Abstract
Introduction
Two methods are currently available for the assay of α-defensin: the enzyme-linked immunosorbent assay (ELISA) and the lateral flow test. We aimed to assess the diagnostic accuracy of synovial fluid α-defensin and to compare the accuracy of the laboratory-based test and the qualitative assessment for the diagnosis of hip and knee prosthetic infection.
Materials and methods
We searched (from inception to May 2018) MEDLINE, Scopus, EMBASE, Web of Science, and Cochrane for studies on α-defensin in the diagnosis of periprosthetic joint infection (PJI). Sensitivity, specificity, positive and negative likelihood ratio (LR), and diagnostic odds ratio were analyzed using the bivariate diagnostic random-effects model. The receiver-operating curve for each method was calculated.
Results
We included 13 articles in our meta-analysis, including 1170 patients who underwent total hip and knee arthroplasties revision; 368 (31%) had a joint infection according to MSIS and MSIS-modified criteria. Considering the false-positive result rate of 8% and false-negative result rate of 3%, pooled sensitivity and specificity were 0.90 (95% CI 0.83–0.94) and 0.95 (0.92–0.96), respectively. The area under the curve (AUC) was 0.94 (0.92–0.94). No statistical differences in terms of sensitivity and specificity were found between the laboratory-based and qualitative test. The pooled sensitivity and specificity of the two alpha-defensin assessment methods were: laboratory-based test 0.97 (95% CI 0.93–0.99) and 0.96 (95% CI 0.94–0.98), respectively; qualitative test 0.83 (95% CI 0.73–0.91) and 0.94 (95% CI 0.89–0.97), respectively.
The diagnostic odds ratio of the α-defensin laboratory based was superior to that of the qualitative test (1126.085, 95% CI 352.172–3600.702 versus 100.9, 95% CI 30.1–338.41; p < 0.001). The AUC for immunoassay and qualitative tests was 0.97 (0.95–0.99) and 0.91 (0.88–0.99), respectively.
Conclusion
Detection of α-defensin is an accurate test for diagnosis of hip and knee prosthetic infections. The diagnostic accuracy of the two alpha-defensin assessment methods is comparable. The lateral flow assay is a valid, rapid, and more available diagnostic tool, particularly to rule out PJI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periprosthetic joint infection (PJI) is one of the most serious complications after total hip arthroplasty (THA) and total knee arthroplasty (TKA). It is currently the most common indication for revision total knee arthroplasty (16.8% of all knee revisions) and the third most common indication for revision total hip arthroplasty (14.8% of all hip revisions) worldwide [1,2,3,4]. Although a definite preoperative diagnosis of septic failure is imperative for proper treatment and management, the diagnosis of PJI still remains a serious clinical challenge [5,6,7,8]. Unfortunately, no gold standard exists and no single test is available with 100% of diagnostic accuracy to detect an infection. In the past few years, the attention of diagnosticians of PJI has been focused on synovial fluid biomarkers and, in particular, on α-defensin [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Due to its promising diagnostic accuracy, synovial fluid α-defensin measurement was recently introduced in the PJI diagnostic workup [24]. The two commercially available methods for measuring α-defensin in synovial fluid are the laboratory-based test (enzyme-linked immunosorbent assay-based Synovasure® α-defensin immunoassay—Citrano Medical Laboratories, CD Diagnostics), and the Synovasure lateral flow test (Zimmer Biomet, Warsaw, Indiana). The lateral flow test kit, which is a qualitative assessment of synovial fluid α-defensin, has the advantage of immediate availability of results so it is useful for intra-operative diagnosis of PJI, with respect to quantitative evaluation. Recently, three meta-analyses compared the accuracy of these two methods for the diagnosis of PJI and concluded that the α-defensin laboratory-based test had superior overall diagnostic value to that of the qualitative test [25,26,27]. Since then, further studies that have evaluated the accuracy of the lateral flow test kit have been published, so a meta-analysis that includes these emerging studies is needed to verify the accuracy of the previous results. Furthermore, we designed the present meta-analysis because the available evidence on these two tests has not yet been investigated exclusively on hip and knee prosthetic infections. Therefore, the aim of this study was to assess the role of synovial fluid α-defensin as a biomarker for infection of hip and knee prostheses and to compare the accuracy of the laboratory-based test and the qualitative test kit to diagnose TKA and THA infections.
Materials and methods
Registration
The protocol was registered online with PROSPERO (International prospective register of systematic reviews with number CRD42017077276) [28] before commencing the review.
Alpha-defensin assessment methods
Two alpha-defensin assessment methods are available. The qualitative test includes a single use cassette, a pre-measured vial of dilution buffer, a Microsafe tube, and a sample cup. The synovial fluid is put in the sample cup and collected by the disposable Microsafe tube. The specimen is added to the pre-measured dilution buffer. Finally, three drops of the diluted synovial fluid sample were applied to the Synovasure PJI testing cassette. Test results were available within 10 min. If the level of α-defensin in the diluted mixture is greater than the cutoff concentration (> 1 mg/l), the test is considered positive.
In the laboratory-based test, aliquots for α-defensin testing were subjected to centrifugation. The immunoassay for synovial fluid α-defensin was generated using specific reagents and measured by standard enzyme-linked immunosorbent assay [22, 23]. The majority of studies have used 5.2 mg/l as the threshold value [15, 17, 22, 23].
Data sources and search strategy
We searched for studies investigating diagnostic accuracy of synovial fluid α-defensin in patients with periprosthetic knee and hip infections in the MEDLINE, Scopus, EMBASE, Web of Science, and Cochrane databases from inception to June, 2018. The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [29] methodology guidance was employed. The search strategy used a combination of the following key words: alfa-defensin OR α-defensin OR alpha defensin OR alpha-defensin OR α-defensin AND PJI OR periprosthetic joint infection OR prosthetic infection, with no language restrictions. The reference lists of selected articles were also searched for any additional articles that were not identified from the database search.
