Abstract
Acne vulgaris is a chronic skin disorder involving hair follicles and sebaceous glands. Multiple factors contribute to the disease, including skin microbes. The skin microbiome in the follicle is composed of a diverse group of microorganisms. Among them, Propionibacterium acnes and Malassezia spp. have been linked to acne development through their influence on sebum secretion, comedone formation, and inflammatory response. Antibiotics targeting P. acnes have been the mainstay in acne treatment for the past four decades. Among them, macrolides, clindamycin, and tetracyclines are the most widely prescribed. As antibiotic resistance becomes an increasing concern in clinical practice, understanding the skin microbiome associated with acne and the effects of antibiotic use on the skin commensals is highly relevant and critical to clinicians. In this review, we summarize recent studies of the composition and dynamics of the skin microbiome in acne and the effects of antibiotic treatment on skin microbes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acne vulgaris is a common and multifactorial skin disease, affecting approximately 85% of adolescents and young adults. |
Propionibacterium acnes is the dominant member of the skin microbiome in the pilosebaceous unit. Certain strains of P. acnes have been linked to acne pathogenesis. Other microorganisms such as Malassezia may also play a role in acne. |
Antibiotics, mainly macrolides, clindamycin, and tetracyclines, have been the mainstay for acne treatment, and influence the composition and dynamics of the skin microbiome. Antibiotic resistance has become increasingly prevalent worldwide, and thus there is an urgent need for new acne therapies. |
1 Introduction
Acne vulgaris (commonly called acne) is a common, chronic skin disease that arises in the hair follicle and often involves inflammation. Approximately 85% of adolescents and young adults are affected by the disease [1], while moderate and severe acne accounts for 15–20% of cases [2]. Based on the data from the Global Burden of Disease study in 2013, acne accounted for 0.29% of all skin conditions, which contributed 1.79% to the global burden of disease. Acne ranks second among the most common dermatological conditions after dermatitis [3].
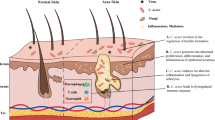
Four factors have been thought to contribute to acne: hyper-secretion of sebum, abnormal proliferation and differentiation of keratinocytes in the hair follicle, bacterial colonization, and host inflammatory response [4]. Among these factors, the skin commensal Propionibacterium acnes is thought to trigger an inflammatory response and lead to subclinical and inflammatory acne lesions [5].
Skin is colonized by hundreds of microorganisms, which occupy different cutaneous environmental niches and form various communities [6]. When the normal flora is disturbed or the host immune defense is weakened, opportunistic microorganisms may trigger or aggravate certain skin diseases [7]. The relationship between skin microorganisms and acne has long been implicated but not fully elucidated. With the rise of the microbiome field in recent years, new findings from studies of the skin microbiome have provided improved understanding of the role of skin microorganisms in health and acne [8,9,10,11,12].
Antibiotics have been an effective and widely used treatment for acne in the past four decades. However, worldwide increase of antibiotic resistance due to frequent and long-term use of antibiotics raises significant concern regarding how the commensal skin microbiome and its protective role for the skin are affected. A better understanding of the relationship among acne, the skin microbiome, and antibiotic treatment may provide new insight on the treatment of the disease while restoring a healthy microbiome.
2 The Skin Microbiome and Acne
The skin is the largest organ in the body, with an average area in adults of 1.8–2 m2. If considering hair follicles, sweat gland ducts, and other skin appendages, the body surface area can reach up to 30 m2 according to Meisel et al. [13]. Various heterogenous communities of microorganisms, including bacteria, viruses, fungi, and mites, occupy different skin environmental niches and appendages [6, 14].
Bacteria are the most dominant and best studied members of the skin microbiome. More than 40 bacterial genera have been identified on human skin, mainly belonging to four phyla: Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes [8,9,10]. The proportions of these bacteria in each community vary depending on individuals, body sites, as well as skin micro-environments [9, 11, 15]. Propionibacteria, Staphylococcus and Corynebacteria, and Gram-negative bacteria are predominant in the sebaceous area, moist skin, and dry skin, respectively. Skin bacteria are not only diverse in taxonomy, but also vary in quantities. Culture-based methods suggest that the total colony-forming units per cm2 skin varies from 3.7 × 104 to 1.2 × 106 [16]. It has been estimated that 106 aerobic bacteria are present per cm2 of moist skin, whereas less than 102 aerobic bacteria and up to 106 anaerobes are present per cm2 of dry skin [17]. The balance of the skin microbiome and its interaction with the host affect the states of skin health and disease.
2.1 Propionibacterium acnes and Acne
P. acnes was first observed by Unna [18] in 1896 and later isolated by Sabouraud [19] from acne lesions in 1897, which led to speculation regarding its involvement in acne pathogenesis. P. acnes was initially named Bacillus acnes, which was then changed to Corynibacterium acnes as it is morphologically similar to Corynibacteria. The name was changed again in the 1940s to P. acnes due to its production of propionic acid [20]. With the identification of distinct phylogenetic groups based on multi-locus sequence typing (MLST) and whole-genome sequencing, it was proposed in 2015 to name the three major types as three subspecies known as P. acnes subsp. acnes, P. acnes subsp. defendens, and P. acnes subsp. elongatum [21]. In 2016, a new genus, Cutibacterium, was proposed for cutaneous propionibacteria [22] and, as such, P. acnes was renamed again to Cutibacterium acnes, although the name P. acnes continues to be used in the field in an effort to reduce the confusion between Cutibacterium and Corynibacterium [23].
