Abstract
Acne is one of the most common skin inflammatory diseases, representing the top three most prevalent skin conditions in the general population worldwide (Johnson and Roberts, Vital Health Stat 11 (212):i–v, 1–72, 1978; Wolkenstein et al., Arch Dermatol 139(12):1614–9, 2003; Rea et al., Br J Prev Soc Med 30(2):107–14, 1976; Bhate and Williams, Br J Dermatol 168(3):474–85, 2013). The complex physiopathology of this dermatosis involves several key players: sebaceous gland, activation of innate immunity and microbiome with a commensal bacteria Cutibacterium acnes. In this book chapter, we propose to give an overview of the most current findings about acne physiopathology and future medical challenges ahead. This chapter will focus on the commensal bacterium Cutibacterium acnes, as it has a pivotal role in acne physiopathology, its interaction with the different microorganisms constituting skin microbiota and the innate immune system. Based on the scientific literature, this book chapter will also review the innovative therapeutic options that will be developed in the future years.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
This book chapter focuses on Cutibacterium acnes, which is a commensal bacterium of the cutaneous microbiome, playing a crucial role in acne development [1,2,3,4]. This chapter will first precisely describe the identity passport of this bacterium and then focus on the interactions existing between C. acnes and the other microorganisms’ resident of the human skin, mainly Staphylococcus epidermidis. This chapter will then describe the interactions existing between C. acnes and the innate immune system of the skin and finally will open on the future potential treatments that will be developed in the next years, to treat acne.
Cutibacterium acnes (Ex – Propionibacterium acnes) Identity Passport
The skin represents a complex ecosystem [5]. A large and diverse community of microorganisms is present on the body. Depending on the ecological niches, the bacterial distribution can vary [6]. Thus, in a lipidic area, Actinobacteria are more represented, and Cutibacterium acnes can represent until 70% [7]. This anaerobic-aerotolerant Gram-positive bacteria is a skin commensal, and its ecological niche is represented by the sebaceous follicles [8,9,10].
Bacteriological Description
Initially, C. acnes was classified as a Corynebacterium [11]. According to the recent literature, the microscopy morphology can be diverse leading to different subtypes [12,13,14]. By direct microscopy examination, the historical phylotypes I, II and III are somehow different [12, 15, 16]. New insights from integration of population community’s analysis, genomic studies and biochemical and host-microorganism interactions lead to a better knowledge of this bacterium involved in inflammatory process [17, 18].
Ecological Niches
C. acnes is a major resident of the normal human skin microbiota and dominates in the pilosebaceous units which can be explained by production of different enzymes [19,20,21]. It can interact with other microorganisms, especially Staphylococcus epidermidis playing an important role in the skin health, educating the innate immune system and maintaining the skin homeostasis [22]. S. epidermidis could be a partner in the pathogenesis of acne, producing antimicrobial substances (bacteriocins) active against C. acnes leading to a disruption (dysbiosis) of the normal skin homeostasis equilibrium [23]. Its involvement in skin disorder, especially acne, has been described, but we also can recover isolates from mouth, gastrointestinal tract, prostate and device-related infections [14].
Taxonomy Modification
Following its discovery in a patient with acne, P. acnes, henceforth C. acnes, underwent a series of taxonomic changes. It was successively placed in the genus Bacillus, followed by Corynebacterium [11]. However, in 1946, Douglas and Gunter were able to demonstrate that this microorganism was more closely related to the Propionibacterium genus members since, like other species of this genus, it ferments lactose to propionic acid in an anaerobic atmosphere maintaining an acid pH on the skin surface and limiting pathogen development [24, 25]. Recently, a significant taxonomic revision was proposed by Scholz et al., placing all Propionibacterium species from the skin microbiota within this new genus Cutibacterium [25]. Henceforth, the main actor of the sebaceous follicles should be named Cutibacterium acnes. Recently, according to the three main phylotypes described at the beginning, subspecies have been proposed. Thus, phylotype I corresponds to the subspecies C. acnes subsp. acnes [26], phylotype II corresponds to the subspecies C. acnes subsp. defendens [27] (due to the presence of a CRISPR system limiting gene transfer or acquisition) [28] and phylotype III corresponds to subspecies C. acnes subsp. elongatum according to its microscopy morphology [26].
Phylogeny
Since 2005, different groups have developed molecular tools to identify if possible clusters or lineages are more involved in different specific diseases. At the beginning, the role of specific C. acnes subgroups in the physiopathology of these diseases was conducted with antibodies [15]. Using different targets such as tly or recA genes, several groups developed different molecular typing methods [29]. Thereafter, phylotype multiplex PCR, different multi-locus sequence typing schemes and a useful single-locus sequence typing method which can be performed directly from samples have been proposed [30,31,32,33]. Nevertheless, to compare the phylogeny of clinical isolates recovered during different diseases, we proposed a consensus with an algorithm to identify subtypes of C. acnes by molecular typing methods [34]. Thus, in moderate to severe acne, different studies have proven to have highly prevalence in skin inflammatory swab specimens of phylotype IA1 [35,36,37,38,39,40,41]. At the opposite, for example, another skin disease is linked to an overrepresentation of phylotype III: progressive macular hypomelanosis [42, 43].
Growth Culture Conditions
Conventional microbial culture of C. acnes from skin samples requires some attention, but in a well-trained microbiology laboratory, it remains easy. Different media can be used, sometimes with supplementation with tween, for example [14]. Schaedler agar, Brucella agar, or chocolate agar plates can be seeded and incubated anaerobically for at least 7–10 days at 37 °C [13]. In acne lesions, different colony aspects can be observed regarding colour and haemolysis [44].
Virulence Factors
C. acnes is able to produce numerous virulence factors [45]. Thus, it produces short-chain fatty acids (leading to a local inflammation); thiopeptides; bacteriocins [46]; degradative enzyme such as lipases [20], endoglyceramidases, sialidase and hyaluronidase [21]; and other molecules with inhibitory properties against pathogens such as Staphylococcus aureus or Streptococcus pyogenes. C. acnes is able to trigger innate immune system via Toll-like receptor 2 (TLR-2) activation. Different TLR-2 ligands can be involved in this immune stimulation: lipoteichoic acids and peptidoglycan fragments [45] but also cell surface proteins like Christie-Atkins-Munch-Petersen (CAMP) factors which have co-haemolytic activity and cytotoxin properties [47, 48]. C. acnes lipase has a crucial role in hydrolysing triglycerides of sebum leading to the release of irritating fatty acids within pilosebaceous follicles which partly explain acne pathogenesis [13]. Interestingly, phylotype IA1 recovered in 80% of acne lesion produces more lipase than other phylotypes [49].
