Abstract
Acne is an immune-mediated chronic inflammatory disease. Although several factors are involved in its pathophysiology, this process is not completely understood. Androgen hormone activity increases sebum production inside the pilosebaceous follicle, adjusting the environment for the development of Propionibacterium acnes which triggers inflammation. Knowing how others factors such as the skin barrier and microbiome are involved in acne, can help in understanding more about the disease and may help to conduct a better treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
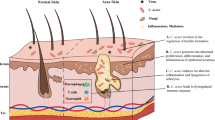
Acne is a chronic inflammatory disease of the pilosebaceous follicles that mainly affects adolescents [5]. In adults, its prevalence and incidence has increased in recent years, especially among women [8]. Many patients develop different types of scars, in addition to presence of inflammatory lesions of variable severity. As this disease mainly affects the face, it is associated with psychological impact with symptoms of depression, anxiety, and anger [60]. Understanding the etiopathogenic mechanisms involved in acne is fundamental to increase the possibilities of prevention, treatment, and management of difficult cases.
In contrast to atopic dermatitis, changes in epidermal barrier are not the first questions that come to mind for a patient with acne. However, research on the contribution of horny layer to skin homeostasis has helped to decipher how the epidermal barrier can be intrinsically changed in acne vulgaris [53]. It is also known that some acne treatments compromise epidermal permeability, and change its physiological activity. In addition, we must consider that the follicular epithelial barrier, directly involved in inflammatory process, may rupture with the evolution of acne vulgaris [55]. Skin microbiota is another factor implicated in acne. It represents the group of microorganisms, which reside on the skin. When in balance with the defense mechanisms, they are essential to health skin activity as they inhibit bacterial hyperproliferation and pathogenic strains involved in different skin diseases. Intrinsically, there is a relationship between the integrity of the skin barrier, microbiota of the epidermis, and sebaceous gland in the pathophysiology of acne.
The cutaneous barrier in acne
The stratum corneum or horny layer in the epidermal barrier acts in the homeostatic control of water, assists in the recognition and neutralization of microorganisms, blocks formation of reactive oxygen species, promotes protection against ultraviolet radiation, and help in the response against the external allergens [1]. When the horny layer is unable to control transepidermal water loss (TEWL), its enzymatic system becomes inefficient, leading to loss of control of permeability and appearance of xerosis, erythema, and desquamation [11]. Although poorly researched, changes in skin barrier have been described even in acne patients without treatment [53].
Kurokawa et al. [33] showed increased expression of fillagrin in follicular keratinocytes in patients with acne. Fillagrin is a protein with key functions in epidermal differentiation and contributes to the structural and functional integrity of the horny layer [33]. During differentiation of the granular layer, fillagrin undergoes a degradation process that giving rise to formation of natural moisturizing factor (NMF) [25]. Jarrousse et al. [28] proved that the presence of the Propionibacterium acnes (P. acnes) increases the expression of fillagrin in keratinocytes in cell culture medium. It is unclear if this fillagrin increase is a primary or secondary event in the development of follicular hyperkeratosis.
There is also an important change in sebum secretion, which is regulated by different factors involved in cell proliferation and differentiation, lipogenesis, hormone metabolism, and cytokine release [63]. In most cases, increased secretion is associated with sebaceous gland hypertrophy [54]. However, not all patients with acne have seborrhea [6].
Studies have shown that patients with acne have qualitative changes in sebum, with increased secretion of squalene and free fatty acid reduction [41, 43]. The increased flow of sebum dilutes the concentration of linoleic acid resulting in relative deficiency which contributes to comedogenesis [15]. Pro-inflammatory sebum lipid fractions (monounsaturated fatty acid-MUFA and lipo-peroxides) are additional factors in the pathogenesis of acne [62]. These substances influence proliferation and differentiation of keratinocytes contributing to infundibular hyperkeratinization [39, 47]. Ethnic differences also influence the composition of sebum, as quantitative changes in specific fractions of ceramides have been demonstrated between caucasian and black americans [40].