In the pilosebaceous unit, where acne arises, P. acnes is the most prevalent and abundant species, accounting for ~ 90% of the microbiome [10, 12]. The scalp and facial skin harbor the highest density of P. acnes (~ 105–106/cm2), followed by the upper limbs and torso, and the lower limbs have the lowest density of P. acnes (~ 102/cm2) [24]. The abundance of P. acnes also varies with age. It is low on the skin of children before puberty, but gradually increases with age, starting from adolescence to adulthood, and then decreases in older persons of age above 50 years [24,25,26].
Several mechanisms of acne pathogenesis involving P. acnes have been proposed, including changes in sebaceous gland activity, comedone formation, and host inflammation.
-
Increasing sebum secretion: the colony-forming units of P. acnes in the pilosebaceous unit are correlated with the total amount and composition of the lipids on the skin. The secreted sebum is used by P. acnes as metabolic substrates to promote its growth [24, 27]. P. acnes further enhances sebum secretion by increasing the activity of diacylglycerol acyltransferase, and exacerbates pre-existing androgen-related seborrhea [28].
-
Promoting comedone formation: P. acnes breaks down triglycerides secreted from sebaceous glands and releases free fatty acids. Porphyrins secreted by P. acnes are catalytic factors for the oxidation of squalene, a main component of sebum. Free fatty acids and oxidized squalene promote comedogenesis [29]. Comedones form due to retention of hyper-proliferating keratinocytes/corneocytes in the follicular duct. Studies have shown that P. acnes not only forms a biofilm to increase keratinocyte adhesion [30, 31], but also activates the insulin-like growth factor 1 (IGF-1)/IGF-1 receptor signaling pathway to up-regulate filaggrin expression. The up-regulation of filaggrin expression leads to increased levels of integrin-α3, -6 s, and -vβ6, and thereby affects keratinocyte proliferation and differentiation [32, 33] and comedone formation.
-
Inducing/aggravating inflammation: upon binding to Toll-like receptor (TLR)-2 and -4 on the surface of keratinocytes, P. acnes induces monocytes and other cells to produce interleukin (IL)-1α, IL1-β, IL-6, IL-8, IL-12, tumor necrosis factor (TNF)-α, interferon, chemotactic factors, β-defensin, and other cytokines and polypeptides, thereby triggering and/or aggravating inflammatory responses [34,35,36,37]. P. acnes also activates the classical and alternative complement pathways to form C3a and C5a, which increase the vascular permeability and the involvement of chemotactic leukocytes in inflammatory responses [38, 39]. Furthermore, P. acnes stimulates sebocytes and promotes the conversion of naïve T cells into T helper (Th) 17 cells by secreting transforming growth factor-β, IL-1β, and IL-6. P. acnes can also activate the NLRP3 inflammasome to induce the release of IL-1β, IL-8, and TNF-α from sebocytes [40]. P. acnes produces lipases, proteases, hyaluronidases, and phosphatases, and induces multiple cells to produce matrix metalloproteinases, thus directly impairing hair follicles, sebaceous glands, and dermal extracellular matrix, and ultimately aggravating inflammation [41,42,43].
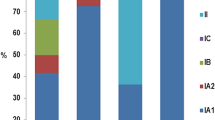
While a causal role of P. acnes in acne pathogenesis remains to be proven, P. acnes is also considered an important commensal for skin health. It releases free fatty acids through triglyceride hydrolysis to maintain low skin pH and inhibits the colonization of pathogenic bacteria, such as Staphylococcus aureus and Streptococcus [44,45,46]. P. acnes typing and genome sequencing efforts suggest that P. acnes can function as a commensal or an opportunistic pathogen depending on the strains and the disease [10, 47, 48]. P. acnes was previously classified into two types, I and II, based on serum lectin response, cell wall sugar content, and susceptibility to phages [49]. Later, an additional phylotype, type III, was defined [50]. Within type I, P. acnes can be further separated into clades IA1, IA2, IB, and IC based on the Belfast MLST scheme [51] or I-1a, I-1b, and I-2 based on the Aarhus MLST scheme [52]. With the whole-genome sequencing effort of a large number of P. acnes isolates [48], higher resolution of the phylogeny became available. Based on the single nucleotide polymorphisms (SNPs) identified throughout the core genome regions, P. acnes can be classified into phylogenetic clades IA-1, IA-2, IB-1, IB-2, IB-3, IC, II, and III [10, 48]. Table 1 summarizes the corresponding nomenclatures of the phylogenetic clades based on the whole-genome sequences and different MLST schemes [48, 51, 52]. Additionally, based on the 16S ribosomal RNA (rRNA) sequences, P. acnes can be classified into multiple ribotypes (RTs) with RTs 1–10 being the most common RTs found in the population [10]. These classifications are useful in understanding the associations between P. acnes strains and disease or healthy skin (Table 1). Strains from clades IA-2, (mainly RT4 and RT5), IB-1 (RT8), and IC (RT5) are strongly associated with acne. Type II strains, including RT2 and RT6, are associated with healthy non-acne skin. Strains from clades IA-1, IB-2, and IB-3 have been found in both healthy individuals and acne patients [10, 48, 53]. Type III strains are rarely found on the facial skin, but are abundant on the back and have been linked to the skin condition progressive macular hypomelanosis [54, 55].