Hyaluronidase is another extracellular enzyme implicated in the bacterial pathogenesis (involvement in penetrating the extracellular matrix) leading to total hyaluronic acid degradation for HYL-IB/II variant versus a partial degradation for the HLY-IA variant [13, 21, 50]. Certain C. acnes strains, especially those involved in acne, belonging to phylotype I can produce haemolysins with cytotoxin properties. Valanne et al. demonstrated the presence of the five CAMP factors in the different C. acnes subgroups. However, the camp2 gene seems to be the most relevant and active co-haemolytic factor but in the IA phylotype C. acnes genetic background [13, 44, 47]. At last, the ability of C. acnes clinical strains to produce biofilm has been largely investigated, especially in device-related infections [51, 52]. In acne field, in 2008, Coenye et al. suggested the impact in acne of sessile C. acnes cells either highly resistant to antimicrobial agents or tolerant to with potential increased production of virulence factors and quorum sensing molecule regulation [53]. In biofilm condition, lipase has a greater extracellular activity [8]. In 2012, the presence of C. acnes macrocolonies within the pilosebaceous follicles has been described. Interestingly, different phylotypes were contained and coexisted [54]. Recently, Kuehnast et al. suggested that biofilm formation correlates with the phylotype, rather than the anatomical isolation site. In their model, phylotype IA1 (SLST types A1 and A2) demonstrated higher biofilm production [55].
Resistance in Acne Context
C. acnes is susceptible to a large range of antibiotics [14]. Nevertheless, in acne context, antibiotics should be used for a short treatment period. Indeed, from 1979, the first resistant strains have been reported [56]. Henceforth, erythromycin resistance is largely higher than tetracycline one [57, 58]. According to antibiotic treatment habits, the epidemiological resistance of C. acnes is different: topical or systemic treatment, doses, combination, duration, etc. Thus, macrolide resistance rate can vary from less than 25% in Columbia to almost 90% in Spain [57]. The tetracycline situation is better with less than 10% in France to almost 50% in India [57]. The mechanism involved in these resistances is systematically point mutation in the chromosomal gene targets: 23S encoding gene and to a lesser extent L4 or L22 proteins for macrolides and 16S encoding genes for tetracycline [14]. Recently, in Japan, the impact of fluoroquinolone topical use has been reported with the emergence of resistant C. acnes strains [59] but also a worrying problem linked to the collateral damages with the impact on resistance in the microbiota and therefore Staphylococcus epidermidis fluoroquinolone selection [60].
Acne in the Genomic Era
As the skin ecosystem is a dynamic and evolving environment with numerous bacterial interactions, genomic, transcriptomic and metabolomic approaches will help us better understand the role of these specific bacterial communities in acne pathogenesis and inflammation (Table 1.1).
Cutibacterium acnes and Cutaneous Microbiome Interactions
The human skin microbiome is a unique and complex mixture of different groups of microorganisms. Human skin harbours bacteria (anaerobic, aerotolerant, or facultative anaerobic), virus, fungi and bacteriophages. Interspecies cross talks exist between these cutaneous microbial communities. These interactions take place through different ways, notably growth regulation, quorum sensing, biofilm synthesis regulation and extracellular vesicles exchanges. This fragile balance between growth and inhibition of each cutaneous species is the guarantor of skin homeostasis and functional skin barrier.
First of all, growth regulation is possible through the production of certain type of bioactive molecules able to kill and/or inhibit the growth of certain bacteria. To illustrate this phenomenon, Christensen et al. showed that Staphylococcus epidermidis strains possess an arsenal of mechanisms to inhibit C. acnes growth. These growth regulations result from the production of bioactive molecules called bacteriocins, such as the epidermin produced by S. epidermidis in that case [22]. These molecules act on the cytoplasmic membrane of Gram-positive bacteria. Another example of bioactive molecule is gallidermin. This molecule was successfully tested in a topical formulation on rat skin showing antibacterial potential against C. acnes and S. aureus [61]. Another example was reported by Wang et al. concerning the inhibitory potential of C. acnes on the growth of methicillin-resistant Staphylococcus aureus , using an in vitro model [62].
Secondly, quorum sensing (QS) is a way to communicate between bacteria enabling the regulation of bacterial gene expression in response to changes in cell density. It permits them to sense bacterial numbers among their population (cell density), integrate and process the environmental parameters and synchronously alter their behaviour by expressing specific target genes [63, 64]. Nowadays, more and more evidences relate interspecies, inter-genera and inter-kingdom communications using largely diffusible small molecules named “quoromones” or “autoinducers” [65]. In Gram-positive bacteria such as Cutibacterium acnes, these molecules are often oligopeptides [65]. On the clinical point of view, it was recently suggested that QS mutants of human pathogens were attenuated for virulence [66, 67] quickly leading to the concept of using QS inhibitors to control some diseases [63]. Then, QS appears as a way to regulate microbial populations among skin microbiome, as previously suggested [68], and even more could be involved in the physiopathology of dermatoses such as acne [69].
Then, interspecies interactions are also described through biofilm synthesis regulation. This kind of mechanism was previously reported between Staphylococcus aureus and C. acnes [70]. In this study, authors demonstrated that C. acnes may have an effect on the behaviour of S. aureus. This study suggests that C. acnes may produce a factor or provide a promoting environment for staphylococcal biofilm formation. Since coproporphyrin III is known to induce S. aureus aggregation in cutaneous isolates, it is possible that this molecule could also induce biofilm formation or there may be a different mechanism currently not described [71].
Finally, extracellular vesicle (EV) exchanges are nowadays considered as a crucial player in bacteria communications [72]. All bacteria are capable of producing this type of natural messenger, including Gram-positive ones [73]. Recently, C. acnes was described as able to produce EVs [74]. These bacterial EVs enable the communication between bacteria themselves but also between them and host cells such as keratinocytes in cutaneous context, notably via TLR2-mediated signalling pathways [75]. Indeed, Choi et al. described that the entry of C. acnes-derived EVs into keratinocytes is mediated by clathrin-dependent endocytosis, and this way, the internal cargo of these EVs can be delivered into keratinocytes. In this example, Choi et al. demonstrated that C. acnes-derived EVs were able to induce an acne-like phenotype in keratinocytes and confirmed their results in a reconstituted human epidermis model. In addition, one specific study reports the possible regulation between bacterial populations from different microbiotas using EV pathway, to protect the skin from inflammation induced by a pathogen. Indeed, it was previously reported that EVs from Lactobacillus plantarum, which is a commensal found in digestive tract, were able to protect from atopic dermatitis induced by S. aureus-derived EVs. Clinical applications are then suggested using L. plantarum-derived EVs, based on their modulation potential towards cutaneous pathogens like S. aureus. Another clinical outlook was suggested in the literature, based on the inhibition of the release of EVs from C. acnes to avoid inflammatory cytokine releases from keratinocytes and acne phenotype occurrence [75].
