Abstract
Purpose
Inflammatory breast cancer (IBC) represents a rare and aggressive form of cancer with negative prognosis and high rate of recurrence. The purpose of this retrospective multi-center study was to evaluate the effect of IBC on overall and disease-free survival. Furthermore we analyzed the influence of hormone and Her2 receptor expression on inflammatory breast cancer cells on the clinical outcome of patients.
Methods
This retrospective German multi-center study included 11,780 patients with primary breast cancer recruited from 1992 to 2008. In this sub-group analysis we focused on 70 patients with IBC.
Results
Despite the relatively small sample size, we could confirm the aggressiveness of inflammatory breast cancer and the different clinical behavior of IBC subtypes. It could be demonstrated that the lack of expression of hormone receptors on tumor cells is associated with a more aggressive clinical course and decreased overall and disease-free survival. Higher incidence of Her2 overexpression, that is typically associated with poor prognostic outcome among women with non-IBC tumors, seems however to have no prognostic significance.
Conclusions
This BRENDA sub-group analysis, on a German cohort of breast cancer patients confirmed the negative outcome of IBC and the different clinical behavior of IBC subtypes. The best management of IBC requires intensive coordination and cooperation between various clinical disciplines involved in the treatment of IBC patients. Moreover there is a need to identify IBC-specific targeted therapies to improve the curing prospects of this subtype of cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diagnosis of breast cancer includes a group of complex and heterogeneous diseases with completely different clinical, morphological, and molecular manifestations. Patients that show typical clinical signs like edema, redness, and swelling, exhibiting a wrinkled and orange-peel appearance of the skin of the breast defined as peau d’orange and that display cancer cells in the subdermal lymphatics, suffer from a breast cancer sub-group called Inflammatory breast cancer (IBC). This term was first introduced by Lee and Tannenbaum in 1924 [1, 2]. IBC is a rare and aggressive form of breast cancer with poor prognosis and high risk of early recurrence. Due to its propensity to rapidly metastasize, IBC is accountable for a high number of breast cancer-related deaths [3, 4]. Although not mandatory for the diagnosis of IBC, the existence of tumor emboli in dermal lymphatics, is a pathological hallmark [5]. Tumor emboli are non-adherent cell clusters that are spread by passive dissemination. This is responsible for both distant metastasis and local recurrence. Moreover IBC is highly angiogenic and angioinvasive, with many tumor cell clusters blocking the dermal lymphatic vessels and by that way causing the inflammatory signs like edema, erythema, pain, breast widening, and induration [6, 7]. Although data from the United States Surveillance, Epidemiology, and End Results (SEER) database had suggested an improvement in 20-year cancer-specific survival for patients with IBC who were treated in 1995 compared to 1975, even today at the time of presentation almost all women with IBC have lymph node involvement and approximately one-third have distant metastases [8–14]. The current therapeutic approach is based on an interdisciplinary treatment consisting of neoadjuvant chemotherapy followed by mastectomy and chest wall radiation therapy (RT). Patients treated with this therapeutic regimen have reported 5 year disease-free survival rates of 20–45 % and overall survival of 30–70 % [13]. Historically, single-modality treatment like radical mastectomy to cure IBC had a very negative outcome. In studies from the 1950s >90 % of IBC patients faced a relapse within 2 years after primary mastectomy; none of the patients survived 5 years [15, 16]. Combining the surgical therapy followed by chest wall radiation resulted in better locoregional control than with one single therapy alone. Overall survival stayed very low however. It was not until the invention of neoadjuvant chemotherapy followed by surgery and radiation in the 1970s that the efficacy of the treatment of this aggressive form of breast cancer became better [17–19]. Today neoadjuvant chemotherapies combining anthracyclines and taxanes lead to the best response [20, 21].
As women with this most aggressive type of breast cancer typically still have poorer prognosis compared to those diagnosed with non-IBC tumors, more effort is afforded to improve diagnosis and tumor therapy for IBC patients.
In this retrospective multi-center study we analyzed the impact of IBC on clinical outcome and evaluated the effect of hormone receptor and Her2 expression on inflammatory breast cancer cells on overall and disease-free survival.
