Abstract
Propionibacterium acnes plays an important role in the pathogenesis of acne and it is established that this bacteria is involved in the induction and maintenance of the inflammatory phase of acne. The aim of our work was to determine if P. acnes extracts could modulate integrins and filaggrin in vitro expression by keratinocytes. Integrins and filaggrin expression was examined using immunohistochemistry technique both on Normal Human Epiderminal Keratinocytes (NHEK) and on deep-frozen sections of normal human skin explants incubated with three different P. acnes extracts. In addition, the expression of filaggrin was investigated on biopsies of acne lesions and by western-blot associated with its precursor profilaggrin. We demonstrated that P. acnes extracts induced β1 integrin expression significantly on both proliferating keratinocytes and differentiated keratinocytes. In addition, P. acnes induced α3, α6s and αVβ6 integrin expression and filaggrin expression on differentiated keratinocytes. Finally P. acnes extracts increased filaggrin expression by suprabasal layer of epidermis of explants. Western-blot confirmed that total amount of filaggrin was increased. These results indicate that P. acnes extracts are directly able to modulate the differentiation of keratinocytes suggesting that this bacteria play a role not only in the development of inflammatory acne lesions but also in the formation of the microcomedo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acne is a skin disorder of the pilosebaceous unit resulting from multiple factors. Microcomedo is considered as the early step of the formation of acne lesions before closed or opened comedones and inflammatory lesions. The mechanisms inducing the formation of comedones are still partially known, but they are believed to involve hyperkeratinization of the follicle secondary to both hyperproliferation of keratinocytes and abnormalities of their differentiation and decreasing desquamation [3, 6].
Bacterial hypercolonization of the sebaceous follicle, especially P. acnes, has been considered as a major causative factor for the development of the inflammatory reaction in acne vulgaris. Indeed, it is widely accepted that inflammation in acne may be mainly mediated by different factors secreted by P. acnes, such as lipase which induce the liberation of free fatty acids from sebum that are pro-inflammatory [17]. Recent studies have also shown that P. acnes triggers anti-microbial peptide and cytokine secretion of keratinocytes in vitro [11, 12]. P. acnes, itself is able to induce cytokines-like such as TNF-α, IL-1α and IL-8 [5]. The genome sequence of P. acnes encodes many factors that may have immunomodulatory potential [2]. However, P. acnes has also been identified at a high concentration in microcomedomes with 105–106 organisms recovered from these preclinical lesions [10]. P. acnes is also able to induce the proliferation of keratinocytes in vitro. Confirmed both by MTT viability test and Ki67 immunolabelling [8, 11]. Thus, the precise role that P. acnes could play in the formation of comedone in addition of its action in the development of the inflammatory reaction remains unclear.
Integrins play an important role in the modulation of both differentiation and proliferation of keratinocytes in epidermis [14, 15]. Integrins are heterodimeric transmembrane receptors consisting of one α and one β subunit. β1 integrins play a crucial role in regulating the balance between epidermal proliferation and terminal differentiation. Keratinocytes with high levels of β1 integrins are less motile than keratinocytes with lower level [16]. α3β1 and α6β4 (laminin receptor) expression is constitutive whereas αVβ6 (receptor for fibronectin and tenascin) is induced in NHEK culture, on wounding and pathological conditions. The conclusion from analysis of mice lacking β1 or α6β4 integrins is that β1 integrins are indeed important for normal epidermal proliferation, whereas the role of α6β4 is primarily one of anchorage. Thus abnormal keratinocytes differentiation induce alterations of the expression of β1, α3, α6 integrins sub-units. αVβ6 was used as keratinocytes suffering marker. Interestingly, on biopsies of acne lesions, it has been demonstrated that the alteration of keratinocytes differentiation is associated with abnormal integrins expression [7].
Filaggrin is a cationic (basic) protein named for its ability to aggregate filaments of keratin into fibers or macrofibrils. Human filaggrin (37,000 apparent molecular weight), is synthesized in the granular cells of the epidermis as a large, highly phosphorylated precursor, profilaggrin, which accumulates in the keratohyalin granules [4]. A number of keratinization disorders are associated with dysregulation in the formation of keratohyalin granules and in the processing of profilaggrin [13]. Kurukawa et al. [9] have shown that seborrhoeic and acne skin revealed considerable amounts of filaggrin in the intermediate layers of the sebaceous duct and the infundibulum indicating a premature terminal keratinization process in these areas. Furthermore they observed by electron microscopic studies an increased number of keratohyaline granules in acne skin.