Taken together, these elements of the literature underline the importance of appropriate interspecies cross talks. Indeed, an imbalance in these microbial interactions could potentially jeopardize the relationships between skin microbiota and host cells and may result in skin inflammatory diseases where dysbiosis is often cited as a potent actor.
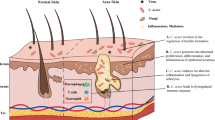
Skin microbiota appears as a complex and multifactorial organ part of the skin, for which modulation is nowadays thought to be able to treat inflammatory dermatoses, as recently suggested in acne context [76]. Indeed, as antibiotic resistance is an increasing phenomenon especially in acne disease [77, 78], probiotic solutions are nowadays considered as an interesting alternative to antibiotic treatments and also a new option added to the current therapeutic arsenal of clinicians (Fig. 1.1).
Cutibacterium acnes and cutaneous microbiome interactions. Summary of the different interactions existing between C. acnes and the different skin microbial communities. (a) Growth regulations are mediated through different bioactive molecules (epidermin, gallidermin). (b) Quorum sensing is one of the pathways possible for interaction between bacteria. (c) Interactions between C. acnes and skin microbiota also take place through biofilm synthesis. Indeed, recent studies reported that C. acnes-derived biofilm was able to promote biofilm synthesis by S. aureus. (d) Extracellular vesicles are able to carry signals promoting interspecies communications and also host/microbiota communications
Cutibacterium acnes and Innate Immunity
The skin with its microbiome develops a wide range of innate immune responses to protect the body against infection. In contrast to the gut microbiome that is physically separated from the epithelium by a dense mucus layer in the colon, the skin microbiome is in close contact with the epidermis. It is important that the immune response is primed to recognize and tailored to respond to an appropriate threat, as any immune reaction towards commensal agents could lead to chronic disease. Keratinocytes and sebocytes are the main cell types of the epidermis and actively participate in innate immunity, as a source of antimicrobial peptides and cytokines that trigger inflammation when the epithelium is exposed to damage-/pathogen-associated molecular patterns (D/PAMP), mainly represented by Toll-like receptor 2, 4 and 6 (TLR) ligands and protease-activated receptor (PAR)-2 ligands that link with the corresponding receptors expressed on/in the keratinocytes and sebocytes [79]. The activation of innate immunity seems different according to the type of the skin and phylotype of C. acnes. In one study, type IC isolated in the normal skin would induce higher secretion of IL-8 in keratinocytes than type IA [80]. In contrast, types IA and IB of C. acnes were found to induce greater levels of the human β-defensin 2 (hBD2) from cultured sebocytes than a type II isolate [81, 82] which demonstrated that C. acnes type III had the highest pro-inflammatory potential by upregulating the expression of PAR-2, TNF-alpha, MMP-13 and TIMP-2, whereas Cutibacterium avidum had the weakest by upregulating only MMP-13 and TIMP-2 [82].
C. acnes can induce IFN-γ from NK cells by mechanism involving the release of RNA and an innate pathway dependent on activation of TLR8 and the secretion of IL-12p40 and IL18 [83]. In addition of IL-8, in the process of inflammation triggered by C. acnes, secretion of IL-1β by monocytes and sebocytes throughout the activation of the key inflammasome gene NLRP3 has been observed [84]. This mechanism is regulated by proteases and reactive oxygen species. Moreover, C. acnes promotes mixed Th17/Th1 responses by inducing the concomitant secretion of IL-17A and IFN-γ from specific CD4+T cells in vitro. Therefore, the presence of IL-17A-positive T cells and the activation of Th17-related cytokines in acne lesions indicate that the Th17 pathway may play a pivotal role in the disease process, possibly offering new targets of therapy [85]. Recently it has been shown that IL-17 was increased in the serum of acne patients [86]. In addition of cytokines, antimicrobial peptides (AMPs) are important modulator of cutaneous inflammation and belong to the innate immunity. There is strong evidence that AMP plays a role in the pathogenesis of inflammatory acne lesions. Skin-derived AMPs comprise the family of β-defensins, S100 proteins, RNases and the cathelicidin LL-37. While some AMPs are constitutively secreted, hBD-2 and hBD-3 and LL-37 are upregulated in acne lesions and induced by culture supernatants of C. acnes in vitro both in keratinocytes [48] and in sebocytes [87]. RIS-1/psoriasin is an epithelial antimicrobial peptide, whose expression is upregulated in inflammatory skin diseases including acne and is induced by retinoids. Inflammation modifies the compartmentation of RIS-1/psoriasin in sebaceous glands and the follicular root sheaths with an increase of its expression, thus making this AMP a new target of acne treatments [88].
Acne is associated with scar development in many patients. Recently, we showed that in the skin of acne patients prone to scars versus not prone to scars, TLR-4, IL-2, IL-10, TIMP-2 and JUN were significantly overexpressed and the MMP-9 protein level was decreased. Similar results were obtained in inflammatory papules, except for TLR-4. Thus, these results suggest a link between the early events of inflammation with levels of activation of innate immunity in the normal epidermis of acne patients and the development of scars showing how crucial it is to treat inflammation in acne to prevent the development of scars [89]. TGF-β1 could also play a role in the development of scars as it is strongly elevated in lesions of acne patients who were prone to scars [90].
A crucial question in the microbiome field is why do cells switch from a state of immunological tolerance to a chronic inflammatory state in the absence of an infection. In the case of acne development, a dynamic shift in the microenvironment of the follicle induced by hyperseborrhea can trigger a different transcriptional response of the microbiome. Thus, culturing C. acnes in a lipid-rich, hypoxic environment similar to that of an occluded hair follicle promotes anaerobic fermentation and production of short-chain fatty acids that activate an epigenetic mechanism to enhance the TLR2-mediated production of IL-6, IL-8 and TNFα in human keratinocytes [91] (Fig. 1.2) .
What Alternatives in the Future?
The development of new treatments against pathology requires a good and strong knowledge of the physiopathology and the pathways involved in order to better target the factors involved in the pathology and by inducing few side effects. Currently, the exact pathophysiological mechanisms of acne are only partially known. The predominant involvement of C. acnes is questionable since the latest knowledge shows that acne state and induced inflammation are governed by complex association of multiple factors. These factors mainly depend on the microbiological microenvironment, gender, age and individual intrinsic factors.