Recent studies of P. acnes and the skin microbiome have shed new light on the strain-level differences in the roles of P. acnes in health and acne. Fitz-Gibbon et al. [10] revealed that certain P. acnes strains were enriched in acne patients, while some other strains were mostly found in healthy individuals. Tomida et al. [48] further compared the genomes of P. acnes strains isolated from healthy individuals and patients with acne, and identified that the non-core genomic regions of P. acnes strains associated with acne contain extra virulence-related genes when compared with other strains. Johnson et al. [56] showed that acne-associated strains produce more porphyrins, which are a group of proinflammatory molecules inducing inflammation in keratinocytes and aggravating tissue damage by producing reactive oxygen species. Kang et al. [57] further demonstrated that vitamin B12 supplementation alters the transcriptional activities and increases porphyrin production in acne-associated P. acnes strains, while health-associated P. acnes strains do not respond to vitamin B12 supplementation. Furthermore, several recent studies have shown that acne-associated P. acnes strains induce significant inflammatory responses in keratinocytes, sebocytes, and peripheral blood mononuclear cells, while health-associated strains do not [58,59,60,61]. These studies suggest that different strains of P. acnes may play different roles in skin health and acne pathogenesis.
Multiple other skin bacteria colonize the external surface of the skin, some of which may play a role in maintaining skin health or exacerbating diseases. Staphylococcus epidermidis, Staphylococcus hominis, and other coagulase-negative staphylococcal species can be found on the skin of healthy and acne individuals [62]. In acne skin, the relative abundance of S. epidermidis increases at the expense of P. acnes [49]. Several studies suggest that P. acnes can be inhibited by S. epidermidis. Wang et al. [63] showed that S. epidermidis strains could produce succinic acid, which has anti-P. acnes activity. The study by Christensen et al. [64] suggested that S. epidermidis possesses a functional ESAT-6 (early secreted antigenic target of 6 kDa) secretion system, which could inhibit P. acnes growth through polymorphic toxins that are antibacterial. Additionally, it was shown that S. epidermidis secretes staphylococcal lipoteichoic acid, which could reduce P. acnes-associated inflammation by inducing expression of miR-143 and inhibiting TLR-2 expression in keratinocytes [65]. These studies suggest that Staphylococci, especially S. epidermidis, may protect skin against acne. However, this hypothesis requires further examination.
2.2 Malassezia and Acne
Malassezia has been thought to induce acne [66]. Malassezia is the most abundant fungal organism on the skin, co-existing with P. acnes and other bacterial species. In a study by Hu et al. [67], acne lesions were significantly reduced after administration of antifungal drugs. The authors suggested that Malassezia, not P. acnes, was potentially the cause of refractory acne [67]. The findings from several other studies are in support of this hypothesis. Song et al. [68] and Numata et al. [69] reported that Malassezia restricta and Malassezia globosa can be isolated from young acne patients. Akaza et al. [70] showed that the lipase activity of Malassezia is ~ 100 times higher than that of P. acnes. Malassezia can also hydrolyze triglycerides in the sebum to produce free fatty acids, which may affect the abnormal keratinization of hair follicular ducts, chemotize polymorphonuclear neutrophils [71, 72], and promote secretion of pro-inflammatory cytokines from keratinocytes and monocytes [73, 74]. The role of Malassezia in acne pathogenesis remains to be further investigated.
3 Antibiotics in Acne Treatment
Bacterial factors and inflammation are both thought to contribute to acne pathogenesis. Although acne is not a typical infectious disease, the use of antibiotics has been the mainstay in acne treatment for over 40 years. Topical antibiotics are largely used for their bactericidal effects against P. acnes. Oral antibiotics have anti-inflammatory effects in addition to antimicrobial effects, which target both P. acnes and host immune response [4, 75, 76].
Based on several treatment guidelines and expert consensus documents, macrolides, clindamycin, and tetracyclines are recommended as the first-line therapy in the acute inflammatory phase of acne [77,78,79,80,81,82]. Erythromycin, clarithromycin, roxithromycin, and azithromycin are macrolides. Clindamycin belongs to lincosamides. Tetracyclines for acne treatment mainly include tetracycline, doxycycline, and minocycline. The effects of macrolides, clindamycin, and tetracyclines on the skin microbiome, including the target bacterium P. acnes and other non-target bacteria, and the associated issue of antibiotic resistance are discussed in Sects. 3.1–3.3. Several other antibiotics, such as trimethoprim–sulfamethoxazole, levofloxacin, rifampin, dapsone, and metronidazole, may also be used in acne treatment. However, current data on the effects of these antibiotics are limited in scope and quality. Additional studies are needed to address multiple knowledge gaps regarding these antibiotics.
3.1 Influence of Antibiotic Use on P. acnes
3.1.1 Effect of Macrolides and Clindamycin
Erythromycin and clindamycin have been widely used in the last 40 years in acne treatment, and are still frequently prescribed by physicians. Long-term use of oral macrolides for acne treatment facilitates the increase of macrolide-resistant P. acnes strains [83]. In recent years, increasing levels of resistance of P. acnes to macrolides and clindamycin have been reported in several regions of the world [83]. In some countries, the resistance of P. acnes to erythromycin is over 50% [83, 84], and the resistance to azithromycin reaches 82–100% [85, 86]. Similarly, the resistance of P. acnes to clindamycin increased from 4% in 1999 to 90.4% in 2016 [85, 87]. There was a high proportion (52%) of acne patients who carried at least one P. acnes strain resistant to clindamycin [88]. When topical clindamycin was administered for 16 weeks for acne treatment, the amount of resistant P. acnes was increased by 16 times from the baseline [89]. After antibiotic treatment is ended, tolerant P. acnes strains may remain on the skin for a considerably long period of time, and the presence of resistant P. acnes strains manifests as a re-occurrence of acne [4]. Furthermore, when patients are treated again with antibiotics, the efficacy of such drugs is reduced or voided [4, 90].