Yamamoto et al. [59] analyzed several parameters in patients with and without acne, and observed an increased TEWL and reduction in horny layer conductance in addition to the increased sebum production, causing impairment to with in hydration. They also found reduced levels of sphingolipids (ceramides and free sphingosine), indicating lipid deficiency in the intercellular membrane. The increased TEWL and reduced hydration of the horny layer correlates directly with the disease severity. The authors concluded that there are cutaneous barrier changes in acne even in patients without treatment [59].
With the inflammatory process progression, there is an increase in glandular structures and progressive activation of enzymes like lipases, proteases, metalloproteinases, and a greater participation of the immune system resulting in thinning of the epidermal follicular wall. This results in glandular rupture causing extravasation of keratin, sebum, bacteria, and cellular debris to the dermis, which triggers foreign body-type reactions along with the formation of nodules and scarring [31, 57].
The skin microbiome and acne
The skin represents a barrier to the development of microorganisms by producing proteases, lisoenzymes, and antimicrobial peptides (AMPs). pH, temperature, humidity, and sebaceous secretions also comprise an intricate medium colonized by a variety of bacteria [32]. Phyla that dominate the skin are: Actinobacteria, Proteobacteria, Bacteroidetes and Firmicutes. Of these, more than 60% of the bacterial species belong to three genera: Staphylococcus (Firmicutes); Corynebacterium, and Propionibacterium (Actinobacteria) [22, 23, 45]. Sites rich in sebaceous secretion are colonized by lipophilic species of Propionibacterium and in wet locations such as folds, by Staphylococcus and Corynebacterium [45]. The sebaceous gland produces and secretes a rich lipid secretion, which forms a hydrophobic layer that protects and lubricates hair and skin. Although the sebum has antibacterial functions, P. acnes hydrolyzes triglycerides present in secretions and releases free fatty acids that promote bacterial adherence by facilitating the colonization of these glands [34].
Microbial variations on the skin may contribute to modifications and diseases in this ecosystem. They are implicated in protection and pathogenesis of various diseases [7, 30]. Many bacterial genes are involved in coding the production of toxins, invasins, adhesins, and thus, the virulence of bacteria. In P. acnes, the detected virulence factors include camp5, gehA, sialidases, neuraminidases, endoglicoceraminidases, lipases, and hemolysins [4]. The relationship between P. acnes and others skin bacteria’s with their host regulate many aspects of the loco-regional immune system [2]. Staphylococcus epidermidis can induce increased skin antimicrobial peptides (AMPs) and reduction in the chance of skin infection by acting on the strains of S. aureus. This occurs by connecting the Langerhans cells that activate the immune system (innate and adaptive defense). Similarly, P. acnes hydrolyzes lipids present in the skin barrier by the action of enzymes, acidifying the environment and thus, preventing the colonization of pathogenic bacteria.
The skin microbiota also controls the expression of interleukin-1 (IL-1), a cytokine involved in initiation and amplification of the immune response. In fact, AMPs and IL-1 represent the oldest arms of innate immune system, suggesting that they could have been initial mediators between host and commensal bacteria [37]. Theories developed in the early 1980s have proposed that the total number of bacteria that inhabit the human body would be equivalent to 10× the number of human cells. This theory lasted until a recent publication, which stated that previous studies overestimated the number of intestinal bacteria, so, the correct ratio of bacterial commensal microbiota and the number of human cells would be 1:1 [46]. Scientific advances that have explained the relationship between the presence of pathogenic and commensal bacteria and their interaction with the immune system have helped to better understand some diseases [32]. Also, new techniques of bacterial identification including analysis with 16S RNA, is improving the understanding of changes in microbiota during human life and its role in different diseases [38].
The mode of childbirth and delivery also plays a key role. Vaginal microbiota during natural childbirth and microbiota from the maternal skin in cesarean section influences the composition of cutaneous microbiome. Also, the neonate contact with the mother’s skin during breastfeeding exerts a role for colonization by bacteria [14, 35].
Several factors regulate microbiome during life, such as: nutritional status and medications, antibiotics, and proton-pump inhibitors. The last one leads to exaggerated bacterial growth in the small intestine, an increase in the markers of systemic inflammation and reduced absorption of important nutrients [19].