In the current therapeutic arsenal, the management of acne varies mainly according to acne severity. Management algorithms are published [92] including topical treatments (antibiotics, retinoids, benzoyl peroxide and salicylic/azelaic acids) and systemic treatments (antibiotics, retinoids, zinc) [93]. Some studies pointed that the main research goal of acne treatment is to target C. acnes and the induced inflammatory status, the sebum hypersecretion and hyperkeratinization [94]. In parallel, antibiotics modulate C. acnes and have an anti-inflammatory effect [95]. Benzoyl peroxide and azelaic acid inhibit C. acnes colonization and have comedolytic and anti-inflammatory/antibacterial effects [96,97,98]. Oral retinoids or isotretinoin are more likely used to treat severe acne. These molecules impact on sebum production and regulate C. acnes/TLR-2-mediated innate immune response [99]. Systemic retinoids might indirectly regulate skin microbes and reduce the number of C. acnes, inducing changes in microbial diversity [93, 100].
Despite some proven efficacy of current treatments, cutaneous side effects of topical products, systemic effects as for isotretinoin, antibiotic-induced bacterial resistance and acne chronicity encourage the research to explore targeted therapies, respecting the microbiome diversity and inducing fewer side effects. Currently, there are four main axes in development: probiotics, vaccines, phages and antimicrobial peptide therapies.
Microbiome and Probiotics Approach
The use of antibiotic therapy to eliminate, as a priority, C. acnes considered for a long time as major acne agent is less and less recommended especially in oral monotherapy [92] for at least two major reasons: development of resistance to antibiotics and disruption of the skin and gut microbiome (bacterial diversity loss) which is a crucial condition in normal healthy status. Furthermore, it is known that phylotype IA1 is overrepresented and involved in moderate to severe acne [37,38,39]. In parallel, dysbiosis in acne patient is associated with a decreased number of S. epidermidis which is able to control C. acnes proliferation via releasing of succinic acid and fatty acid fermentation product [23]; this way, the systematic eradication of C. acnes no longer seems a relevant strategy. In consequence, it will now be necessary to take into account the other types of bacteria that constitute the skin microbiome. The steady state of the microbiome and its preservation is complex and little known. Recently, data from a clinical study showed that Propionibacteriaceae and Staphylococcaceae family were significantly overrepresented respectively in healthy controls and acne patient [101].
Without targeting only C. acnes, the new research orientations aim at the development new per os treatments or topical formulations based on probiotics. These innovative approaches aim to restore skin microbiome diversity and eliminate pathogenic species and induced inflammation in acne and other inflammatory diseases [79, 93, 102].
Recent knowledge demonstrated that microbial dysbiosis in the skin and the gut was implicated in many chronic inflammatory diseases. The improvement of dysbiosis and restoration of a normal skin microbiome are promising therapeutic strategies that have been tested in intestinal dysbiosis by oral administration of probiotics, living microorganisms that are beneficial to the host’s health or by faecal transplantation with a pill which encapsulates stool of a healthy donor containing its intestinal microbiota. Faecal transplantation has been used in Clostridium difficile infections, in the irritable bowel syndrome or in inflammatory colitis. The faecal microbiome transplants have been demonstrated to be safe and effective for patients with Clostridium difficile infections [103].
The therapeutic approach for cutaneous dysbiosis is currently poorly developed, and some trials have been conducted in inflammatory conditions such as atopic dermatitis, psoriasis and acne [76, 104]. Topical treatment consisting of the commensal bacterium Vitreoscilla filiformis used in patients with atopic dermatitis showed significant clinical improvement with decreasing SCORAD (scoring atopic dermatitis) score and pruritus [104]. Moreover, the approach based on specific bacterial strains selected from the skin microbiome to treat atopic dermatitis patients has been shown to eliminate S. aureus and restore a balanced microbiome [105].
Some data have shown that probiotics could induce C. acnes inhibition with antimicrobial proteins such as Streptococcus salivarius which suppresses the growth of C. acnes by secreting a bacteriocin-like inhibitory substance [106]. Topical treatment with cream containing Streptococcus thermophiles was shown to display antimicrobial activity against C. acnes by ceramide production [107]. Probiotics could also act on immune response by inhibiting pro-inflammatory cytokine IL-8 from keratinocytes [108], by suppression of substance P-induced skin inflammation [109].
Some clinical trials have been conducted in acne patients to investigate the clinical benefit of probiotics [93]. Topical Enterococcus faecalis treatment has shown significant reduction of inflammatory acne lesions versus placebo [110]. Lactobacillus plantarum treatment also induces a decrease of acne severity and associated erythema [111]. Interestingly, association of freeze-dried Bifidobacterium bifidum and L. acidophilus used as a supplement to acne treatment showed greater resolution of acne compared with the non-supplemented group [112].
The new concept in acne drug development, despite C. acnes implication in acne, takes into account that C. acnes might also play a protective role in the skin by preserving a permanent low level of innate immunity activation, and thus therapeutic options that respect C. acnes equilibrium are an adequate alternative to treat acne [94]. An ongoing clinical study investigates the role of the skin microbiome and the potential use of a topical probiotic cream (YUN ACN cream) for acne treatment [113].
Recently some data postulated the beneficial effect of S. epidermidis in the physiopathology of acne by limiting C. acnes-induced colonization of the skin and inflammation [23]. However, overexpression of S. epidermidis could induce nosocomial infections. Therefore, to respect the balanced skin homeostasis, future treatments may be based on probiotics derived from S. epidermidis to allow a restoration of the normal skin microbiota and to target the regulation of the host’s AMP mediators, without increasing S. epidermidis population [23].
Phage Therapy Approach
The development of phage therapy in acne would be suitable to target the specific C. acnes strain implicated in acne and preserve microbiome diversity profile of the healthy skin. This is based on the fact that in acne patients, skin C. acnes phages are more present than in the skin from healthy patients [18] and that an increase amount of phage with increasing age would be related to disappearance of acne in older individuals. Bacteriophages, the least understood component of the human microbiome, are viruses that can infect and kill bacteria. Interestingly it has been shown that type I strains of C. acnes appear to be more susceptible to phage infections compared to those from the type II phylogroup [114]. This interesting effect of phage on C. acnes type I has recently been confirmed and more detailed by Liu et al. who challenged genetically distinct C. acnes strains with 15 different phages and found that strains from types IA1 and IA2 phylogroups were more sensitive to infection, while those from types IB, II and III phylogroups appeared to be more resistant [18].
These data suggest that antiviral strategies based on certain strains of C. acnes could normalize the cutaneous microbiota and allow a potential personalized therapy based on a well-selected phage. While this approach seems to be attractive, few data are available on phage treatments essentially in acne.
Vaccine Approach
C. acnes is able to produce many virulence factors which are either secreted or anchored in the cell wall and which stimulate adjacent host cells, triggering inflammation and cell damages. Among them is the CAMP factor, a secretory virulence factor that constitutes an essential source of inflammation in acne physiopathology [115].