Different P. acnes strains exhibit a varying degree of antibiotic resistance. Based on multiple recent studies of P. acnes isolates collected from different geographic areas including Italy, Sweden, UK, Australia, USA [10, 47, 51, 91], Denmark [88], and Greece [92], RT4 and RT5 strains, which are mostly clonal complex 3 (CC3) strains and some CC18 strains based on MLST [51, 88], accounted for 85–95% of the antibiotic-resistant strains [47, 51, 91, 92]. The underlying molecular mechanisms for resistance include point mutations G2057A, A2058G, and A2059G in the domain V of 23S rRNA, as well as the presence of erm(X) gene [92,93,94,95,96]. It is common that resistance to erythromycin correlates with resistance to clindamycin [93]. Cross-resistance to erythromycin and clindamycin of P. acnes isolates from acne patients varies from 11.6 to 100%, as reported in different studies [51, 97,98,99,100].
To reduce the emergence of antibiotic resistance, it is currently recommended that topical antibiotics be used in combination with benzoyl peroxide (BPO) or retinoid in acne treatment [83]. Studies have shown that combining clindamycin with BPO or retinoid for topical application not only significantly reduced the total number of P. acnes on the skin, but also lowered antibiotic resistance of P. acnes to erythromycin and clindamycin [101].
3.1.2 Effect of Tetracyclines
Tetracyclines are another class of antibiotics frequently used for treating moderate to severe acne. Although this group of antibiotics is still largely active against the majority of P. acnes isolates, antibiotic resistance is rising and needs the attention of the medical field. The rate of resistance to tetracycline differed from 2 to 30% in studies from different geographic regions in recent years [86, 97, 99, 101]. In parallel, resistance to doxycycline among isolated P. acnes strains varied between 2 and 44.2% [85, 86, 97, 101]. The combined resistance to tetracycline and doxycycline ranged from 1.2 to 100% in different groups of patients [99, 102]. In contrast to this high resistance rate to tetracycline and doxycycline, a lower resistance rate to minocycline (< 2%) was observed in Europe, Latin America, Northern America, and parts of Asia. This makes minocycline the most effective agent in the tetracycline class for acne treatment [85, 92, 97]. The resistance mechanism against tetracyclines is a G1058C mutation in P. acnes 16S rRNA gene [96]. Additionally, an amino acid substitution in the ribosomal S10 protein contributes to reduced doxycycline susceptibility [103].
3.2 Influence of Antibiotic Use on Other Skin Bacteria
3.2.1 Effect of Macrolides and Clindamycin
The use of macrolides and clindamycin in acne treatment results in resistance in other skin bacteria in addition to P. acnes. At least 30% of S. epidermidis isolates from acne patients were resistant to erythromycin, roxithromycin, and clindamycin [104]. Harkaway et al. [105] reported that after 12-week treatment with topical erythromycin alone, erythromycin-tolerant S. epidermidis became predominant on the skin surface, and the relative abundance of S. aureus at nostrils rose from 15 to 40%. Similarly, Mills et al. [106] reported that in acne treatment with topical erythromycin, the proportion of patients with erythromycin-tolerant staphylococci on the face was 87% at baseline, and increased to 98% at week 12 of treatment. Furthermore, the proportion of patients with resistant staphylococci was only slightly reduced 12 weeks after drug withdrawal. The average density of tolerant bacteria at non-treated sites, such as the back, increased at the end of the treatment. Transmission of such bacteria to different sites may cause serious consequences [106]. Like macrolides, clindamycin exerts selection pressure on both P. acnes and staphylococci. The study by Nakase et al. [95], which analyzed the correlation of antimicrobial resistance between P. acnes and S. epidermidis, reported that clindamycin-resistant S. epidermidis strains were isolated from more than 80% of the patients who also carried clindamycin-resistant P. acnes.
3.2.2 Effect of Tetracyclines
There are few established data on the effect of tetracyclines on the skin bacteria besides P. acnes. Doxycycline 40 mg modified release has been used for the treatment of inflammatory lesions in moderate and severe acne. Limited evidence suggested that this dose showed no effect on the normal skin flora as well as the rate of antibiotic resistance while being effective in reducing acne lesions [76, 107].
Lymecyclin and sarecycline are new members of tetracyclines in acne therapy. A recent study based on 16S rRNA sequencing demonstrated that at 6 weeks after lymecyclin treatment, the relative abundance of Propionibacterium on the cheeks of patients decreased, but the relative abundances of Streptococcus, Staphylococcus, Micrococcus, and Corynebacterium increased. Changes in this microbial community after drug withdrawal were not investigated [108]. Sarecycline (two phase III clinical trials completed in 2017) has a narrow antibacterial spectrum relative to other tetracyclines. It might have less selective pressure on enteric Gram-negative bacteria, but there are no data available on its influence on the skin microbiome [109].
3.3 Applications of Antibiotics in Acne Treatment
The growing prevalence of antibiotic resistance in P. acnes and other skin commensal bacteria is becoming increasingly alarming. For mild-to-moderate acne, topical antibiotic monotherapy is not recommended. Topical retinoid, BPO, or a combination therapy (topical retinoid + BPO, topical antibiotic + BPO, topical retinoid + topical antibiotic + BPO) is recommended [110]. For moderate-to-severe acne, the recommended first-line treatment is oral antibiotics combined with BPO and/or a topical retinoid. Oral antibiotic monotherapy is not recommended [111]. To reduce antibiotic-resistant microorganisms on the skin, as alternative treatments, BPO can be used for at least 5–7 days between antibiotic courses [5]. Oral contraceptives or anti-androgens may be another alternative for some female patients. To obtain better clinical efficacy and reduce antibiotic resistance, information on past exposure to macrolides or clindamycin should suggest avoidance of prescription of these antibiotics. Given that some acne patients are colonized by antibiotic-resistant P. acnes strains, Sinnott et al. [112] recommended that swabbing, culturing, and testing for resistant strains may be one way to help avoid long-term use of ineffective antibiotics.