Methods
For the analysis of this retrospective study, data from 11,780 patients was collected between 1992 and 2008. Patients were treated or diagnosed at the Department of Gynecology and Obstetrics at the University of Ulm and 16 partner clinics. All clinics are certified by the German Society of Cancer as breast cancer centers. In this context the newly established, comprehensive patient database BRENDA was used for the analysis of these retrospective data.
BRENDA is a multi-center clinical study investigating the influence of guideline adherence of the treatment of breast cancer patients on disease-free and overall survival.
The main interest of this study is the different therapeutic decisions about primary treatment of breast cancer: Breast-conserving therapy, axillary lymph node removal, radiotherapy, adjuvant chemotherapy, and anti-hormonal therapy. In contrast to other randomized clinical studies all breast cancer patients are monitored and tracked without selection.
The effect of the participation in randomized trials as well as the treatment in accordance with clinical guidelines deserve particular attention. Therefore, BRENDA records various patient and tumor-specific data regarding TNM stage, grading, histological subtype, hormone receptor expression, Her2 expression, date of primary diagnosis, lymphatic and vascular invasion.
Moreover BRENDA collects multiple data concerning therapeutical regimen including operative therapy (date of surgery, BCT, mastectomy, sentinel-node biopsy, and axillary lymph node dissection), adjuvant systemic chemo- and endocrine therapy, and precise information on the applied radiotherapy. Additionally BRENDA records follow-up data for each patient, concerning the date of first recurrences, secondary primary tumors, and date and cause of death. Therefore, physicians responsible for the follow-up care received questionnaires moreover the local death registries as well as patients were contacted to determine date and site of first recurrences, life style factors, general health data as well as date and cause of death.
To guarantee a high quality of the recorded data, specially trained medical assistants performed the compilation of all data at the university department in Ulm under strict and continuous quality checks [22].
For each patient included in this retrospective study a written consent form was obtained.
The inclusion criteria were histologically confirmed invasive breast cancer in a female patient.
The exclusion criteria were carcinoma in situ, sarcoma, bilateral breast cancer, primary occult disease, phyllodes tumor, incomplete follow-up, missing data on variables used as covariates in the survival analyses, respectively.
Furthermore if patients had more than one tumor and only one of these was inflammatory, only the inflammatory tumor was included. In this case the date of diagnosis for these patients was the date of diagnosis for the inflammatory tumor.
If patients had two tumors that were diagnosed within 30 days of each other, and both were inflammatory, the one that was diagnosed first is included and the other one was excluded.
We defined inflammatory breast cancer as a clinical-pathologic entity characterized by edema (peau d’orange) and diffuse erythema, involving one-third or more of the skin of the breast and displaying cancer cells in the subdermal lymphatics.
Our definition is based on the recommendations of the American Joint Committee on Cancer and the International Union for Cancer Control (AJCC-UICC) in 2010 [23].
Statistical analysis
Multivariate Cox proportional hazard models were used to estimate hazard ratios (HRs) and confidence intervals (CIs). The primary end points were defined as recurrence-free survival (RFS) and overall survival (OS). All Cox regressions were adjusted for the Nottingham Prognostic Index, which was calculated from the tumor size, grading, and positive lymph nodes of tumors. In case any of these three were missing, the missing value was replaced by the average for all inflammatory and non-inflammatory cases, respectively. Comparisons between groups on continuous variables were carried out using t tests and comparisons on categorical variables using χ 2-tests.
Ethical approval
This study and the BRENDA project have been approved by the Ethics Committee of the University of Ulm, which covers all participating breast cancer centers of the BRENDA network.
Results
The investigated cohort consisted of 11,780 female breast cancer patients; 76 were inflammatory. Six patients with IBC had to be excluded from this group as the inflammatory tumor was diagnosed secondly after diagnosis of a non-inflammatory breast cancer. Finally there remains 70 cases of IBC.
The median age at diagnosis was 58.9 years (range 35–93 years) for IBC patients and 61.5 years (range 22–101 years) for non-IBC patients. 86.8 % of IBCs were nodal positive at the time of diagnosis vs. 41.2 % for patients with non-IBC tumors. Moreover 75.9 % of IBC tumors were histological grade III.
Regarding IBC prevalence, significantly higher level of patients with IBC was determined at the university department compared to the participating breast cancer centers (62.9 vs. 31.2 %), respectively (Table 1).