The various C. acnes phylotypes release various CAMP factors which could explain the pathogenic potential of the different phylotypes. The genome of C. acnes contains five genes encoding five CAMP homologs including CAMP factor 2, a major active co-haemolytic factor of C. acnes [116].
It has been shown that C. acnes CAMP factor is immunogenic [117] and that mice vaccinated by CAMP factor overexpressed in Escherichia coli experienced therapeutic protection against C. acnes [117,118,119]. Furthermore, the mutation of CAMP factor leads to a less effect on the inflammation induced by C. acnes in mice, demonstrating the essential role of CAMP factor in the cytotoxicity of C. acnes [115]. Incubation of ex vivo acne explants with an antibody targeting CAMP factor has shown to decrease IL-8 and IL-1β, usually expressed at higher levels in acne lesions. It has also been published that vaccination approach by using surface sialidase [120] or heat-killed C. acnes [121] as an antigen significantly decreases the inflammation induced by C. acnes.
All these data bring a valuable rational to consider the vaccination using C. acnes CAMP factor as a promising target for acne immunotherapy. As C. acnes phylotype IA1 is widely known to be associated with acne, in parallel, higher expression of CAMP2 was detected in phylotype IA compared with other phylotypes, CAMP2 seems to be the best eligible and the most effective virulence factor to be targeted by the vaccine strategy.
It has been suggested that as CAMP2 is expressed by all other strains, it also might be important for the normal existence of the commensals that vaccination targeting CAMP2 may also affect C. acnes strains involved in the skin homeostasis and could induce colonization by pathogenic agents. Consequently, the ideal vaccination targets should be highly specific to avoid unwanted side effects due to the elimination of the needed bacteria. Although it is currently admitted that C. acnes phylotype IA1 is highly associated with acne, recently our group demonstrated that acne severity would rather be dependent on the basal level of active innate immunity in patients prone to severe acne [36, 89]. Moreover, recent studies reported that severe acne was associated with an important C. acnes phylotype diversity loss and that this diversity loss was capable of inducing a cutaneous inflammatory response [37, 122]. Considering these data, it may be more suitable and relevant to target secreted virulence factors than focusing on vaccination strategy aiming to eradicate C. acnes or targeting a surface antigen. The specific inhibition of secreted virulence factors should limit the risk of unwanted targeting of nonpathogenic bacteria and overcome a possible selection of resistant bacteria [116].
Although CAMP2 vaccination approach seems to be attractive, complementary studies are needed to investigate the effects of such vaccination on the microbiota and also to demonstrate that such approach will not induce bacterial dysbiosis, leading to cutaneous pathologies.
Conclusion
In the last 3 years, a lot of new data have been associated with C. acnes deeply changing the pathophysiology of acne. First, it changed the name from P. acnes to C. acnes. Its role as commensal bacteria is more and more well-known. In addition, at the same time, its role in the pathophysiology of acne has also evolved. C. acnes is now well recognized as able to produce numerous virulence factors and thus to be one of the most pro-inflammatory bacteria of the skin. Moreover, the six main different phylotypes of C. acnes are able to activate differently the innate immunity which continually interacts with C. acnes through cytokines, antimicrobial peptides and specific receptors expressed by keratinocytes and other skin cells (TLR, PAR). Until recently, the severity of inflammatory lesions in acne was considered directly related to the proliferation of the bacteria. But now, the inflammation is considered in link with the severity of the dysbiosis of the microbiome with a diversity loss of the phylotypes of C. acnes combined with the overrepresentation of the phylotype IA1. At the therapeutic level, the consequences are crucial as the objective of innovative treatments is not to eradicate C. acnes but to rebalance the microbiome to make it as close as possible of the microbiome of a normal skin. Consequently, new approaches with vaccines, antimicrobial peptides, probiotics, and phage therapy are developed in acne.
Abbreviations
- AMP:
-
Antimicrobial peptide
- CAMP:
-
Christie-Atkins-Munch-Petersen (e.g. CAMP2, etc.)
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- D/PAMP:
-
Damage-/pathogen-associated molecular pattern
- EVs:
-
Extracellular vesicles
- hBD2:
-
Human β-defensin 2
- HYL-IA:
-
Variant of hyaluronidase (HYL) found in phylotype IA
- HYL-IB and II:
-
Variant of hyaluronidase (HYL) found in phylotypes IB and II
- IFN-γ:
-
Interferon- γ
- IL:
-
Interleukin (e.g. IL-8, IL-6, etc.)
- MMPs:
-
Matrix metalloproteinases (e.g. MMP-9, MMP-13, etc.)
- NK cells:
-
Natural killer cells
- NLRP3:
-
NOD-like receptor family, pyrin domain containing 3
- PAR-2:
-
Protease-activated receptor-2
- PCR:
-
Polymerase chain reaction
- QS:
-
Quorum sensing
- RIS-1/psoriasin:
-
Retinoic acid-inducible skin-specific gene
- RNA:
-
Ribonucleic acid
- RNases:
-
Ribonucleases
- SCORAD:
-
Scoring atopic dermatitis
- SLST:
-
Single-locus sequence typing
- TGF-β:
-
Transforming growth factor-β
- Th17/Th1:
-
T helper 17/T helper 1 cells
- TIMP-2:
-
Tissue inhibitor of metalloproteinases (e.g. TIMP-2, TIMP-4, etc.)
- TLRs:
-
Toll-like receptors (e.g. TLR-2, TLR-4, etc.)
- TNF-α:
-
Tumour necrosis factor-α
References
Johnson MT, Roberts J. Skin conditions and related need for medical care among persons 1–74 years. United States, 1971–1974. Vital Health Stat 11. 1978;(212):i–v, 1–72.
Wolkenstein P, Grob J-J, Bastuji-Garin S, Ruszczynski S, Roujeau J-C, Revuz J, et al. French people and skin diseases: results of a survey using a representative sample. Arch Dermatol. 2003;139(12):1614–9; discussion 1619.
Rea JN, Newhouse ML, Halil T. Skin disease in Lambeth. A community study of prevalence and use of medical care. Br J Prev Soc Med. 1976;30(2):107–14.
Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–85.
Oh J, Byrd AL, Deming C, Conlan S, NISC Comparative Sequencing Program, Kong HH, et al. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514(7520):59–64.
Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143–55.
Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244–53.
Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32(Suppl 2):5–14.
Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31(Suppl 5):8–12.
Rocha MA, Bagatin E. Skin barrier and microbiome in acne. Arch Dermatol Res. 2018;310(3):181–5.
Cummins CS, Johnson JL. Corynebacterium parvum: a synonym for Propionibacterium acnes? J Gen Microbiol. 1974;80(2):433–42.