The recommended minimum course of acne treatment with oral antibiotics is 6–8 weeks. Oral antibiotics may continue to be used after taking effect, but should not be used for longer than 12 weeks [83, 113]. However, it is reported that in practice, 17.5% of antibiotic treatment courses last ≥ 6 months and 7% of the treatments last over 9 months, with an average treatment time of 125–129 days [114, 115]. The long-term use of antibiotics may significantly alter the skin microbiome and increase drug resistance. Future longitudinal studies of long-term use of antibiotics may shed light on its effect on the composition and dynamics of the microbiome [83].
4 Conclusions and Outlook
Although the pathogenesis mechanisms of acne have not yet been fully elucidated, it is recognized that P. acnes and inflammatory response play important roles in the development of the disease. The use of bactericidal and anti-inflammatory antibiotics remains an important strategy for treating acne. Thus, rational selection of antibiotics according to the classification of P. acnes strains and corresponding drug susceptibility is preferred. However, this recommendation has not yet gained sufficient attention in clinical practice.
Given the rapid emergence of antibiotic resistance on the global scale and considering the effects of antibiotic use on the human microbiome, alternative clinical practice to antibiotic prescription in treating microbe-related diseases has become critical. A recently published study suggested a potential vaccination approach against acne by targeting Christie–Atkins–Munch–Petersen (CAMP) factor as an antigen [116]. Meanwhile, other studies showed promise in microbiome-based therapies, which may shift the balance among the microbial members, influence the function of immune cells, and prevent diseases while restoring a healthy microbiome [117,118,119]. In one such study, Nakatsuji et al. [119] showed that reintroduction of coagulase-negative Staphylococcus (CoNS) strains, which produce antimicrobial peptides, to patients with atopic dermatitis decreased S. aureus colonization on the skin. The study demonstrated how commensal skin bacteria can defend against pathogens and suggested that correcting microbiome dysbiosis may potentially be used to treat or improve certain conditions. Future studies on how to effectively reduce the load of pathogenic microorganisms and inflammation while preserving the balance of the commensal microflora may lead to potential new therapies.
References
White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34–7.
Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474–85.
Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease study 2013. JAMA Dermatol. 2017;153:406–12.
Thiboutot D, Gollnick H, Bettoli V, Dreno B, Kang S, Leyden JJ, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60:S1–50.
Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49:S1–37.
Barnard E, Li H. Shaping of cutaneous function by encounters with commensals. J Physiol. 2017;595:437–50.
Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–9.
Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA. 2007;104:2927–32.
Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–2.
Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133:2152–60.
Oh J, Byrd AL, Deming C, Conlan S, Program NCS, Kong HH, et al. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514:59–64.
Barnard E, Shi B, Kang D, Craft N, Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep. 2016;6:39491.
Meisel JS, Sfyroera G, Bartow-McKenney C, Gimblet C, Bugayev J, Horwinski J, et al. Commensal microbiota modulate gene expression in the skin. Microbiome. 2018;6:20.
Liu J, Yan R, Zhong Q, Ngo S, Bangayan NJ, Nguyen L, et al. The diversity and host interactions of Propionibacterium acnes bacteriophages on human skin. ISME J. 2015;9:2078–93.
Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–7.
Dekio I, Hayashi H, Sakamoto M, Kitahara M, Nishikawa T, Suematsu M, et al. Detection of potentially novel bacterial components of the human skin microbiota using culture-independent molecular profiling. J Med Microbiol. 2005;54:1231–8.
Leyden JJ, McGinley KJ, Nordstrom KM, Webster GF. Skin microflora. J Invest Dermatol. 1987;88:65s–72s.
Unna PG. The histopathology of the diseases of the skin (Translated by N. Walker). New York: Macmillan & Co; 1896.
La Sabouraud H. seborrhee grasse et la pelade. Ann Inst Pasteur Lilly. 1897;11:134–59.
Douglas HC, Gunter SE. The taxonomic position of Corynebacterium acnes. J Bacteriol. 1946;52:15–23.
McDowell A, Barnard E, Liu J, Li H, Patrick S. Emendation of Propionibacterium acnes subsp. acnes (Deiko et al. 2015) and proposal of Propionibacterium acnes type II as Propionibacterium acnes subsp. defendens subsp. nov. Int J Syst Evol Microbiol. 2016;66:5358–65.
Scholz CF, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016;66:4422–32.
Alexeyev OA, Dekio I, Layton AM, Li H, Hughes H, Morris T, et al. Why we continue to use the name Propionibacterium acnes. Br J Dermatol. 2018;179:1227.
Leyden JJ, McGinley KJ, Vowels B. Propionibacterium acnes colonization in acne and nonacne. Dermatology. 1998;196:55–8.
Perkins AC, Maglione J, Hillebrand GG, Miyamoto K, Kimball AB. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21:223–30.
Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, Foster KW, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56–9.
McGinley KJ, Webster GF, Ruggieri MR, Leyden JJ. Regional variations in density of cutaneous propionibacteria: correlation of Propionibacterium acnes populations with sebaceous secretion. J Clin Microbiol. 1980;12:672–5.
Iinuma K, Sato T, Akimoto N, Noguchi N, Sasatsu M, Nishijima S, et al. Involvement of Propionibacterium acnes in the augmentation of lipogenesis in hamster sebaceous glands in vivo and in vitro. J Invest Dermatol. 2009;129:2113–9.
