Pathol Lab Med 130(4):465–473. doi:10.1043/1543-2165(2006)130[465:MCOHCU]2.0.CO;2
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. doi:10.1038/35021093
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol Off J Eur Soc Med Oncol ESMO 22(8):1736–1747. doi:10.1093/annonc/mdr304
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol Off J Am Soc Clin Oncol 30(15):1796–1804. doi:10.1200/JCO.2011.38.8595
Parise CA, Caggiano V (2014) Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J Cancer Epidemiol 2014:469251. doi:10.1155/2014/469251
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol Off J Eur Soc Med Oncol ESMO 24(9):2206–2223. doi:10.1093/annonc/mdt303
Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen TO, Perou CM (2013) Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 31(2):203–209. doi:10.1200/JCO.2012.43.4134
Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini AM, Fasola G, Puglisi F (2015) Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis 32(2):125–133. doi:10.1007/s10585-015-9697-2
Kast K, Link T, Friedrich K, Petzold A, Niedostatek A, Schoffer O, Werner C, Klug SJ, Werner A, Gatzweiler A, Richter B, Baretton G, Wimberger P (2015) Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat 150(3):621–629. doi:10.1007/s10549-015-3341-3
Wenners A, Berlin L, Alkatout I, van Mackelenbergh M, Jonat W, Mundhenke C (2015) Clinical implications of first and multiple locoregional breast cancer recurrences. Arch Gynecol Obstet 292(1):165–173. doi:10.1007/s00404-014-3586-9
Blanco G, Holli K, Heikkinen M, Kallioniemi OP, Taskinen P (1990) Prognostic factors in recurrent breast cancer: relationships to site of recurrence, disease-free interval, female sex steroid receptors, ploidy and histological malignancy grading. Br J Cancer 62(1):142–146
Savci-Heijink CD, Halfwerk H, Hooijer GK, Horlings HM, Wesseling J, van de Vijver MJ (2015) Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat 150(3):547–557. doi:10.1007/s10549-015-3352-0
Park HS, Kim S, Kim K, Yoo H, Chae BJ, Bae JS, Song BJ, Jung SS (2012) Pattern of distant recurrence according to the molecular subtypes in Korean women with breast cancer. World J Surg Oncol 10:4. doi:10.1186/1477-7819-10-4
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA (2009) Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115(2):423–428. doi:10.1007/s10549-008-0086-2
Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F (2013) Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol Off J Am Soc Clin Oncol 31(25):3083–3090. doi:10.1200/JCO.2012.46.1574
Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ (1991) Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer J Int Du Cancer 49(5):650–655
Paluch-Shimon S, Ben-Baruch N, Wolf I, Zach L, Kopolovic J, Kruglikova A, Modiano T, Yosepovich A, Catane R, Kaufman B (2009) Hormone receptor expression is associated with a unique pattern of metastatic spread and increased survival among HER2-overexpressing breast cancer patients. Am J Clin Oncol 32(5):504–508. doi:10.1097/COC.0b013e3181967d72
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol Off J Am Soc Clin Oncol 28(20):3271–3277. doi:10.1200/JCO.2009.25.9820
Brouckaert O, Wildiers H, Floris G, Neven P (2012) Update on triple-negative breast cancer: prognosis and management strategies. Int J Women’s Health 4:511–520. doi:10.2147/IJWH.S18541
Andre F, Zielinski CC (2012) Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann oncol off j Eur Soc Med Oncol ESMO 23(Suppl 6):vi46–vi51. doi:10.1093/annonc/mds195