Initially, the impact of IBC on survival parameters was analyzed. Therefore we compared the IBC sub-group with non-IBC patients. The IBC sub-group showed significantly decreased overall and disease-free survival (OAS/DFS) values compared to the non-IBC population (Fig. 1).
To further determine the aggressiveness of IBC we analyzed the annual percentage of deceased patients after primary diagnosis of IBC and non-IBC patients (Table 2).
Mortality especially in the first years after diagnosis of IBC are striking. Five years after primary diagnosis 4.5 times more patients died from IBC than from non-inflammatory breast cancer despite intense therapy. Women who survived the first 6 years after primary diagnosis of IBC had however a good chance to not to die from IBC.
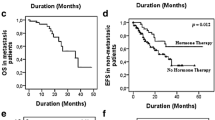
The fatal outcome of inflammatory tumors becomes even more evident as Table 3 points out that significantly more IBC vs. non-IBC patients have metastasis at time of primary diagnosis: 27 vs. 6 % (χ 2 = 279.2; p < 0.001). Moreover, patients with metastasis suffering from IBC have significantly decreases OAS (Fig. 2).
A lack of hormone receptor expressions among IBC tumors has shown to be associated with a more aggressive clinical course and with a decreased overall and breast cancer-specific survival [24]. Therefore we investigated the expression of hormone and Her2 receptor status on IBC and non-IBC tumor cells. The analysis clearly demonstrates that IBC tumors are frequently Her2 positive: 34 vs. 17 % (χ 2 = 11.9; p < 0.001) whereas the expression of estrogen or progesterone receptors is significantly decreased: 62.5 vs. 34.3 % (χ 2 = 37.2; p < 0.001) (Table 1).
Furthermore we analyzed the effects of hormone receptor expression on survival parameters. The IBC hormone receptor-negative sub-group showed a significant decrease in OAS and disease-free survival (DFS) compared to the hormone receptor-positive sub-group of IBC (Figure 3).
Moreover we analyzed the effect of Her2 expression on OAS and DFS. In our study group 36 patients suffering from IBC were determined as Her2 negative and 20 patients as Her2 positive. For 14 patients Her2 status remains unknown. There are no differences in overall and recurrence-free survival between Her2-positive and Her2-negative inflammatory breast cancer patients (Fig. 4).
Discussion
Inflammatory breast cancer (IBC) still appears to be a very aggressive subtype of breast cancer with a poor clinical outcome and strong metastatic potential [7]. Up to now, a definitive molecular or pathological diagnostic criteria for IBC could not be identified. For that reason clinical findings like: erythema and edema of the skin of the breast, rapid onset of symptoms, and signs are still decisive for the diagnosis of IBC [25, 26].
The main reasons of delayed diagnosis and insufficient management of this aggressive subtype of breast cancer is the lack of specific diagnostics and that many women with IBC are misdiagnosed with mastitis. In the synopsis of our analysis it becomes clear that most patients suffering from IBC have a very unfavorable and palliative prognosis at the time of diagnosis. As metastasized breast cancer—IBC as well as non-IBC—is incurable, it becomes evident that the diagnosis and therapy for IBC is mostly done too late to give patients a chance of cure.
In our patient collective, almost 30 % of women with IBC were metastatic at the time of diagnosis and about 60 % of patients died within 2 years after primary manifestation. In the following years mortality of IBC stays relatively stable in contrast to non-IBC tumors (Table 2). Our findings are in line with the literature. Dawood et al. [27] demonstrates in a multivariable model that increasing year of diagnosis is associated with a decreasing risk of death from IBC. In context with our finding Dawood et al. and Gogia et al. presented similar level of primary metastatic disease of inflammatory breast cancer. These findings emphasis the importance of correct and early diagnosis and therapy at a time point when IBC cells have not spread into the body [27, 28].
A review by Kim et al. confirms that the main cause for the dramatical variability concerning the treatment and differences in terms of clinical outcome for IBC were the inconsistent criteria used to identify IBC [29]. For the diagnosis of IBC we tried to apply the criteria given by the American Joint Committee on Cancer and the International Union for Cancer Control (AJCC-UICC) [23]. However, the definition of IBC depends strongly on the experience of the medical clinician as well as the pathologist in terms of interpretation of clinical symptoms and the pathological diagnosis. This results in a heterogeneous study population and is therefore a big challenge for researchers [30, 31]. This problem could also explain the relatively small sample size of 70 IBC patients in our study group of 11,780 breast cancer patients. 70 IBC patients is significantly less than the number of inflammatory cases that could be expected.