Saint-Leger D, Bague A, Cohen E, Chivot M. A possible role for squalene in the pathogenesis of acne. I. In vitro study of squalene oxidation. Br J Dermatol. 1986;114:535–42.
Burkhart CG, Burkhart CN. Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol. 2007;57:722–4.
Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br J Dermatol. 2012;167:50–8.
Jarrousse V, Castex-Rizzi N, Khammari A, Charveron M, Dreno B. Modulation of integrins and filaggrin expression by Propionibacterium acnes extracts on keratinocytes. Arch Dermatol Res. 2007;299:441–7.
Isard O, Knol AC, Aries MF, Nguyen JM, Khammari A, Castex-Rizzi N, et al. Propionibacterium acnes activates the IGF-1/IGF-1R system in the epidermis and induces keratinocyte proliferation. J Invest Dermatol. 2011;131:59–66.
Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–41.
Nakatsuji T, Liu YT, Huang CP, Zouboulis CC, Gallo RL, Huang CM. Vaccination targeting a surface sialidase of P. acnes: implication for new treatment of acne vulgaris. PLoS One. 2008;3:51.
Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–32.
Sugisaki H, Yamanaka K, Kakeda M, Kitagawa H, Tanaka K, Watanabe K, et al. Increased interferon-gamma, interleukin-12p40 and IL-8 production in Propionibacterium acnes-treated peripheral blood mononuclear cells from patient with acne vulgaris: host response but not bacterial species is the determinant factor of the disease. J Dermatol Sci. 2009;55:47–52.
Scott DG, Cunliffe WJ, Gowland G. Activation of complement-a mechanism for the inflammation in acne. Br J Dermatol. 1979;101:315–20.
Terui T, Rokugo M, Kato T, Tagami H. Analysis of the proinflammatory property of epidermal cyst contents: chemotactic C5a anaphylatoxin generation. Arch Dermatol Res. 1989;281:31–4.
Li ZJ, Choi DK, Sohn KC, Seo MS, Lee HE, Lee Y, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747–56.
Puhvel SM, Reisner RM. The production of hyaluronidase (hyaluronate lyase) by Corynebacterium acnes. J Invest Dermatol. 1972;58:66–70.
Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6:555–8.
Ingham E, Holland KT, Gowland G, Cunliffe WJ. Purification and partial characterization of an acid phosphatase (EC 3.1.3.2) produced by Propionibacterium acnes. J Gen Microbiol. 1980;118:59–65.
Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–53.
Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y, et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS One. 2013;8:e55380.
Wang Y, Dai A, Huang S, Kuo S, Shu M, Tapia CP, et al. Propionic acid and its esterified derivative suppress the growth of methicillin-resistant Staphylococcus aureus USA300. Benef Microbes. 2014;5:161–8.
Kasimatis G, Fitz-Gibbon S, Tomida S, Wong M, Li H. Analysis of complete genomes of Propionibacterium acnes reveals a novel plasmid and increased pseudogenes in an acne associated strain. Biomed Res Int. 2013;2013:918320.
Tomida S, Nguyen L, Chiu BH, Liu J, Sodergren E, Weinstock GM, et al. Pan-genome and comparative genome analyses of propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. MBio. 2013;4:e00003–13.
Johnson JL, Cummins CS. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J Bacteriol. 1972;109:1047–66.
McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57:218–24.
McDowell A, Barnard E, Nagy I, Gao A, Tomida S, Li H, et al. An expanded multilocus sequence typing scheme for propionibacterium acnes: investigation of ‘pathogenic’, ‘commensal’ and antibiotic resistant strains. PLoS One. 2012;7:e41480.
Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS One. 2010;5:e12277.
McDowell A, Nagy I, Magyari M, Barnard E, Patrick S. The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS One. 2013;8:e70897.
Barnard E, Liu J, Yankova E, Cavalcanti SM, Magalhaes M, Li H, et al. Strains of the Propionibacterium acnes type III lineage are associated with the skin condition progressive macular hypomelanosis. Sci Rep. 2016;6:31968.
Petersen RL, Scholz CF, Jensen A, Bruggemann H, Lomholt HB. Propionibacterium acnes phylogenetic type III is associated with progressive macular hypomelanosis. Eur J Microbiol Immunol (Bp). 2017;7:37–45.
Johnson T, Kang D, Barnard E, Li H. Strain-level differences in porphyrin production and regulation in Propionibacterium acnes elucidate disease associations. mSphere. 2016;1:e00015–23.
Kang D, Shi B, Erfe MC, Craft N, Li H. Vitamin B12 modulates the transcriptome of the skin microbiota in acne pathogenesis. Sci Transl Med. 2015;7:293ra103.
Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–8.
Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–205.
Agak GW, Kao S, Ouyang K, Qin M, Moon D, Butt A, et al. Phenotype and antimicrobial activity of Th17 cells induced by Propionibacterium acnes strains associated with healthy and acne skin. J Invest Dermatol. 2018;138:316–24.
Yu Y, Champer J, Agak GW, Kao S, Modlin RL, Kim J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Invest Dermatol. 2016;136:2221–8.
Kloos WE, Musselwhite MS. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol. 1975;30:381–5.
Wang Y, Kuo S, Shu M, Yu J, Huang S, Dai A, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014;98:411–24.
Christensen GJ, Scholz CF, Enghild J, Rohde H, Kilian M, Thurmer A, et al. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genom. 2016;17:152.
Xia X, Li Z, Liu K, Wu Y, Jiang D, Lai Y. Staphylococcal LTA-induced miR-143 inhibits Propionibacterium acnes-mediated inflammatory response in skin. J Invest Dermatol. 2016;136:621–30.