During adolescence, androgen hormones [testosterone, dehydroepiandrosterone sulfate, and insulin growth factor type-1 (IGF-1)], lead to an increase production of sebum and a high colonization of P. acnes inside the sebaceous gland. The qualitative changes and the increased number of P. acnes are important factors in the pathophysiology of acne [19].
In addition to P. acnes, additional microbiota in acne patients involved in its pathophysiology include: S. epidermidis, S. aureus, Streptococcus pneumoniae, Enterobacter and Klebsiella pneumoniae [20, 26, 44]. Although, P. acnes is the main factor in acne development [3], other bacteria act as indirect contributors to the inflammatory process. Staphylococcus epidermidis increases the incidence of acne to 70% compared with the control group [42]. Their virulence factors are lipases and delta-hemolysins [61].
Recently, different strains of P. acnes were identified, allowing the speculation that apart from numerical increase, its subtypes could also be important in disease development and chronicity. Fitz-Gibbon et al. demonstrated that patients with acne have a greater presence of ribotypes 4 and 5 (RT4 and RT5), while in the control group there is a predominance of RT6 [20]. In another study with a different classification, the P. acnes strain III showed greater ability to stimulate inflammation, increasing the expression of proteinase-activated receptor 2, TNF, and matrix metalloproteinase 13; suggesting greater virulence of this subtype [29].
The importance of the cutaneous barrier and microbiome in the treatment of acne
Acne treatments affect skin barrier and microbiota, causing changes in the permeability and the skin functions of defense. These treatments include topical medications, systemic and physical treatments like peelings, and microdermabrasion. It may lead to increase in TEWL and in many cases, xerosis and irritant contact dermatitis.
The topical retinoids exhibit great efficacy and safety for the treatment of acne [21, 27]. They are the most commonly used drugs alone or in association, to treat all degrees of acne [27]. They reduce the expression of TLR-2 receptors (Toll-like receptor-2), have comedolytic and intrinsic anti-inflammatory effects. These transmembrane receptors release inflammatory cytokines when activated by P. acnes [17, 58]. In the first days of use, many patients develop irritant dermatitis which lasts about 2–4 weeks with subsequent improvement. If the desquamation persists, with a reduction in the thickness of the stratum corneum, the epidermal permeability barrier function is compromised [50]. Moisturizers associated with topical retinoids have shown reduction in dermatitis, without changing the effectiveness of the treatment [52]. Another study that analyzed the efficacy of retinoid-associated moisturizers before and during the treatment of photoaging, concluded that there was less change in TEWL with the association [16].
Systemic treatment with isotretinoin often causes xerosis and desquamation. Its action markedly reduces the production of sebum and induces change in skin microflora, with an increased tendency of staphylococcal colonization [13]. Modifications in epidermal permeability induced by topical and oral retinoids were not related to changes in the intercellular lipids of the stratum corneum [17]. Topical treatment with benzoyl peroxide is also widely indicated in different degrees of acne. It has anti-inflammatory, antibacterial effects and is able to eliminate drug-resistant strains of P. acnes [12, 21, 51]. The use of benzoyl peroxide (BP) may cause skin irritation, although this effect appears to be dependent on different factors including concentration of the agent, vehicle, cutaneous adjuvant treatment, and concomitant use of other anti-acne medications [45]. Weber et al. [56] showed 1.8 times increase in TEWL using a formulation with 10%. BP dermatitis induced by irritant contact is more frequent than allergy with the use of this product [24].
The use of oral antibiotics for extended periods of time has been associated with the development of resistance strains of P. acnes, increased incidence of folliculitis by gram-negative bacteria, and increase in bacterial pharyngitis. These result from changes in the microbial environment of the skin and airways [18].