As shown earlier, the overexpression of Her2 on tumor cells is of significant importance for diagnosis and therapy of breast cancer in general, we analyzed the expression of Her2 on tumor cells in our study population. For non-IBC tumors the Her2 overexpression is mostly associated with an aggressive form of breast cancer, whereas the prognostic value of Her2 overexpression among women with IBC tumors currently remains unclear [32].
Regarding the California Cancer Registry comprising >2000 women with IBC there was only a marginal association observed for breast cancer-specific survival and Her2 overexpression. This analysis showed a slightly better outcome for women with Her2-positive IBC tumors compared to Her2-negative tumors [24]. On the other hand a retrospective study including 179 women with IBC showed no difference in recurrence-free survival between women with Her2-positive and -negative IBC tumors [32].
For the therapeutic regimen a recent prospective study that randomized women with IBC, to a chemotherapeutic regimen including an anthracycline with or without 1 year of trastuzumab revealed increased pCR rates following combined therapy with trastuzumab [33].
Cristofanilli et al. [34] could demonstrate that the use of lapatinib, a reversible inhibitor of Her1 and Her2 showed a good clinical response in the preoperative setting.
Although the prognostic role of Her2 expression among IBC tumors is not fully understood, there is some evidence indicating that patients with Her2 overexpression benefit from a combined therapy consisting of trastuzumab and systemic chemotherapy [24, 35–39].
In contrast to non-IBC tumors, clinical studies revealed a higher frequency of hormone receptor-negative status among IBC breast cancer. Up to 83 % of IBC tumors were reported to be negative for estrogen or progesterone receptor expression [7, 30, 31]. A different analysis of population-based data has demonstrated an improvement in median survival among female patients with hormone receptor-positive IBC in comparison to those with hormone negative IBC [7, 24]. Analysis of population-based data from the Surveillance, Epidemiology, and End Results database showed a statistically significant improvement in median survival among female patients with hormone receptor-positive IBC and anti-hormonal treatment compared to patients suffering from IBC with negative hormone receptor expression [7].
Women with hormone receptor-positive IBC should therefore receive at least 5 years of anti-hormone therapy. Depending on their menopausal status, a hormone receptor modulator or an aromatase inhibitor should be applied [34].
In our study group we could confirm the lower expression of hormone receptors on inflammatory breast cancer cells as well as the higher level of Her2 expression. Moreover our data confirm the negative effect of low estrogen and progesterone receptor expression on OAS and DFS.
Concerning the therapeutic regimen of IBC our data show that patients were not always treated in accordance with clinical guidelines for IBC consisting of neoadjuvant chemotherapy, ablation, radiotherapy, and where appropriate anti-Her2 or anti-hormonal therapy. Wöckel et al. and Schwentner et al. discussed the reasons for the lack of guideline adherence in terms of breast cancer therapy and the negative effect for clinical outcome of breast cancer patients. Age, comorbidities as well as the rejection of the therapeutic recommendations by the patients can explain these guideline violations [40, 41].
As guideline violations have negative effects for the clinical outcome, our data underline that the adherence to guideline conform therapy of inflammatory breast cancer needs to be improved.
We adjusted our data for the most important prognostic parameters using the Nottingham Prognostic index. Of course adjusting for other confounding factors would have been the preferable option. However due to the small absolute number of IBC patients in our large cohort, it was not possible to do so. Obviously we therefore cannot completely answer the question whether additional confounding prognostic variables might influence the outcome for IBC. Yet the Nottingham Prognostic index (NPI) is arguably the most informative single variable for indicating prognosis. Our results in which we compared sub-groups of IBC patients did not change in important ways between analyses in which we did, versus did not adjust for the NPI. We are therefore convinced that these results would have been robust as well against adjusting for other prognostic factors that are each less informative than the NPI. In the analysis in which we compared IBC with non-IBC patients, the hazard ratios became even larger when we did not adjust for NPI. Adjusting for more patient and tumor characteristics might lead to smaller differences in OAS and DFS between IBC and non-IBC patients than the ones reported here.