Akaza N, Akamatsu H, Numata S, Yamada S, Yagami A, Nakata S, et al. Microorganisms inhabiting follicular contents of facial acne are not only Propionibacterium but also Malassezia spp. J Dermatol. 2016;43:906–11.
Hu G, Wei YP, Feng J. Malassezia infection: is there any chance or necessity in refractory acne? Chin Med J (Engl). 2010;123:628–32.
Song YC, Hahn HJ, Kim JY, Ko JH, Lee YW, Choe YB, et al. Epidemiologic study of Malassezia yeasts in acne patients by analysis of 26S rDNA PCR-RFLP. Ann Dermatol. 2011;23:321–8.
Numata S, Akamatsu H, Akaza N, Yagami A, Nakata S, Matsunaga K. Analysis of facial skin-resident microbiota in Japanese acne patients. Dermatology. 2014;228:86–92.
Akaza N, Akamatsu H, Takeoka S, Sasaki Y, Mizutani H, Nakata S, et al. Malassezia globosa tends to grow actively in summer conditions more than other cutaneous Malassezia species. J Dermatol. 2012;39:613–6.
Katsuta Y, Iida T, Inomata S, Denda M. Unsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermis. J Invest Dermatol. 2005;124:1008–13.
Webster GF. Inflammation in acne vulgaris. J Am Acad Dermatol. 1995;33:247–53.
Kesavan S, Walters CE, Holland KT, Ingham E. The effects of Malassezia on pro-inflammatory cytokine production by human peripheral blood mononuclear cells in vitro. Med Mycol. 1998;36:97–106.
Akaza N, Akamatsu H, Takeoka S, Mizutani H, Nakata S, Matsunaga K. Increased hydrophobicity in Malassezia species correlates with increased proinflammatory cytokine expression in human keratinocytes. Med Mycol. 2012;50:802–10.
Dreno B, Thiboutot D, Gollnick H, Bettoli V, Kang S, Leyden JJ, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330–4.
Skidmore R, Kovach R, Walker C, Thomas J, Bradshaw M, Leyden J, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Arch Dermatol. 2003;139:459–64.
Eichenfield LF, Krakowski AC, Piggott C, Del Rosso J, Baldwin H, Friedlander SF, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131(Suppl 3):S163–86.
Goh CL, Abad-Casintahan F, Aw DC, Baba R, Chan LC, Hung NT, et al. South-East Asia study alliance guidelines on the management of acne vulgaris in South-East Asian patients. J Dermatol. 2015;42:945–53.
Gollnick HP, Bettoli V, Lambert J, Araviiskaia E, Binic I, Dessinioti C, et al. A consensus-based practical and daily guide for the treatment of acne patients. J Eur Acad Dermatol Venereol. 2016;30:1480–90.
Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945–973.e33.
Hayashi N, Akamatsu H, Iwatsuki K, Shimada-Omori R, Kaminaka C, Kurokawa I, et al. Japanese Dermatological Association Guidelines: guidelines for the treatment of acne vulgaris 2017. J Dermatol. 2018;45:898–935.
Thiboutot DM, Dreno B, Abanmi A, Alexis AF, Araviiskaia E, Barona Cabal MI, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78:S1–S23.e21.
Walsh TR, Efthimiou J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:e23–33.
Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14:742–50.
Sardana K, Gupta T, Kumar B, Gautam HK, Garg VK. Cross-sectional pilot study of antibiotic resistance in Propionibacterium Acnes strains in Indian acne patients using 16S-RNA polymerase chain reaction: a comparison among treatment modalities including antibiotics, benzoyl peroxide, and isotretinoin. Indian J Dermatol. 2016;61:45–52.
Gonzalez R, Welsh O, Ocampo J, Hinojosa-Robles RM, Vera-Cabrera L, Delaney ML, et al. In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in northern Mexico. Int J Dermatol. 2010;49:1003–7.
Kurokawa I, Nishijima S, Kawabata S. Antimicrobial susceptibility of Propionibacterium acnes isolated from acne vulgaris. Eur J Dermatol. 1999;9:25–8.
Lomholt HB, Kilian M. Clonality and anatomic distribution on the skin of antibiotic resistant and sensitive Propionibacterium acnes. Acta Derm Venereol. 2014;94:534–8.
Cunliffe WJ, Holland KT, Bojar R, Levy SF. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117–33.
Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395–403.
Liu J, Cheng A, Bangayan NJ, Barnard E, Curd E, Craft N, et al. Draft genome sequences of Propionibacterium acnes type strain ATCC6919 and antibiotic-resistant strain HL411PA1. Genome Announc. 2014;2:e00740-14.
Giannopoulos L, Papaparaskevas J, Refene E, Daikos G, Stavrianeas N, Tsakris A. MLST typing of antimicrobial-resistant Propionibacterium acnes isolates from patients with moderate to severe acne vulgaris. Anaerobe. 2015;31:50–4.
Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, Bettoli V, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003;148:467–78.
El-Mahdy TS, Abdalla S, El-Domany R, Mohamed MS, Ross JI, Snelling AM. Detection of a new erm(X)-mediated antibiotic resistance in Egyptian cutaneous propionibacteria. Anaerobe. 2010;16:376–9.
Nakase K, Nakaminami H, Takenaka Y, Hayashi N, Kawashima M, Noguchi N. Relationship between the severity of acne vulgaris and antimicrobial resistance of bacteria isolated from acne lesions in a hospital in Japan. J Med Microbiol. 2014;63:721–8.
Ross JI, Snelling AM, Eady EA, Cove JH, Cunliffe WJ, Leyden JJ, et al. Phenotypic and genotypic characterization of antibiotic-resistant Propionibacterium acnes isolated from acne patients attending dermatology clinics in Europe, the U.S.A., Japan and Australia. Br J Dermatol. 2001;144:339–46.