While topical therapy remains a fundamental approach in the management of acne, it is associated with side effects that compromise the stratum corneum layer and its functions, affecting patient compliance. The adjunctive use of mild cleansers and non-comedogenic moisturizers reduces the side effects of treatment, reducing the frequency of irritant contact dermatitis and increases therapeutic efficacy [10, 49]. Ideally, the cleansers and moisturizers should be non-acnegenic, non-comedogenic, non- irritant and without allergens [48]. Its chemical composition should comply with the pH of the skin because high pH results in increase in TEWL, causing xerosis. In addition, high pH can facilitate microbial growth and exacerbate acne [9]. Excessive cleansing can also change the skin’s natural defense system. The use of detergents that degrade the lipid barrier and induce reduction of AMPs, allow the proliferation of S. epidermidis [36]. Moisturizers must be effective in preventing and reducing water evaporation, relieving xerosis, and maintaining integrity of the skin barrier without leaving the skin oily.
Conclusion
In conclusion, we know that acne is fundamentally an inflammatory and immune-mediated disease, even if at subclinical stages. Many factors already discovered and others in research contribute to the activation and maintenance of this process. According to the latest data, it is essential to maintain the integrity of the epidermal barrier and balance of the skin’s microbiota to control the release of some pro-inflammatory cytokines. Care with cleaning, moisturizing, and photoprotection must be integrated with any adopted therapeutic approach.
References
Baroni A, Buommino E, De Gregorio V et al (2012) Structure and function of epidermis related to barrier properties. Clin Dermatol 30:257–262
Belkaid Y, Segre JA (2014) Dialogue between skin microbiota and immunity. Science 346:954–959
Brüggemann H, Henne A, Hoster F et al (2004) The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 305:671–673
Brüggemann H (2005) Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg 24:67–72
Burton JL, Cunliffe WJ, Stafford L (1971) The prevalence of acne vulgaris in adolescence. Br J Dermatol 85:119–26
Choi CW, Choi JW, Park KC, Youn SW (2013) Facial sebum affects the development of acne, especially the distribution of inflammatory acne. J Eur Acad Dermatol Venereol 27:301–306
Cogen AL, Nizet V, Gallo RL (2008) Skin microbiota: a source of disease or defense? Br J Dermatol 158:442–455
Collier CN, Harper JC, Cafardi JA, Wang W, Foster KW (2008) The prevalence of acne in adults 20 years and older. J Am Acad Dermatol 58:56–9
Decker A, Graber EM (2012) Over-the-counter acne treatments: a review. J Clin Aesthet Dermatol 5(5):32–40
Del Rosso JQ (2009) Moisturizers: functions, formulation, and clinical applications. In: Draelos ZD (ed) Cosmeceuticals. Saunders Elsevier, Philadelphia, pp 97–102
Del Rosso JQ, Levin J (2011) The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol 4(9):22–42
Del Rosso JQ (2011) Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF (eds) Acne vulgaris. Informa Healthcare, London 95–104
Del Rosso JQ (2013) Clinical relevance of skin barrier changes associated with the use of oral isotretinoin: the importance of barrier repair therapy in patient management. J Drugs Dermatol 12:626–631
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 107(26):11971–11975
Downing D, Stewart M, Wertz P, Strauss J (1986) Essential fatty acids and acne. J Am Acad Dermatol 14:221–225
Draelos ZD, Ertel KD, Berge CE (2006) Facilitating facial retinization through barrier improvement. Cutis 78:275–281
Elias PM (1986) Epidermal effects of retinoids: supramolecular observations and clinical implications. J Am Acad Dermatol 15(4 Pt 2):797–809
Farrah G, Tan E (2016) The use of oral antibiotics in treating acne vulgaris: a new approach. Dermatol Ther 29(5):377–384