Overall our data confirms the clinical impact and the aggressiveness of IBC and the importance of further research to improve the diagnosis and tumor-specific treatment of patients suffering from this rare and fatal form of breast cancer.
Therefore, the molecular characterization of IBC is important. In this context genome profiling was done to a retrospective series of clinical IBC samples for a better understanding of this aggressive disease at the molecular level [42–45]. Because of the rarity of IBC and the small size of diagnostic biopsies it is difficult to perform molecular studies. For that reason the molecular evidence for the aggressiveness of IBC is poorly understood. The most persuading results in terms of molecular analysis could be achieved on the RNA level [45].
Different molecular methods like: miRNA, DNA, proteomics, or CDA were only investigated in small clinical samples or in IBC cell lines [46]. Being able to draw clinical conclusions, large series of clinical samples would however be inevitable. As mentioned above, higher incidence of certain molecular alterations could be investigated in IBC: low expression of estrogen and progesterone receptors, overexpression HER2, high level of TP53 mutations, high proliferation and angiogenesis levels, overexpression of E-cadherin, dysfunction of MUC1 as well as the overexpression of chemokines and chemokine receptors [45, 47].
Despite some new findings in the area of molecular tumor analysis, the treatment of IBC is limited to the use of standardized therapeutics. Some new targeted therapies are under investigation such as lapatinib or bevacizumab [34, 48]. For the improvement of therapeutic strategies for IBC it remains one of the most important challenges to collect prospectively more IBC samples through international collaborations. Applying new analysis strategies like high-throughput molecular analyses and next generation sequencing could help to better define the differences between IBC and other types of breast cancer. Moreover research should focus on the pattern of histone modifications in IBC and non-IBC as well as the role of alternative splicing [49–51]. Just the molecular analysis of tumor biology will offer the chance for new specific targeted therapy strategies.
References
Dawood S, Ueno NT, Valero V, Woodward WA, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM, Cristofanilli M (2012) Identifying factors that impact survival among women with inflammatory breast cancer. Ann Oncol Off J Euro Soc Med Oncol/ESMO 23(4):870–875. doi:10.1093/annonc/mdr319
Lee BJTN (1924) Inflammatory carcinoma of the breast: a report of twenty-eight cases from the breast clinic of Memorial Hospital. Surg Gynecol Obst 39:580–595
Dawood S, Cristofanilli M (2011) Inflammatory breast cancer: what progress have we made? Oncology (Williston Park) 25(3):264–270, 273
Palangie T, Mosseri V, Mihura J, Campana F, Beuzeboc P, Dorval T, Garcia-Giralt E, Jouve M, Scholl S, Asselain B et al (1994) Prognostic factors in inflammatory breast cancer and therapeutic implications. Eur J Cancer 30A(7):921–927
Dawood S, Ueno NT, Valero V, Woodward WA, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM, Cristofanilli M (2011) Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer 117(9):1819–1826. doi:10.1002/cncr.25682
Bonnier P, Charpin C, Lejeune C, Romain S, Tubiana N, Beedassy B, Martin PM, Serment H, Piana L (1995) Inflammatory carcinomas of the breast: a clinical, pathological, or a clinical and pathological definition? Int J Cancer J Int du Cancer 62(4):382–385
Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH (2005) Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 97(13):966–975. doi:10.1093/jnci/dji172