Thus, the aim of this work was to determine if P. acnes is directly able to modulate integrins and filaggrin expression by keratinocytes which will be new arguments to think that this bacteria could play a role not only in the formation of inflammatory lesions but also of retentional lesions in acne.
Materials and methods
Materials
Keratinocytes culture
Normal Human Epidermal Keratinocytes (NHEK) from healthy prepuces were obtained from pediatric surgery department of Nantes Hospital and were grown at 37°C in a humid 5% CO2 atmosphere, in serum free keratinocyte growth medium KSFM (Invitrogen, Cergy-Pontoise, France) supplemented with 25 μg/ml bovine pituitary extract (BPE), 0.5 ng/ml of recombinant epidermal growth factor (EGF), 2.5 μg/ml fungizone (Bristol-Myers Squibb, Paris, France) and Penicilline 100 UI/ml-Streptomycine 100 μg/ml. Cells were used after a limited number (n = 2) of subcultures. NHEK were seeded at the density of 20,000 per well in four-wells chamber slides (Dutscher, Brumath, France) in low calcium (CaCL2 0.09 mM) defined medium KBM (keratinocyte basal medium) (Promocell, Heidelberg, Germany) with HC (hydrocortisone) supplemented with 0.4% of BPE, EGF 0.125 ng/ml, insulin 5 μg/ml, transferrine 10 μg/ml, epinephrine 0.1 μg/ml. The medium was replaced by KBM without HC 24 h before starting the experiment and NHEK were used at 80% of confluence.
Skin explants
Explants were prepared from skin abdominal plasties received after plastic surgery. After fat removing, explants (4 mm diameter) were incubated at 37°C in presence of P. acnes extracts in order to cause stimulation or in control medium (KBM without HC).The explants were submerged and P. acnes extracts added to the culture medium. After incubation, explants were removed from the culture medium and frozen in liquid nitrogen before storage at −80°C.
Inflammatory acne lesions
Five frozen biopsies of inflammatory acne lesions (papulo-pustules) were obtained from Immunodermatology Laboratory. They were kept in liquid nitrogen before storage at −80°C.
Bacterial extracts
We used three extracts of Propionibacterium acnes (IP 53113) provided by Pierre Fabre Laboratories (Toulouse, France). This reference strain was first described in 1968.
Supernatant A (SA) and pellet C were obtained after bacterial culture centrifugation, freeze/thawing of the cell pellet and a last centrifugation at 4,000 rpm during 15 min. Supernatant B (SB) was obtained after a second centrifugation (4,000 rpm, 15 min) of pellet C. The membrane fraction (FM) extract was obtained after reconstitution of pellet resuspended in KBM without HC.
The membrane fraction (FM) contained peptidoglycan and lipoteic acid. Supenatant A (SA) contained cytosolic proteins and supernatant B (SB) was rich in membrane proteins.
Preliminary experiments were carried out in our laboratory to determine the effect of different dilutions of P. acnes extracts on keratinocytes viability (MTT test). Following extracts dilutions: FM diluted at 1/2, SA and SB diluted at 1/5 were considered to be the most appropriated [8].
NHEK and cutaneous explants incubation with P. acnes extracts
The different P. acnes extract (FM 1/2, SA 1/5 and SB 1/5) were deposited on cutaneous explants for 3 (Immunohistochemistry and Western immunoblotting) or 24 h (Western immunoblotting).
Antibodies used for immunohistochemistry
They are listed in table 1.
Methods
Labelling of smears and biopsies (Immunohistochemistry)
Sections, 5 μm thick, were obtanined from skin explants frozen in liquid nitrogen and fixed in acetone for 10 min. Immunostaining was performed using an immunoperoxidase technique using phosphate-buffered saline (PBS)/bovine serum albumin (BSA) 0.1% for dilution of antibodies and Tris-buffered saline (TBS)/Tween 20 0.05% (w/v)/BSA 0.1% w/v for washes.