Corvec S, Dagnelie M-A, Khammari A, Dréno B. Taxonomy and phylogeny of Cutibacterium (formerly Propionibacterium) acnes in inflammatory skin diseases. Ann Dermatol Venereol. 2019;146(1):26–30.
Corvec S. Clinical and biological features of cutibacterium (Formerly Propionibacterium) avidum, an underrecognized microorganism. Clin Microbiol Rev. 2018;31(3):e00064-17.
Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Méd Mal Infect. 2014;44(6):241–50.
McDowell A, Valanne S, Ramage G, Tunney MM, Glenn JV, McLorinan GC, et al. Propionibacterium acnes types I and II represent phylogenetically distinct groups. J Clin Microbiol. 2005;43(1):326–34.
McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218–24.
McDowell A, Nagy I, Magyari M, Barnard E, Patrick S. The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS One. 2013;8(9):e70897.
Liu J, Yan R, Zhong Q, Ngo S, Bangayan NJ, Nguyen L, et al. The diversity and host interactions of Propionibacterium acnes bacteriophages on human skin. ISME J. 2015;9(9):2078–93.
Allhorn M, Arve S, Brüggemann H, Lood R. A novel enzyme with antioxidant capacity produced by the ubiquitous skin colonizer Propionibacterium acnes. Sci Rep. 2016;6:36412.
Miskin JE, Farrell AM, Cunliffe WJ, Holland KT. Propionibacterium acnes, a resident of lipid-rich human skin, produces a 33 kDa extracellular lipase encoded by gehA. Microbiol Read Engl. 1997;143(Pt 5):1745–55.
Nazipi S, Stødkilde-Jørgensen K, Scavenius C, Brüggemann H. The skin bacterium Propionibacterium acnes employs two variants of hyaluronate lyase with distinct properties. Microorganisms. 2017;5(3):57.
Christensen GJM, Scholz CFP, Enghild J, Rohde H, Kilian M, Thürmer A, et al. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genomics. 2016;17:152.
Claudel J-P, Auffret N, Leccia M-T, Poli F, Corvec S, Dréno B. Staphylococcus epidermidis: a potential new player in the physiopathology of acne? Dermatology. 2019;235(4):287–94.
Douglas HC, Gunter SE. The taxonomic position of corynebacterium acnes. J Bacteriol. 1946;52(1):15–23.
Scholz CFP, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016;66(11):4422–32.
Dekio I, Culak R, Misra R, Gaulton T, Fang M, Sakamoto M, et al. Dissecting the taxonomic heterogeneity within Propionibacterium acnes: proposal for Propionibacterium acnes subsp. acnes subsp. nov. and Propionibacterium acnes subsp. elongatum subsp. nov. Int J Syst Evol Microbiol. 2015;65(12):4776–87.
McDowell A, Barnard E, Liu J, Li H, Patrick S. Proposal to reclassify Propionibacterium acnes type I as Propionibacterium acnes subsp. acnes subsp. nov. and Propionibacterium acnes type II as Propionibacterium acnes subsp. defendens subsp. nov. Int J Syst Evol Microbiol. 2016;66(12):5358–65.
Brüggemann H, Lomholt HB, Tettelin H, Kilian M. CRISPR/cas loci of type II Propionibacterium acnes confer immunity against acquisition of mobile elements present in type I P. acnes. PLoS One. 2012;7(3):e34171.
McDowell A. Over a decade of recA and tly gene sequence typing of the skin bacterium Propionibacterium acnes: what have we learnt? Microorganisms. 2017;6(1):1.
Barnard E, Nagy I, Hunyadkürti J, Patrick S, McDowell A. Multiplex touchdown PCR for rapid typing of the opportunistic pathogen Propionibacterium acnes. J Clin Microbiol. 2015;53(4):1149–55.
McDowell A, Barnard E, Nagy I, Gao A, Tomida S, Li H, et al. An expanded multilocus sequence typing scheme for Propionibacterium acnes: investigation of “pathogenic”, “commensal” and antibiotic resistant strains. PLoS One. 2012;7(7):e41480.
Scholz CFP, Jensen A, Lomholt HB, Brüggemann H, Kilian M. A novel high-resolution single locus sequence typing scheme for mixed populations of Propionibacterium acnes in vivo. PLoS One. 2014;9(8):e104199.
Kilian M, Scholz CFP, Lomholt HB. Multilocus sequence typing and phylogenetic analysis of Propionibacterium acnes. J Clin Microbiol. 2012;50(4):1158–65.
Dagnelie M-A, Khammari A, Dréno B, Corvec S. Cutibacterium acnes molecular typing: time to standardize the method. Clin Microbiol Infect. 2018;24(11):1149–55.
Saint-Jean M, Frenard C, Le Bras M, Aubin GG, Corvec S, Dréno B. Testosterone-induced acne fulminans in twins with Kallmann’s syndrome. JAAD Case Rep. 2015;1(1):27–9.
Paugam C, Corvec S, Saint-Jean M, Le Moigne M, Khammari A, Boisrobert A, et al. Propionibacterium acnes phylotypes and acne severity: an observational prospective study. J Eur Acad Dermatol Venereol. 2017;31(9):e398–9.
Dagnelie M-A, Corvec S, Saint-Jean M, Bourdès V, Nguyen J-M, Khammari A, et al. Decrease in diversity of Propionibacterium acnes phylotypes in patients with severe acne on the back. Acta Derm Venereol. 2018;98(2):262–7.
Saint-Jean M, Corvec S, Nguyen J-M, Le Moigne M, Boisrobert A, Khammari A, et al. Adult acne in women is not associated with a specific type of Cutibacterium acnes. J Am Acad Dermatol. 2019;81(3):851–2.
Lomholt HB, Scholz CFP, Brüggemann H, Tettelin H, Kilian M. A comparative study of Cutibacterium (Propionibacterium) acnes clones from acne patients and healthy controls. Anaerobe. 2017;47:57–63.
Kwon HH, Yoon JY, Park SY, Suh DH. Analysis of distribution patterns of Propionibacterium acnes phylotypes and Peptostreptococcus species from acne lesions. Br J Dermatol. 2013;169(5):1152–5.
Sadhasivam S, Sinha M, Saini S, Kaur SP, Gupta T, Sengupta S, et al. Heterogeneity and antibiotic resistance in Propionibacterium acnes isolates and its therapeutic implications: blurring the lines between commensal and pathogenic phylotypes. Dermatol Ther. 2016;29(6):451–4.
Barnard E, Liu J, Yankova E, Cavalcanti SM, Magalhães M, Li H, et al. Strains of the Propionibacterium acnes type III lineage are associated with the skin condition progressive macular hypomelanosis. Sci Rep. 2016;6:31968.