Kleer CG, van Golen KL, Merajver SD (2000) Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast cancer Res BCR 2(6):423–429. doi:10.1186/bcr89
Giordano SH, Hortobagyi GN (2003) Inflammatory breast cancer: clinical progress and the main problems that must be addressed. Breast Cancer Res BCR 5(6):284–288. doi:10.1186/bcr608
Perez CA, Fields JN, Fracasso PM, Philpott G, Soares RL Jr, Taylor ME, Lockett MA, Rush C (1994) Management of locally advanced carcinoma of the breast. II. inflammatory carcinoma. Cancer 74(1 Suppl):466–476
Thoms WW Jr, McNeese MD, Fletcher GH, Buzdar AU, Singletary SE, Oswald MJ (1989) Multimodal treatment for inflammatory breast cancer. Int J Radiat Oncol Biol Phys 17(4):739–745
Baldini E, Gardin G, Evagelista G, Prochilo T, Collecchi P, Lionetto R (2004) Long-term results of combined-modality therapy for inflammatory breast carcinoma. Clin Breast Cancer 5(5):358–363
Liauw SL, Benda RK, Morris CG, Mendenhall NP (2004) Inflammatory breast carcinoma: outcomes with trimodality therapy for nonmetastatic disease. Cancer 100(5):920–928. doi:10.1002/cncr.20083
Harris EE, Schultz D, Bertsch H, Fox K, Glick J, Solin LJ (2003) Ten-year outcome after combined modality therapy for inflammatory breast cancer. Int J Radiat Oncol Biol Phys 55(5):1200–1208
Haagensen CD, Stout AP (1951) Carcinoma of the breast. III. Results of treatment, 1935-1942. Ann Surg 134(2):151–172
Lamb CC, Eberlein TJ, Parker LM, Silver B, Harris JR (1991) Results of radical radiotherapy for inflammatory breast cancer. Am J Surg 162(3):236–242
Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, Theriault RL, Strom EA, Wasaff BJ, Asmar L, Frye D, Hortobagyi GN (1997) Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol 40(4):321–329. doi:10.1007/s002800050664
Koh EH, Buzdar AU, Ames FC, Singletary SE, McNeese MD, Frye D, Holmes FA, Fraschini G, Hug V, Theriault RL et al (1990) Inflammatory carcinoma of the breast: results of a combined-modality approach–M.D. Anderson Cancer Center experience. Cancer Chemother Pharmacol 27(2):94–100
Singletary SE, Ames FC, Buzdar AU (1994) Management of inflammatory breast cancer. World J Surg 18(1):87–92
Cristofanilli M, Buzdar AU, Sneige N, Smith T, Wasaff B, Ibrahim N, Booser D, Rivera E, Murray JL, Valero V, Ueno N, Singletary ES, Hunt K, Strom E, McNeese M, Stelling C, Hortobagyi GN (2001) Paclitaxel in the multimodality treatment for inflammatory breast carcinoma. Cancer 92(7):1775–1782
Cristofanilli M, Gonzalez-Angulo AM, Buzdar AU, Kau SW, Frye DK, Hortobagyi GN (2004) Paclitaxel improves the prognosis in estrogen receptor negative inflammatory breast cancer: the M. D. Anderson Cancer Center experience. Clin Breast Cancer 4(6):415–419
Schouten LJ, Jager JJ, van den Brandt PA (1993) Quality of cancer registry data: a comparison of data provided by clinicians with those of registration personnel. Br J Cancer 68(5):974–977
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474. doi:10.1245/s10434-010-0985-4
Zell JA, Tsang WY, Taylor TH, Mehta RS, Anton-Culver H (2009) Prognostic impact of human epidermal growth factor-like receptor 2 and hormone receptor status in inflammatory breast cancer (IBC): analysis of 2,014 IBC patient cases from the California Cancer Registry. Breast Cancer Res BCR 11(1):R9. doi:10.1186/bcr2225
Gonzalez-Angulo AM, Hennessy BT, Broglio K, Meric-Bernstam F, Cristofanilli M, Giordano SH, Buchholz TA, Sahin A, Singletary SE, Buzdar AU, Hortobagyi GN (2007) Trends for inflammatory breast cancer: is survival improving? Oncologist 12(8):904–912. doi:10.1634/theoncologist.12-8-904
Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, Dirix LY, Levine PH, Lucci A, Krishnamurthy S, Robertson FM, Woodward WA, Yang WT, Ueno NT, Cristofanilli M (2011) International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol Off J Euro Soc Med Oncol/ESMO 22(3):515–523. doi:10.1093/annonc/mdq345