Findley K, Grice EA (2014) The skin microbiome: a focus on pathogens and their association with skin disease. PLoS Pathog 10(10):e1004436
Fitz-Gibbon S, Tomida S, Chiu BH et al (2013) Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol 133:2152–2160
Gollnick H, Cunliffe W, Berson D et al (2003) Management of acne: a report from the global alliance to improve outcomes in acne. J Am Acad Dermatol 49(Suppl 1):S1–S38
Grice EA, Kong HH, Renaud G et al (2008) A diversity profile of the human skin microbiota. Genome Res 18:1043–1050
Grice EA, Segre JA (2011) The skin microbiome. Nat Rev Microbiol 9:244–253
Haustein UF, Tegetmeyer L, Ziegler V (1985) Allergic and irritant potential of benzoyl peroxide. Contact Dermat 13(4):252–257
Heughebaert C, Shalita A. Comedogenesis (2011) In: Shalita AR, Del Rosso JQ, Webster GF (eds) Acne vulgaris. Informa Healthcare, New York, pp 28–42
Hsieh M, Chen C (2011) Delivery of pharmaceutical agents to treat acne vulgaris: current status and perspectives. J Med Biol Eng 32(4):215–224
Hui A, Shalita A (2011) Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF (eds) Acne vulgaris. Informa Healthcare, London, pp 86–94
Jarrousse V, Castex-Rizzi N, Khammari A et al (2007) Modulation of integrins and filaggrin expression by Propionibacterium acnes extracts on keratinocytes. Arch Dermatol Res 299:441–447
Jasson F, Nagy I, Knol AC, Zuliani T, Khammari A, Dréno B (2013) Different strains of Propionibacterium acnes modulate differently the cutaneous innate immunity. Exp Dermatol 22:587–592
Kong HH (2011) Skin microbiome: genomics-based insights into the diversity and role of skin microbes. Trend Mol Med 17:320–328
Krishna S, Kim C, Kim J (2011) Innate immunity in the pathogenesis of acne vulgaris. In: Shalita AR, Del Rosso JQ, Webster GF (eds) Acne vulgaris. Informa Healthcare, London, pp 12–27
Kumar B, Pathak R, Mary PB et al (2016) New insights into acne pathogenesis: exploring the role of acne-associated microbial populations. Dermatol Sin 34(2):67–73
Kurokawa I, Mayer-da-Silva A, Gollnick H et al (1988) Monoclonal antibody labeling for cytokeratins and filaggrin in the human pilosebaceous unit of normal, seborrhoeic and acne skin. J Invest Dermatol 91:566–571
Marples RR, Downing DT, Kligman AM (1971) Control of free fatty acids in human surface lipids by Corynebacterium acnes. J Invest Dermatol 56:127–131
Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG (2015) The infant microbiome development: mom matters. Trend Mol Med 21(2):109–17
Muszer M, Noszczyńska M, Kasperkiewicz K, Skurnik M (2015) Human microbiome: when a friend becomes an enemy. Arch Immunol Ther Exp (Warsz) 63(4):287–98
Naik S, Bouladoux N, Wilhelm C et al (2012) Compartmentalized control of skin immunity by resident commensals. Science 337:1115–1119
Nakamura M, Kametani IS, Higaki S, Yamagishi T (2003) Identification of Propionibacterium acnes by polymerase chain reaction for amplification of 16S ribosomal RNA and lipase genes. Anaerobe 9(1):5–10
Ottaviani M, Alestas T, Flori E, Mastrofrancesco A, Zouboulis CC, Picardo M (2006) Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: a possible role in acne vulgaris. J Invest Dermatol 126:2430–2437
Pappas A, Fantasia J, Chen T (2013) Age and ethnic variations in sebaceous lipids. Dermato-endocrinology 5:319–324
Pappas A, Johnsen S, Liu JC et al (2009) Sebum analysis of individuals with and without acne. Dermato-endocrinology 1(3):157–161
Pathak R, Kasama N, Kumar R, Gautman HK (2013) Staphylococcus epidermidis in human skin microbiome associated with acne: a cause of disease or defence? Res J Biotech 8(12):78–82
Plewig G, Kligman AM (2000) Sebaceous glands. In: Plewig G, Kligman AM (eds) Acne and rosacea, vol 70, 3rd edn. Springer, Berlin
Ritvo E, Del Rosso J, Stillman MA et al (2011) Psychosocial judgments and perceptions of adolescents with acne vulgaris: a blinded, controlled comparison of adult and peer evaluations. Biopsychosoc Med 5:11–26