Bramati A, Girelli S, Torri V, Farina G, Galfrascoli E, Piva S, Moretti A, Dazzani MC, Sburlati P, La Verde NM (2014) Efficacy of biological agents in metastatic triple-negative breast cancer. Cancer Treat Rev 40(5):605–613. doi:10.1016/j.ctrv.2014.01.003
Clark O, Botrel TE, Paladini L, Ferreira MB (2014) Targeted therapy in triple-negative metastatic breast cancer: a systematic review and meta-analysis. Core Evid 9:1–11. doi:10.2147/CE.S52197
Wockel A, Wolters R, Wiegel T, Novopashenny I, Janni W, Kreienberg R, Wischnewsky M, Schwentner L (2014) The impact of adjuvant radiotherapy on the survival of primary breast cancer patients: a retrospective multicenter cohort study of 8935 subjects. Ann Oncol Off J Eur Soc Med Oncol ESMO 25(3):628–632. doi:10.1093/annonc/mdt584
Wockel A, Kurzeder C, Geyer V, Novasphenny I, Wolters R, Wischnewsky M, Kreienberg R, Varga D (2010) Effects of guideline adherence in primary breast cancer–a 5-year multi-center cohort study of 3976 patients. Breast 19(2):120–127. doi:10.1016/j.breast.2009.12.006
Schwentner L, Wolters R, Koretz K, Wischnewsky MB, Kreienberg R, Rottscholl R, Wockel A (2012) Triple-negative breast cancer: the impact of guideline-adherent adjuvant treatment on survival–a retrospective multi-centre cohort study. Breast Cancer Res Treat 132(3):1073–1080. doi:10.1007/s10549-011-1935-y
Diessner J, Van Ewijk R, Weiss CR, Janni W, Wischnewsky MB, Kreienberg R, Hancke K, Blettner M, Wockel A, Schwentner L (2015) Identifying the impact of inflammatory breast cancer on survival: a retrospective multi-center cohort study. Arch Gynecol Obstet 292(3):655–664. doi:10.1007/s00404-015-3691-4
Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379(9814):432–444. doi:10.1016/S0140-6736(11)61625-5
Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378(9804):1707–1716. doi:10.1016/S0140-6736(11)61629-2
Redig AJ, McAllister SS (2013) Breast cancer as a systemic disease: a view of metastasis. J Intern Med 274(2):113–126. doi:10.1111/joim.12084
van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415(6871):530–536. doi:10.1038/415530a
Yan M, Shield-Artin K, Byrne D, Deb S, Waddell N, Haviv I, Fox SB (2015) Comparative microRNA profiling of sporadic and BRCA1 associated basal-like breast cancers. BMC Cancer 15:506. doi:10.1186/s12885-015-1522-4
Spellman PT, Gray JW (2014) Detecting cancer by monitoring circulating tumor DNA. Nat Med 20(5):474–475. doi:10.1038/nm.3564
McInnes LM, Jacobson N, Redfern A, Dowling A, Thompson EW, Saunders CM (2015) Clinical implications of circulating tumor cells of breast cancer patients: role of epithelial-mesenchymal plasticity. Front Oncol 5:42. doi:10.3389/fonc.2015.00042
Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J, Caldas C, Gazzaniga P, Manso L, Zamarchi R, de Lascoiti AF, De Mattos-Arruda L, Ignatiadis M, Lebofsky R, van Laere SJ, Meier-Stiegen F, Sandri MT, Vidal-Martinez J, Politaki E, Consoli F, Bottini A, Diaz-Rubio E, Krell J, Dawson SJ, Raimondi C, Rutten A, Janni W, Munzone E, Caranana V, Agelaki S, Almici C, Dirix L, Solomayer EF, Zorzino L, Johannes H, Reis-Filho JS, Pantel K, Pierga JY, Michiels S (2014) Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 15(4):406–414. doi:10.1016/S1470-2045(14)70069-5
Diessner J, Bruttel V, Stein RG, Horn E, Hausler SF, Dietl J, Honig A, Wischhusen J (2014) Targeting of preexisting and induced breast cancer stem cells with trastuzumab and trastuzumab emtansine (T-DM1). Cell Death Dis 5:e1149. doi:10.1038/cddis.2014.115