Dawood S, Lei X, Dent R, Gupta S, Sirohi B, Cortes J, Cristofanilli M, Buchholz T, Gonzalez-Angulo AM (2014) Survival of women with inflammatory breast cancer: a large population-based study. Ann Oncol Off J Euro Soc Med Oncol/ESMO 25(6):1143–1151. doi:10.1093/annonc/mdu121
Gogia A, Raina V, Deo SV, Shukla NK, Mohanti BK, Sharma DN (2014) Inflammatory breast cancer: a single centre analysis. Asian Pacific J Cancer Pre APJCP 15(7):3207–3210
Kim T, Lau J, Erban J (2006) Lack of uniform diagnostic criteria for inflammatory breast cancer limits interpretation of treatment outcomes: a systematic review. Clin Breast Cancer 7(5):386–395. doi:10.3816/CBC.2006.n.055
Harvey HA, Lipton A, Lawrence BV, White DS, Wells SA, Blumenschein G, Lee D (1982) Estrogen receptor status in inflammatory breast carcinoma. J Surg Oncol 21(1):42–44
Nguyen DM, Sam K, Tsimelzon A, Li X, Wong H, Mohsin S, Clark GM, Hilsenbeck SG, Elledge RM, Allred DC, O’Connell P, Chang JC (2006) Molecular heterogeneity of inflammatory breast cancer: a hyperproliferative phenotype. Clin Cancer Res Off J Am Assoc Cancer Res 12(17):5047–5054. doi:10.1158/1078-0432.CCR-05-2248
Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau SW, Islam R, Aldape KD, Yu TK, Hortobagyi GN, Gonzalez-Angulo AM (2008) Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol Off J Euro Soc Med Oncol/ESMO 19(7):1242–1248. doi:10.1093/annonc/mdn036
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375(9712):377–384. doi:10.1016/S0140-6736(09)61964-4
Boussen H, Cristofanilli M, Zaks T, DeSilvio M, Salazar V, Spector N (2010) Phase II study to evaluate the efficacy and safety of neoadjuvant lapatinib plus paclitaxel in patients with inflammatory breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 28(20):3248–3255. doi:10.1200/JCO.2009.21.8594
Parton M, Dowsett M, Ashley S, Hills M, Lowe F, Smith IE (2004) High incidence of HER-2 positivity in inflammatory breast cancer. Breast 13(2):97–103. doi:10.1016/j.breast.2003.08.004
Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P, Pauletti G, Powell JE, Pegram MD, Slamon DJ (2006) Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 24(12):1831–1838. doi:10.1200/JCO.2005.02.8886
Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, Parker LM, Ellisen LW, Kuter I, Gadd MA, Christian RL, Kennedy PR, Borges VF, Bunnell CA, Younger J, Smith BL, Winer EP (2003) Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol Off J Am Soc Clin Oncol 21(1):46–53
Guerin M, Gabillot M, Mathieu MC, Travagli JP, Spielmann M, Andrieu N, Riou G (1989) Structure and expression of c-erbB-2 and EGF receptor genes in inflammatory and non-inflammatory breast cancer: prognostic significance. Int J Cancer J Int du Cancer 43(2):201–208
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32. doi:10.1016/S1470-2045(11)70336-9
Wockel A, Kurzeder C, Geyer V, Novasphenny I, Wolters R, Wischnewsky M, Kreienberg R, Varga D (2010) Effects of guideline adherence in primary breast cancer—a 5-year multi-center cohort study of 3976 patients. Breast 19(2):120–127. doi:10.1016/j.breast.2009.12.006
Schwentner L, Wockel A, Konig J, Janni W, Ebner F, Blettner M, Kreienberg R, Van Ewijk R (2013) Adherence to treatment guidelines and survival in triple-negative breast cancer: a retrospective multi-center cohort study with 9,156 patients. BMC Cancer 13:487. doi:10.1186/1471-2407-13-487
Bertucci F, Ueno NT, Finetti P, Vermeulen P, Lucci A, Robertson FM, Marsan M, Iwamoto T, Krishnamurthy S, Masuda H, Van Dam P, Woodward WA, Cristofanilli M, Reuben JM, Dirix L, Viens P, Symmans WF, Birnbaum D, Van Laere SJ (2014) Gene expression profiles of inflammatory breast cancer: correlation with response to neoadjuvant chemotherapy and metastasis-free survival. Ann Oncol Off J Euro Soc Med Oncol/ESMO 25(2):358–365. doi:10.1093/annonc/mdt496