Petersen RLW, Scholz CFP, Jensen A, Brüggemann H, Lomholt HB. Propionibacterium Acnes phylogenetic type III is associated with progressive macular hypomelanosis. Eur J Microbiol Immunol. 2017;7(1):37–45.
Corvec S, Luchetta J, Aubin GG. Is hemolysis a clinical marker of Propionibacterium acnes orthopedic infection or a phylogenetic marker? Am J Orthop Belle Mead NJ. 2015;44(3):E61–2.
Christensen GJM, Brüggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes. 2014;5(2):201–15.
Fujimura S, Nakamura T. Purification and properties of a bacteriocin-like substance (acnecin) of oral Propionibacterium acnes. Antimicrob Agents Chemother. 1978;14(6):893–8.
Valanne S, McDowell A, Ramage G, Tunney MM, Einarsson GG, O’Hagan S, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiol Read Engl. 2005;151(Pt 5):1369–79.
Lheure C, Grange PA, Ollagnier G, Morand P, Désiré N, Sayon S, et al. TLR-2 recognizes Propionibacterium acnes CAMP factor 1 from highly inflammatory strains. PLoS One. 2016;11(11):e0167237.
Holland C, Mak TN, Zimny-Arndt U, Schmid M, Meyer TF, Jungblut PR, et al. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol. 2010;10:230.
Tyner H, Patel R. Hyaluronidase in clinical isolates of Propionibacterium acnes. Int J Bacteriol. 2015;2015:218918.
Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of Rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885–91.
Achermann Y, Goldstein EJC, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27(3):419–40.
Coenye T, Peeters E, Nelis HJ. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res Microbiol. 2007;158(4):386–92.
Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br J Dermatol. 2012;167(1):50–8.
Kuehnast T, Cakar F, Weinhäupl T, Pilz A, Selak S, Schmidt MA, et al. Comparative analyses of biofilm formation among different Cutibacterium acnes isolates. Int J Med Microbiol. 2018;308(8):1027–35.
Leyden JJ, McGinley KJ, Cavalieri S, Webster GF, Mills OH, Kligman AM. Propionibacterium acnes resistance to antibiotics in acne patients. J Am Acad Dermatol. 1983;8(1):41–5.
Sardana K, Gupta T, Garg VK, Ghunawat S. Antibiotic resistance to Propionobacterium acnes: worldwide scenario, diagnosis and management. Expert Rev Anti-Infect Ther. 2015;13(7):883–96.
Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e23–33.
Nakase K, Sakuma Y, Nakaminami H, Noguchi N. Emergence of fluoroquinolone-resistant Propionibacterium acnes caused by amino acid substitutions of DNA gyrase but not DNA topoisomerase IV. Anaerobe. 2016;42:166–71.
Nakase K, Yoshida A, Saita H, Hayashi N, Nishijima S, Nakaminami H, et al. Relationship between quinolone use and resistance of Staphylococcus epidermidis in patients with acne vulgaris. J Dermatol. 2019;46(9):782–6.
Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Götz F, Manosroi W, et al. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm. 2010;392(1–2):304–10.
Wang Y, Dai A, Huang S, Kuo S, Shu M, Tapia CP, et al. Propionic acid and its esterified derivative suppress the growth of methicillin-resistant Staphylococcus aureus USA300. Benef Microbes. 2014;5(2):161–8.
Greenberg EP. Bacterial communication and group behavior. J Clin Invest. 2003;112(9):1288–90.
Nealson KH, Hastings JW. Bacterial bioluminescence: its control and ecological significance. Microbiol Rev. 1979;43(4):496–518.
Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiol Read Engl. 2007;153(Pt 12):3923–38.
Pearson JP, Feldman M, Iglewski BH, Prince A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect Immun. 2000;68(7):4331–4.
Pirhonen M, Flego D, Heikinheimo R, Palva ET. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993;12(6):2467–76.
Williams MR, Costa SK, Zaramela LS, Khalil S, Todd DA, Winter HL, et al. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med. 2019;11(490):eaat8329.
Lwin SM, Kimber I, McFadden JP. Acne, quorum sensing and danger. Clin Exp Dermatol. 2014;39(2):162–7.
Tyner H, Patel R. Propionibacterium acnes biofilm – a sanctuary for Staphylococcus aureus? Anaerobe. 2016;40:63–7.
Wollenberg MS, Claesen J, Escapa IF, Aldridge KL, Fischbach MA, Lemon KP. Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. mBio. 2014;5(4):e01286–14.
Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17(1):13–24.
Lee E-Y, Choi D-Y, Kim D-K, Kim J-W, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9(24):5425–36.
Jeon J, Mok HJ, Choi Y, Park SC, Jo H, Her J, et al. Proteomic analysis of extracellular vesicles derived from Propionibacterium acnes. Proteomics Clin Appl. 2017;11(1–2):1600040.
Choi E-J, Lee HG, Bae I-H, Kim W, Park J, Lee TR, et al. Propionibacterium acnes-derived extracellular vesicles promote acne-like phenotypes in human epidermis. J Invest Dermatol. 2018;138(6):1371–9.
Paetzold B, Willis JR, Pereira de Lima J, Knödlseder N, Brüggemann H, Quist SR, et al. Skin microbiome modulation induced by probiotic solutions. Microbiome. 2019;7(1):95.
Chien AL, Tsai J, Leung S, Mongodin EF, Nelson AM, Kang S, et al. Association of systemic antibiotic treatment of acne with skin microbiota characteristics. JAMA Dermatol. 2019;155(4):425–34.
Nakase K, Hayashi N, Akiyama Y, Aoki S, Noguchi N. Antimicrobial susceptibility and phylogenetic analysis of Propionibacterium acnes isolated from acne patients in Japan between 2013 and 2015. J Dermatol. 2017;44(11):1248–54.
O’Neill AM, Gallo RL. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome. 2018;6(1):177.
Nagy I, Pivarcsi A, Koreck A, Széll M, Urbán E, Kemény L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124(5):931–8.
Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8(8):2195–205.
Jasson F, Nagy I, Knol AC, Zuliani T, Khammari A, Dréno B. Different strains of Propionibacterium acnes modulate differently the cutaneous innate immunity. Exp Dermatol. 2013;22(9):587–92.
Do TH, Modlin R. 906 Cutibacterium acnes RNA activates the human inflammatory response via TLR8. J Invest Dermatol. 2019;139(5):S156.
Guy R, Green MR, Kealey T. Modeling acne in vitro. J Invest Dermatol. 1996;106(1):176–82.
Kistowska M, Meier B, Proust T, Feldmeyer L, Cozzio A, Kuendig T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135(1):110–8.