Rosenthal M, Goldberg D’Aiello A et al (2011) Skin microbiota: microbial community structure and its potential association with health and disease. Infect Genet Evol 11:839–848
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14(8):e1002533
Smith RN, Braue A, Varigos GA, Mann NJ (2008) The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci 50:41–52
Solomon BA, Shalita AR (1996) Effect of detergents on acne. Clin Dermatol 14:95–9
Subramanyan K (2004) Role of mild cleansing in management of patient skin. Dermatol Ther 17:26–34
Tagami H (2008) Location-related differences in structure and function of the stratum corneum with special emphasis on facial skin. Int J Cosmet Sci 30:413–434
Tanghetti EA, Popp KF (2009) A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin 27:17–24
Tanghetti E, Dhawan S, Green L et al (2010) Randomized comparison of the safety and efficacy of tazarotene 0.1% cream and adapalene 0.3% gel in the treatment of patients with at least moderate facial acne vulgaris. J Drugs Dermatol 9(5):549–58
Thiboutot D, Del Rosso JQ (2013) Acne vulgaris and the epidermal barrier. Is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? Do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol 6(1):18–24
Thiboutot D, Gilliland K, Light J (1999) Androgen metabolism in sebaceous glands from subjects with and without acne. Arch Dermatol 135:1041–1045
Toyoda M, Morohashi M (2001) Pathogenesis of acne. Med Electron Microsc 34(1):29–40
Weber SU, Thiele JJ, Han N et al (2003) Topical tocotrienol supplementation inhibits lipid peroxidation but fails to mitigate increased transepidermal water loss after benzoyl peroxide treatment of human skin. Free Radic Biol Med 34:170–176
Webster GF, Kim J (2008) Immunology of acne. In: Gaspari AA, Tyring SK (eds) Clinical and basic immunodermatology. Springer, London, pp 217–222
Wolf JE Jr (2002) Potential anti-inflammatory effects of topical retinoids and retinoid analogues. Adv Ther 19(3):109–118
Yamamoto A, Takenouchi K, Ito M (1995) Impaired water barrier function in acne vulgaris. Arch Dermatol Res 287(2):214–218
Yazici K, Baz K, Yazici A (2004) Disease-specificquality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol 18:435–439
Zhang YQ, Ren SX, Li HL et al (2003) Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol Microbiol 49:1577–1593
Zouboulis CC (2004) Acne and sebaceous gland function. Clin Dermatol 22:360–366
Zouboulis CC, Schagen S, Alestas T (2008) The sebocyte culture: a model to study the pathophysiology of the sebaceous gland in sebostasis, seborrhoea and acne. Arch Dermatol Res 300:397–413
Related articles recently published in Archives of Dermatological Research (selected by the journal’s editorial staff):
Kong F, Galzote C, Duan Y (2017) Change in skin properties over the first 10 years of life: a cross-sectional study. Arch Dermatol Res. https://doi.org/10.1007/s00403-017-1764-x
Li WH, Zhang Q, Flach CR, Mendelsohn R, Southall MD, Parsa R (2017) In vitro modeling of unsaturated free fatty acid-mediated tissue impairments seen in acne lesions. Arch Dermatol Res. https://doi.org/10.1007/s00403-017-1747-y
Maguire M, Maguire G (2017) The role of microbiota, and probiotics and prebiotics in skin health. Arch Dermatol Res 309:411–421
Patwardhan SV, Richter C, Vogt A, Blume-Peytavi U, Canfield D, Kottner J (2017) Measuring acne using Coproporphyrin III, Protoporphyrin IX, and lesion-specific inflammation: an exploratory study. Arch Dermatol Res 309:159–167
Younis S, Blumenberg M, Javed Q (2016) Resistin gene polymorphisms are associated with acne and serum lipid levels, providing a potential nexus between lipid metabolism and inflammation. Arch Dermatol Res 308:229–237
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest.
Funding
None.
Rights and permissions
About this article
Cite this article
Rocha, M.A., Bagatin, E. Skin barrier and microbiome in acne. Arch Dermatol Res 310, 181–185 (2018). https://doi.org/10.1007/s00403-017-1795-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-017-1795-3