Ledig S, Hiort O, Scherer G, Hoffmann M, Wolff G, Morlot S, Kuechler A, Wieacker P (2010) Array-CGH analysis in patients with syndromic and non-syndromic XY gonadal dysgenesis: evaluation of array CGH as diagnostic tool and search for new candidate loci. Hum Reprod 25(10):2637–2646. doi:10.1093/humrep/deq167
Fernandez SV, Robertson FM, Pei J, Aburto-Chumpitaz L, Mu Z, Chu K, Alpaugh RK, Huang Y, Cao Y, Ye Z, Cai KQ, Boley KM, Klein-Szanto AJ, Devarajan K, Addya S, Cristofanilli M (2013) Inflammatory breast cancer (IBC): clues for targeted therapies. Breast Cancer Res Treat 140(1):23–33. doi:10.1007/s10549-013-2600-4
Bertucci F, Finetti P, Vermeulen P, Van Dam P, Dirix L, Birnbaum D, Viens P, Van Laere S (2014) Genomic profiling of inflammatory breast cancer: a review. Breast 23(5):538–545. doi:10.1016/j.breast.2014.06.008
Zhang EY, Cristofanilli M, Robertson F, Reuben JM, Mu Z, Beavis RC, Im H, Snyder M, Hofree M, Ideker T, Omenn GS, Fanayan S, Jeong SK, Paik YK, Zhang AF, Wu SL, Hancock WS (2013) Genome wide proteomics of ERBB2 and EGFR and other oncogenic pathways in inflammatory breast cancer. J Proteome Res 12(6):2805–2817. doi:10.1021/pr4001527
Charafe-Jauffret E, Tarpin C, Viens P, Bertucci F (2008) Defining the molecular biology of inflammatory breast cancer. Semin Oncol 35(1):41–50. doi:10.1053/j.seminoncol.2007.11.015
Pierga JY, Petit T, Delozier T, Ferrero JM, Campone M, Gligorov J, Lerebours F, Roche H, Bachelot T, Charafe-Jauffret E, Pavlyuk M, Kraemer S, Bidard FC, Viens P (2012) Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol 13(4):375–384. doi:10.1016/S1470-2045(12)70049-9
Schmitt MW, Kennedy SR, Salk JJ, Fox EJ, Hiatt JB, Loeb LA (2012) Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci USA 109(36):14508–14513. doi:10.1073/pnas.1208715109
Sanmiguel P (2011) Next-generation sequencing and potential applications in fungal genomics. Methods Mol Biol 722:51–60. doi:10.1007/978-1-61779-040-9_4
Chatterjee N, Wang WL, Conklin T, Chittur S, Tenniswood M (2013) Histone deacetylase inhibitors modulate miRNA and mRNA expression, block metaphase, and induce apoptosis in inflammatory breast cancer cells. Cancer Biol Ther 14(7):658–671. doi:10.4161/cbt.25088
Acknowledgments
We gratefully thank the following people for their contributions to the BRENDA study: Karsten Gnauert (Ostalbklinikum, Aalen), Steffen Fritz (Kreisklinik Biberach), Ulf Göretzlehner (Kreiskrankenhaus Ehingen), Hans-Walter Vollert (Städt. Krankenhaus Friedrichshafen), Peter Jakob Albert (Klinikum Heidenheim), Ricardo Felberbaum (Klinikum Kempten), Andreas Zorr (Klinikum Konstanz), Felix Flock (Klinikum Memmingen), Erik Schlicht (Stauferklinik, Mutlangen), Martina Gropp-Meier (Oberschwabenklinik Ravensburg), Gerhard Bartzke (Kreiskrankenhaus Rottweil), Andreas Rempen (Diakonie-Krankenhaus, Schwäbisch Hall), Edgar Schelble (Kreiskrankenhaus Sigmaringen), Theodor Dinkelacker (Helfenstein-Klinik Geislingen), Andreas Grüneberger (Oberschwabenklinik Wangen), and Thorsten Kühn (Städt. Kliniken, Esslingen). This work was supported by the German Federal Ministry of Education and Research (BMBF-Grant-01ZP0505).
Conflict of interest
All authors declare that there are no potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations that could inappropriately influence this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diessner, J., Van Ewijk, R., Weiss, C.R. et al. Identifying the impact of inflammatory breast cancer on survival: a retrospective multi-center cohort study. Arch Gynecol Obstet 292, 655–664 (2015). https://doi.org/10.1007/s00404-015-3691-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3691-4