Ebrahim AA, Mustafa AI, El-Abd AM. Serum interleukin-17 as a novel biomarker in patients with acne vulgaris. J Cosmet Dermatol. 2019;18(6):1975–9.
Lee D-Y, Yamasaki K, Rudsil J, Zouboulis CC, Park GT, Yang J-M, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill propionibacterium acnes. J Invest Dermatol. 2008;128(7):1863–6.
Zouboulis CC, Beutler C, Merk HF, Baron JM. RIS-1/psoriasin expression in epithelial skin cells indicates their selective role in innate immunity and in inflammatory skin diseases including acne. Dermatoendocrinology. 2017;9(1):e1338993.
Saint-Jean M, Khammari A, Jasson F, Nguyen J-M, Dréno B. Different cutaneous innate immunity profiles in acne patients with and without atrophic scars. Eur J Dermatol. 2016;26(1):68–74.
Moon J, Yoon JY, Yang JH, Kwon HH, Min S, Suh DH. Atrophic acne scar: a process from altered metabolism of elastic fibres and collagen fibres based on transforming growth factor-β1 signalling. Br J Dermatol. 2019;181(6):1226–37.
Sanford JA, Zhang L-J, Williams MR, Gangoiti JA, Huang C-M, Gallo RL. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci Immunol. 2016;1(4):eaah4609.
Thiboutot DM, Dréno B, Abanmi A, Alexis AF, Araviiskaia E, Barona Cabal MI, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 Suppl 1):S1–S23, e1.
Lee YB, Byun EJ, Kim AHS. Potential role of the microbiome in acne: a comprehensive review. J Clin Med. 2019;8(7):987.
Valente Duarte De Sousa IC. New and emerging drugs for the treatment of acne vulgaris in adolescents. Expert Opin Pharmacother. 2019;20(8):1009–24.
Dreno B. Topical antibacterial therapy for acne vulgaris. Drugs. 2004;64(21):2389–97.
Cong T-X, Hao D, Wen X, Li X-H, He G, Jiang X. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311(5):337–49.
Kircik LH. The role of benzoyl peroxide in the new treatment paradigm for acne. J Drugs Dermatol. 2013;12(6):s73–6.
Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient--a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34(3):321–30.
Dispenza MC, Wolpert EB, Gilliland KL, Dai JP, Cong Z, Nelson AM, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132(9):2198–205.
McCoy WH, Otchere E, Rosa BA, Martin J, Mann CM, Mitreva M. Skin ecology during sebaceous drought-how skin microbes respond to isotretinoin. J Invest Dermatol. 2019;139(3):732–5.
Dagnelie M-A, Montassier E, Khammari A, Mounier C, Corvec S, Dréno B. Inflammatory skin is associated with changes in the skin microbiota composition on the back of severe acne patients. Exp Dermatol. 2019;28(8):961–7.
McLaughlin J, Watterson S, Layton AM, Bjourson AJ, Barnard E, McDowell A. Propionibacterium acnes and acne vulgaris: new insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms. 2019;7(5):128.
Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2017;152(4):799–811, e7.
Gueniche A, Knaudt B, Schuck E, Volz T, Bastien P, Martin R, et al. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled clinical study. Br J v. 2008;v(6):1357–63.
Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378):eaah4680.
Bowe WP, Filip JC, DiRienzo JM, Volgina A, Margolis DJ. Inhibition of propionibacterium acnes by bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. J Drugs Dermatol. 2006;5(9):868–70.
Pavicic T, Wollenweber U, Farwick M, Korting HC. Anti-microbial and -inflammatory activity and efficacy of phytosphingosine: an in vitro and in vivo study addressing acne vulgaris. Int J Cosmet Sci. 2007;29(3):181–90.
Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, et al. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun. 2008;76(9):4163–75.
Gueniche A, Benyacoub J, Philippe D, Bastien P, Kusy N, Breton L, et al. Lactobacillus paracasei CNCM I-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur J Dermatol. 2010;20(6):731–7.
Kang BS, Seo J-G, Lee G-S, Kim J-H, Kim SY, Han YW, et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol. 2009;47(1):101–9.
Muizzuddin N, Maher W, Sullivan M, Schnittger S, Mammone T. Physiological effect of a probiotic on skin. J Cosmet Sci. 2012;63(6):385–95.
Marchetti F, Capizzi R, Tulli A. Efficacy of regulators of the intestinal bacterial flora in the therapy of acne vulgaris. Clin Ter. 1987;122(5):339–43.
Studying the skin microbiome and the potential of a topical probiotic cream for patients with acne – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Aug 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT03469076
Webster GF, Cummins CS. Use of bacteriophage typing to distinguish Propionibacterium acne types I and II. J Clin Microbiol. 1978;7(1):84–90.
Wang Y, Hata TR, Tong YL, Kao M-S, Zouboulis CC, Gallo RL, et al. The anti-inflammatory activities of Propionibacterium acnes CAMP factor-targeted acne vaccines. J Invest Dermatol. 2018;138(11):2355–64.
Contassot E. Vaccinating against acne: benefits and potential pitfalls. J Invest Dermatol. 2018;138(11):2304–6.
Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang C-M. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One. 2011;6(4):e14797.
Liu P-F, Nakatsuji T, Zhu W, Gallo RL, Huang C-M. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine. 2011;29(17):3230–8.
Lo C-W, Lai Y-K, Liu Y-T, Gallo RL, Huang C-M. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting β-hemolysin and CAMP factor. J Invest Dermatol. 2011;131(2):401–9.
Nakatsuji T, Liu Y-T, Huang C-P, Zouboulis CC, Gallo RL, Huang C-M. Vaccination targeting a surface sialidase of P. acnes: implication for new treatment of acne vulgaris. PLoS One. 2008;3(2):e1551.
Nakatsuji T, Liu Y-T, Huang C-P, Zoubouis CC, Gallo RL, Huang C-M. Antibodies elicited by inactivated propionibacterium acnes-based vaccines exert protective immunity and attenuate the IL-8 production in human sebocytes: relevance to therapy for acne vulgaris. J Invest Dermatol. 2008;128(10):2451–7.
Dagnelie M-A, Corvec S, Saint-Jean M, Nguyen J-M, Khammari A, Dréno B. Cutibacterium acnes phylotypes diversity loss: a trigger for skin inflammatory process. J Eur Acad Dermatol Venereol. 2019;33(12):2340–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dagnelie, MA., Corvec, S., Khammari, A., Dréno, B. (2021). Update on Cutibacterium acnes. In: Suh, D.H. (eds) Acne. Updates in Clinical Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-030-68996-4_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-68996-4_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68995-7
Online ISBN: 978-3-030-68996-4
eBook Packages: MedicineMedicine (R0)