Explants sections and chamber slides were incubated with the primary monoclonal antbodies at room temperature and in darkness for 30 min. The slides were then washed in TBS/BSA 0,1% and incubated with a second biotinylated antibody for 30 min, and then washed again and further incubated with streptavidine/peroxidase (DAKO) for 30 min. After a final wash, reactions products were revealed using hydrogen peroxide/aminoethylcarbazole (AEC) (DAKO) peroxidase substrate. The reaction was stopped with distilled water (10 min) and counter-staining was done with Mayer haemalun (VWR International, Strasbourg, France) for about 1 min. The slides were rinsed with distilled water, and mounted in an aqueous medium and observed with a Leitz microscope (20× magnification).
Negative controls included irrelevant monoclonal antibody of the same isotype as the primary antibody. The intensity of the labelling was quantified according to a semi-quantitative scale: none (0), weak (1), moderate (2) and strong (3). Three slides from a total of three donors were examined. For each slide, the intensity of the labelling was determined using three fields and two different examiners read the slides.
Western immunoblotting
Explants cultured in control medium or with P. acnes explants for 3 h were lysed using Sample grinding Kit (Amersham Biosciences, Freiburg, Germany). Samples extracts mixed with an equal volume of 2× Laemmli Sample Buffer (Biorad-Rad, Marnes la Coquette, France) containing 50 mM 2β-mercaptoethanol were boiled for 5 min. Equal amount of protein from each explants lysate were electrophoresed on 8% SDS-polyacrylamide gel and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). Membranes were blocked overnight using blocking reagent (BM chemiluminescence kit, Roche Diagnostic GmbH, Indianapolis, IN) and then incubated for 1 h with anti-human filaggrin primary antibody: AHF2 or AHF7 (kindly provided by M.Simon, Toulouse [14]) diluted at 1/2,500. After four washes in TBS containing 0.05% Tween 20, membranes were incubated with a horseradish peroxidase-conjugated secondary mix anti-mouse/anti-rabbit antibody dilute at 1/5,000 (BM chemiluminescence kit). Blots were developed using a classic chemiluminescence detection system (BM chemiluminescence kit).
Statistical analysis
Results were expressed as mean ± SEM. Statistical analyses were performed using the paired non parametric Wilcoxon test. A P value less than 0.05 were considered to be significant.
Results
Effects of P. acnes extracts on integrin expression by NHEK [Ca2+ 0.09 mM ] and by skin explants
Concerning NHEK (proliferating keratinocytes)
The expression of β1 integrins was increased at the limit of significativity (P = 0.06) on keratinocytes incubated with SB extract (3 ± 0 versus 2.25 ± 0.5 in control) (Fig. 1).
Expression of β1, α3, α6, αVβ6 integrins by NHEK incubated with P. acnes extracts (FM membrane fraction, SA supernatant A, SB supernatant B). Histograms represent the average intensity of immunolabelling: none (0), weak (1), moderate (2) and strong (3). The results represent the main of three experiments. *P < 0.05 (Student test)
The expression of α3 integrins was unchanged in presence of the three P. acnes extracts. The expression of α6 integrins was not significantly increased by SA and SB P. acnes extracts (1.5 ± 0.57). The expression of αVβ6 integrins was not modified in presence of P. acnes extracts.
Concerning skin explants (ex vivo model with epidermis)
We observed that the expression of β1 integrins was significantly increased with FM (2.5 ± 0.5), SA (2.33 ± 0.58) and SB extract (2 ± 1), and extracts compared to control (2 ± 0) (Fig. 2). The expressions of α3 integrins, α6 integrins were not significantly increased in presence of P. acnes extracts. Concerning, αVβ6 integrins the expression was not significantly induced with SB (1 ± 1.17).
Effects of P. acnes extracts on filaggrin expression by NHEK [Ca2+ 0.09 mM] and by skin explants
Concerning NHEK (proliferating keratinocytes)
We observed that the three types of P. acnes extracts did not induced an expression of filaggrin by in vitro proliferating keratinocytes (Fig. 3).
Concerning skin explants (ex vivo model with epidermis)
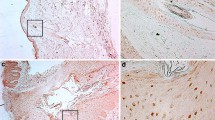
Immunohistochemistry using commercial anti-filaggrin (Fig. 4)
We noted that the three types of P. acnes extracts induced a filaggrin expression on both suprabasal layer (granular and spinous) of epidermis (the strongest effect was observed with SA and SB extracts) (Fig. 4c, d, e).