Grech I. Susceptibility profiles of Propionibacterium acnes isolated from patients with acne vulgaris. J Glob Antimicrob Resist. 2014;2:35–8.
Mendoza N, Hernandez PO, Tyring SK, Haitz KA, Motta A. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol. 2013;52:688–92.
Luk NM, Hui M, Lee HC, Fu LH, Liu ZH, Lam LY, et al. Antibiotic-resistant Propionibacterium acnes among acne patients in a regional skin centre in Hong Kong. J Eur Acad Dermatol Venereol. 2013;27:31–6.
Schafer F, Fich F, Lam M, Garate C, Wozniak A, Garcia P. Antimicrobial susceptibility and genetic characteristics of Propionibacterium acnes isolated from patients with acne. Int J Dermatol. 2013;52:418–25.
Jackson JM, Fu JJ, Almekinder JL. A randomized, investigator-blinded trial to assess the antimicrobial efficacy of a benzoyl peroxide 5%/clindamycin phosphate 1% gel compared with a clindamycin phosphate 1.2%/tretinoin 0.025% gel in the topical treatment of acne vulgaris. J Drugs Dermatol. 2010;9:131–6.
Dumont-Wallon G, Moyse D, Blouin E, Dreno B. Bacterial resistance in French acne patients. Int J Dermatol. 2010;49:283–8.
Nakase K, Nakaminami H, Takenaka Y, Hayashi N, Kawashima M, Noguchi N. Propionibacterium acnes is developing gradual increase in resistance to oral tetracyclines. J Med Microbiol. 2017;66:8–12.
Nishijima S, Kurokawa I, Katoh N, Watanabe K. The bacteriology of acne vulgaris and antimicrobial susceptibility of Propionibacterium acnes and Staphylococcus epidermidis isolated from acne lesions. J Dermatol. 2000;27:318–23.
Harkaway KS, McGinley KJ, Foglia AN, Lee WL, Fried F, Shalita AR, et al. Antibiotic resistance patterns in coagulase-negative staphylococci after treatment with topical erythromycin, benzoyl peroxide, and combination therapy. Br J Dermatol. 1992;126:586–90.
Mills O Jr, Thornsberry C, Cardin CW, Smiles KA, Leyden JJ. Bacterial resistance and therapeutic outcome following three months of topical acne therapy with 2% erythromycin gel versus its vehicle. Acta Derm Venereol. 2002;82:260–5.
Toossi P, Farshchian M, Malekzad F, Mohtasham N, Kimyai-Asadi A. Subantimicrobial-dose doxycycline in the treatment of moderate facial acne. J Drugs Dermatol. 2008;7:1149–52.
Kelhala HL, Aho VTE, Fyhrquist N, Pereira PAB, Kubin ME, Paulin L, et al. Isotretinoin and lymecycline treatments modify the skin microbiota in acne. Exp Dermatol. 2018;27:30–6.
Leyden JJ, Sniukiene V, Berk DR, Kaoukhov A. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17:333–8.
Adler BL, Kornmehl H, Armstrong AW. Antibiotic resistance in acne treatment. JAMA Dermatol. 2017;153:810–1.
Nast A, Dreno B, Bettoli V, Bukvic Mokos Z, Degitz K, Dressler C, et al. European evidence-based (S3) guideline for the treatment of acne+update 2016-short version. J Eur Acad Dermatol Venereol. 2016;30:1261–8.
Sinnott SJ, Bhate K, Margolis DJ, Langan SM. Antibiotics and acne: an emerging iceberg of antibiotic resistance? Br J Dermatol. 2016;175:1127–8.
Bienenfeld A, Nagler AR, Orlow SJ. Oral antibacterial therapy for acne vulgaris: an evidence-based review. Am J Clin Dermatol. 2017;18:469–90.
Lee YH, Liu G, Thiboutot DM, Leslie DL, Kirby JS. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014;71:70–6.
Straight CE, Lee YH, Liu G, Kirby JS. Duration of oral antibiotic therapy for the treatment of adult acne: a retrospective analysis investigating adherence to guideline recommendations and opportunities for cost-savings. J Am Acad Dermatol. 2015;72:822–7.
Wang Y, Hata TR, Tong YL, Kao MS, Zouboulis CC, Gallo RL, et al. The anti-inflammatory activities of Propionibacterium acnes CAMP factor-targeted acne vaccines. J Invest Dermatol. 2018;138:2355–64.
Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell. 2018;172(784–796):e718.
Nakatsuji T, Chen TH, Butcher AM, Trzoss LL, Nam SJ, Shirakawa KT, et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv. 2018;4:eaao4502.
Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9:eaah4680.
Acknowledgements
We thank He Yanyan, Zeng Rong, and Liu Yuzhen from Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College (CAMS&PUMC) for their input.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by National Institutes of Health (NIH) grant (R01GM099530) from National Institute of General Medical Sciences (NIGMS), National Nature Science Foundation of China (81703148, 81673087, 81502739), CAMS Innovation Fund for Medical Sciences (CIFMS 2016-I2 M-1-003), and Innovation Research on Critical Diseases (2016ZX320014).
Conflict of interest
The Regents of the University of California is the owner of three patent applications related to acne and/or healthy skin, which list H.L. as one of the inventors. H.L. is a co-founder and shareholder of SkinomiX Biosciences Inc. and Naked Biome Inc. H.X. states no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, H., Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am J Clin Dermatol 20, 335–344 (2019). https://doi.org/10.1007/s40257-018-00417-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-018-00417-3