Diessner J, Bruttel V, Becker K, Pawlik M, Stein R, Hausler S, Dietl J, Wischhusen J, Honig A (2013) Targeting breast cancer stem cells with HER2-specific antibodies and natural killer cells. Am J Cancer Res 3(2):211–220
Verhaegh W, van Ooijen H, Inda MA, Hatzis P, Versteeg R, Smid M, Martens J, Foekens J, van de Wiel P, Clevers H, van de Stolpe A (2014) Selection of personalized patient therapy through the use of knowledge-based computational models that identify tumor-driving signal transduction pathways. Cancer Res 74(11):2936–2945. doi:10.1158/0008-5472.CAN-13-2515
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. doi:10.1038/nri2506
Engel JB, Honig A, Kapp M, Hahne JC, Meyer SR, Dietl J, Segerer SE (2014) Mechanisms of tumor immune escape in triple-negative breast cancers (TNBC) with and without mutated BRCA 1. Arch Gynecol Obstet 289(1):141–147. doi:10.1007/s00404-013-2922-9
Pogoda K, Niwinska A, Murawska M, Pienkowski T (2013) Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol 30(1):388. doi:10.1007/s12032-012-0388-4
Sihto H, Lundin J, Lundin M, Lehtimaki T, Ristimaki A, Holli K, Sailas L, Kataja V, Turpeenniemi-Hujanen T, Isola J, Heikkila P, Joensuu H (2011) Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res BCR 13(5):R87. doi:10.1186/bcr2944
Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, van de Wouw AJ, Peters FP, van Riel JM, Peters NA, de Boer M, Borm GF, Tjan-Heijnen VC (2013) Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat 141(3):507–514. doi:10.1007/s10549-013-2711-y
Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW (2008) Subtypes of breast cancer show preferential site of relapse. Cancer Res 68(9):3108–3114. doi:10.1158/0008-5472.CAN-07-5644
Yardley DA, Tripathy D, Brufsky AM, Rugo HS, Kaufman PA, Mayer M, Magidson J, Yoo B, Quah C, Ulcickas Yood M (2014) Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer 110(11):2756–2764. doi:10.1038/bjc.2014.174
Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, Ro J (2008) Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res BCR 10(1):R20. doi:10.1186/bcr1870
Lee SJ, Park S, Ahn HK, Yi JH, Cho EY, Sun JM, Lee JE, Nam SJ, Yang JH, Park YH, Ahn JS, Im YH (2011) Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res Treat Off J Korean Cancer Assoc 43(2):89–95. doi:10.4143/crt.2011.43.2.89
Stebbing J, Payne R, Reise J, Frampton AE, Avery M, Woodley L, Di Leo A, Pestrin M, Krell J, Coombes RC (2013) The efficacy of lapatinib in metastatic breast cancer with HER2 non-amplified primary tumors and EGFR positive circulating tumor cells: a proof-of-concept study. PLoS One 8(5):e62543. doi:10.1371/journal.pone.0062543
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367(19):1783–1791. doi:10.1056/NEJMoa1209124
Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, Moliterni A, Vazquez F, Byakhov MJ, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Magazzu D, Heinzmann D, Steinseifer J, Valagussa P, Baselga J (2014) Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 15(6):640–647. doi:10.1016/S1470-2045(14)70080-4
Duan XF, Dong NN, Zhang T, Li Q (2013) The prognostic analysis of clinical breast cancer subtypes among patients with liver metastases from breast cancer. Int J Clin Oncol Jpn Soc Clin Oncol 18(1):26–32. doi:10.1007/s10147-011-0336-x
Eichbaum MH, Kaltwasser M, Bruckner T, de Rossi TM, Schneeweiss A, Sohn C (2006) Prognostic factors for patients with liver metastases from breast cancer. Breast Cancer Res Treat 96(1):53–62. doi:10.1007/s10549-005-9039-1
Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, Robertson JF, Evans AJ (2003) Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer 89(2):284–290. doi:10.1038/sj.bjc.6601038
Ge QD, Lv N, Kong YN, Xie XH, He N, Xie XM, Wei WD (2012) Clinical characteristics and survival analysis of breast cancer molecular subtypes with hepatic metastases. Asian Pac J Cancer Prev APJCP 13(10):5081–5086
Yhim HY, Han SW, Oh DY, Han W, Im SA, Kim TY, Kim YT, Noh DY, Chie EK, Ha SW, Park IA, Bang YJ (2010) Prognostic factors for recurrent breast cancer patients with an isolated, limited number of lung metastases and implications for pulmonary metastasectomy. Cancer 116(12):2890–2901. doi:10.1002/cncr.25054
Heitz F, Harter P, Lueck HJ, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A, du Bois A (2009) Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer 45(16):2792–2798. doi:10.1016/j.ejca.2009.06.027
Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, Valabrega G, Aglietta M, Montemurro F (2014) Metastatic breast cancer subtypes and central nervous system metastases. Breast 23(5):623–628. doi:10.1016/j.breast.2014.06.009
Arslan UY, Oksuzoglu B, Aksoy S, Harputluoglu H, Turker I, Ozisik Y, Dizdar O, Altundag K, Alkis N, Zengin N (2011) Breast cancer subtypes and outcomes of central nervous system metastases. Breast 20(6):562–567. doi:10.1016/j.breast.2011.07.017
Coumans FA, Siesling S, Terstappen LW (2013) Detection of cancer before distant metastasis. BMC Cancer 13:283. doi:10.1186/1471-2407-13-283
Ryu EB, Chang JM, Seo M, Kim SA, Lim JH, Moon WK (2014) Tumour volume doubling time of molecular breast cancer subtypes assessed by serial breast ultrasound. Eur Radiol 24(9):2227–2235. doi:10.1007/s00330-014-3256-0
Fornvik D, Lang K, Andersson I, Dustler M, Borgquist S, Timberg P (2015) Estimates of breast cancer growth rate from mammograms and its relation to tumour characteristics. Radiat Prot Dosim. doi:10.1093/rpd/ncv417
Acknowledgements
We express our thanks to the following persons for their contributions to the BRENDA study: Karsten Gnauert (Ostalbklinikum, Aalen), Steffen Fritz (Kreisklinik Biberach), Ulf Göretzlehner (Kreiskrankenhaus Ehingen), Hans-Walter Vollert (Städt. Krankenhaus Friedrichshafen), Peter Jakob Albert (Klinikum Heidenheim), Ricardo Felberbaum (Klinikum Kempten), Andreas Zorr (Klinikum Konstanz), Felix Flock (Klinikum Memmingen), Erik Schlicht (Stauferklinik, Mutlangen), Martina Gropp-Meier (Oberschwabenklinik Ravensburg), Gerhard Bartzke (Kreiskrankenhaus Rottweil), Andreas Rempen (Diakonie-Krankenhaus, Schwäbisch Hall), Edgar Schelble (Kreiskrankenhaus Sigmaringen), Theodor Dinkelacker (Helfenstein-Klinik Geislingen), Andreas Grüneberger (Oberschwabenklinik Wangen) and Thorsten Kühn (Städt. Kliniken, Esslingen).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The Ethics Committee of the University of Ulm, which covers all participating breast cancer centers of the BRENDA network, has approved this study and the BRENDA project.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding source
This work was supported by the German Federal Ministry of Education and Research (BMBF-Grant-01ZP0505).
Rights and permissions
About this article
Cite this article
Bartmann, C., Wischnewsky, M., Stüber, T. et al. Pattern of metastatic spread and subcategories of breast cancer. Arch Gynecol Obstet 295, 211–223 (2017). https://doi.org/10.1007/s00404-016-4225-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4225-4