Expression of filaggrin on cutaneous (commercial antibody) explants incubated with P. acnes extracts: FM (c), SA (d) and SB (e), compared to normal skin (a) and acne lesion (b). All sample were treated in same conditions excepted acne’s biopsie. This figure is representative slide from one donor. Note that P. acnes extracts induce filaggrin expression by keratinocytes of suprabasal (granular and spinous) on explants skin model
On in vivo biopsies from of a retentional acne lesion we confirmed that the expression of filaggrin was extended to the lower part of epidermis (Fig. 4b) in a similar manner than we obtained with our skin explants incubated with P. acnes extracts.
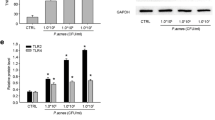
Western-blot using non-commercial anti-filaggrin AHF2 and AHF7 (Fig. 5)
Western-blot confirmed that total amount of filaggrin was increased after 3 or 24 h of incubation with P. acnes compared to control.
Filaggrin (non-commercial antibody) immunoblot on cutaneous explants incubated with P. acnes extracts during 3 and 24 h. The blots show results obtain with AHF2 antibody, which interact both with profilaggrin and filaggrin (a) and AHF7 antibody, which interact only with filaggrin (b). This figure represents results obtained with 3 donors. Note that total amount of filaggrin protein was increased in explants incubated with P. acnes extracts compared to control
Discussion
We show for the first time that P. acnes extracts modulate the expression of β1 integrin and filaggrin using two in vitro models. Explants have the advantage to be a model very closed to in vivo conditions. Thus it is more specifically of interest to study modulations of differentiation of keratinocytes. In addition, the model of NHEK has the advantage to study keratinocytes alone eliminating interactions with other cells of the skin. We do not use whole P. acnes bacteria, but the three extracts represent the different composants of P. acnes which in vivo can be directly in contact with the keratinocytes (protein wall) or indirectly by secretion.
P. acnes induces an increase (but not significant) of β1 integrins expression by proliferating keratinocytes (NHEK [0.09 mM]) and a significant expression of filaggrin on a differentiated epidermis . Moreover, an induction of the expression of integrins (β1, α3, α6 and αVβ6) is also noted on differentiated epidermis (explants) but not at a significant level.
Loss of β1 integrin by keratinocytes has been shown to decrease the proliferation of basal keratinocytes by about 70% [1]. Thus, the induction of β1 integrin by P. acnes, by modulating the proliferation of keratinocytes can play a role in the formation of micro-comedon.
Concerning α3 and α6 integrins, an in vivo study focusing on follicles of acne patients [7] has shown an aberrant α2 and α3 integrins expression around comedones and uninvolved pilosebaceous follicles from acne lesions. Our results suggest that P. acnes which is present in the sebaceous follicle could play a role in the modulation of these integrins, specifically α3 and α6.
Our work also demonstrates that P. acnes extracts induce filaggrin expression on epidermis (suprabasal) of explants skin model whereas no induction of filaggrin expression was observed in NHEK model where keratinocytes were not differentiated. Concerning this part of our study, one of weaknesses is that we cannot directly transfer our in vitro results in vivo even if explants are closed to in vivo conditions. The other one, is the large error bars in Fig. 1 and 2, which can be explained by the variability between donors.
By western-blot, we confirmed that total amount of filaggrin was increased in explants incubated with P. acnes extracts compared to control. Moreover, western-blot, show that only filaggrin is increased in the presence of P. acnes extracts, whereas profilaggrin is totally processed in filaggrin. Thus, these results suggest that P. acnes modulates the terminal phase of differentiation of keratinocytes. Interestingly, this effect was observed with the three extracts of P. acnes. FM extract contains peptidoglycan and lipoteichoic acid, SA extract contain cytosolic proteins and SB is rich in membrane proteins. Thus, P. acnes modulates filaggrin expression on keratinocytes both via proteins localized on cell wall and cytoplasmic components excreted. Our results are in agreement with those obtained in vivo by Kurokawa et al, on pilosebaceous follicle in acne patients. Investigating the distribution of cytokeratins and filaggrin in human pilosebaceous unit in specimens obtained from normal, seborrhoeic, and acne skin [9], they show that both seborrhoeic and acne lesions displayed considerable amounts of filaggrin in the intermediate layers of the sebaceous duct and the infundibulum, indicating a premature terminal keratinization process in these areas. Furthermore they observed by electron microscopic studies an increased number of keratohyaline granules in acne skin. Our results argue for a role of P. acnes in the induction of filaggrin in the infundibulum.
Finally in a previous paper, we described that FM extract of P. acnes increased the proliferation of keratinocytes confirmed both by MTT viability test and by Ki67 immunolabelling [8]. Our hypothesis is that subsequently to sebum increase, P. acnes localized in pilosebaceous follicle proliferates which could be associated with two steps. At an early step P. acnes secretes inflammatory factors inducing the formation of inflammatory infiltrates surrounding follicles as demonstrated by Jeremy et al. [7]. Then, in a second step, the interaction between P. acnes and keratinocytes induce modulation of both proliferation and differentiation of keratinocytes as shown in our study, resulting in the formation of comedo.
In conclusion, our results suggest that P. acnes is implicated in acne lesion as soon as the step of formation of the microcomedo: Thus, they confirm that P. acnes is a key component in acne physiopathology.
References
Brakebusch C, Fassler R (2005) β1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev 24:403–411
Bruggemann H, Henne A, Hoster F et al (2004) The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 305:671–673
Cunliffe WJ, Holland DB, Clark SM, Stables GI (2000) Comedogenesis: some new aetiological, clinical and therapeutic strategies. Br J Dermatol 142:1084–1091
Dale BA (1985) Filaggrin, the matrix protein of keratin. Am J Dermatopathol 7:65–68
Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E (2004) Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol 150:421–428
Guy R, Kealey T (1998) The effects of inflammatory cytokines on the isolated human sebaceous infundibulum. J Invest Dermatol 110:410–415
Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ (2003) Inflammatory events are involved in acne lesion initiation. J Invest Dermatol 121:20–27
Jugeau S, Tenaud I, Knol AC et al (2005) Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol 153:1105–1113
Kurokawa I, Mayer-da-Silva A, Gollnick H, Orfanos CE (1988) Monoclonal antibody labeling for cytokeratins and filaggrin in the human pilosebaceous unit of normal, seborrhoeic and acne skin. J Invest Dermatol 91:566–571
Leyden JJ, McGinley KJ, Vowels B (1998) Propionibacterium acnes colonization in acne and nonacne. Dermatology 196:55–58
Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L (2005) Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol 124:931–938
Schaller M, Loewenstein M, Borelli C et al (2005) Induction of a chemoattractive proinflammatory cytokine response after stimulation of keratinocytes with Propionibacterium acnes and coproporphyrin III. Br J Dermatol 153:66–71
Simon M, Sebbag M, Haftek M et al (1995) Monoclonal antibodies to human epidermal filaggrin, some not recognizing profilaggrin. J Invest Dermatol 105:432–437
van der Flier A, Sonnenberg A (2001) Function and interactions of integrins. Cell Tissue Res 305:285–298
Watt FM (2002) Role of integrins in regulating epidermal adhesion, growth and differentiation. Embo J 21:3919–3926
Watt FM (2002) The stem cell compartment in human interfollicular epidermis. J Dermatol Sci 28:173–180
Zouboulis CC (2001) Is acne vulgaris a genuine inflammatory disease? Dermatology 203:277–279
Acknowledgments
Financial support and P. acnes extracts were provided by Pierre Fabre Laboratories (Toulouse, France). No conflicts of interest exist. We thank surgeons from the plastic surgery Department of Nantes Hospital for abdominal skin samples and the pediatric surgery Department of Nantes Hospital for foreskin samples. The authors thank Simon M. (Toulouse) for antibodies AHF2 and AHF7 gift.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jarrousse, V., Castex-Rizzi, N., Khammari, A. et al. Modulation of integrins and filaggrin expression by Propionibacterium acnes extracts on keratinocytes. Arch Dermatol Res 299, 441–447 (2007). https://doi.org/10.1007/s00403-007-0774-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-007-0774-5