Eligibility criteria
Studies evaluated the diagnostic accuracy of Synovial fluid α-defensin measured either by the immunoassay and lateral flow test in the diagnosis of PJI of the hip and/or knee joint were included. Papers considered MSIS and later modified MSIS criteria as reference standard for the diagnosis of in knee and Hip prosthetic infection were also included. We excluded: (1) studies that do not report quantitative values of sensitivity, specificity or likelihood ratios, or diagnostic accuracy; (2) studies including joint prosthesis infection different from THA and TKA and in which we cannot extrapolate the data of TKA and THA; and (3) studies considering different diagnostic criteria in respect to MSIS and modified MSIS as reference standard to rule out PJI.
Study assessment and data extraction
Initial screening of titles and abstracts was performed by two pairs of independent reviewers. The full text was obtained for all abstracts that appeared to meet the inclusion criteria or where there was any uncertainty. Each article was assessed by two independent reviewers using the inclusion criteria and any discrepancies regarding the eligibility of an article were resolved with a third author. Relevant data were extracted from each included study. Two authors performed quality assessment of each study using the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool [30]. The QUADAS score consists of four domains: (1) patient selection, (2) index test, reference standard, (3) flow, and (4) timing. The risk of bias assessment of the four domains and the clinical applicability of the first three domains were assessed with signaling questions. Questions were answered “yes” for low risk of bias/concerns, “no” for high risk of bias/concerns, or “unclear”.
Statistical analysis
Sensitivity, specificity, positive and negative likelihood ratio, and diagnostic odds ratio (DOR) were meta-analyzed using the bivariate diagnostic random-effects model described by Reitsma et al. [31], using two separate models for the laboratory-based test and the qualitative assay. Zero counts were adjusted by 0.5, which was added to all cells if any one cell in any one study was 0. Heterogeneity between studies was tested using the I2 statistic (0–40% = not relevant; 30–60% = moderate; 50–90% = substantial; 75–100% = considerable) [32]. The DOR, which combines sensitivity and specificity, without a dependence on prevalence, was evaluated to compare the performances of the two assays.
Chi square tests were used to test the significance of differences of all diagnostic outcomes between the laboratory-based and the qualitative test. A summary receiver-operating characteristic (ROC) curve was constructed based on Monte Carlo simulations [33]. The area under the curve (AUC) was calculated using the trapezoidal rule, including the extrapolated points of the ROC curve. All analysis was conducted using the OpenMetaAnalyst software version 12.11.14 (Brown University, Providence, RI, USA) and SPSS version 23 (SPSS Inc., Chicago, IL, USA) for all statistical analyses. p ≤ 0.05 was considered significant.
Results
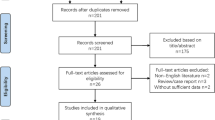
The flow diagram of our search strategy is reported in Fig. 1. Computer search and manual screening of reference lists of relevant studies identified 483 potentially relevant citations. After initial screening of titles and abstracts, the full text of 59 articles was evaluated. After detailed assessment, we excluded 46 references. The remaining 13 articles were included in our meta-analysis. Table 1 summarizes the characteristics of the included studies. In total, 1170 patients who underwent total hip and knee arthroplasties revision were evaluated, among whom 368 (31%; range 19–50%) were confirmed to have a joint infection according to MSIS and MSIS-modified criteria. The number of hip and knee arthroplasty included was explained in 11 papers. The sensitivity, specificity, positive and negative likelihood ratio, and DOR of included studies, and their corresponding pooled indices, are shown in Table 2. The AUC was 0.94 (95% CI 0.92–0.94). The results of the QUADAS-2 assessment for each study are listed in Table 3.
Diagnostic accuracy of laboratory-based vs qualitative assessment of α-defensin in synovial fluid
The pooled sensitivity of the laboratory-based and qualitative tests was 0.97 (95% CI 0.93–0.99) and 0.83 (95% CI 0.73–0.91), respectively (Fig. 2). Heterogeneity among studies for lateral flow assay was 49.9%; no heterogeneity (I2 = 0%; p = 0.99) was recorded for studies evaluating ELISA. The pooled specificity for lateral flow assay was 0.94 (95% CI 0.89–0.97), with moderate heterogeneity among studies (I2 = 40%, p = 0.123). Better results in term of specificity (0.96, 95% CI 0.94–0.98) with no heterogeneity (I2 = 0%, p = 0.75) were reported for laboratory-based tests (Fig. 3). No statistical differences in terms of sensitivity and specificity were found between the two assays. The laboratory-based α-defensin assessment had had a superior DOR value to that of the qualitative test (1126.085, 95% CI 352.172–3600.702 versus 100.9, 95% CI 30.1–338.41; p < 0.001), with a higher positive likelihood ratio (25.879, 95% CI 15.478–43.270 versus 14.530, 95% CI 7.142–29.460; p < 0.001). A lower value of negative LR was retrieved for the laboratory-based test (0.028, 95% CI 0.017–0.047 versus 0.159, 95% CI 0.074–0.313; p < 0.001). In addition, the AUC for laboratory-based and qualitative assessment was 0.97 (95% CI 0.95–0.99) and 0.91 (95% CI 0.88–0.99), respectively (Fig. 4).
Discussion
The synovial fluid analysis plays a key role in the complex diagnostic workup of prosthetic infection [34,35,36]. α-Defensin is considered the most promising biomarkers for the diagnosis of PJI. Recent meta-analyses reported on the high diagnostic accuracy of synovial α-defensin [37, 38]. The pooled estimation of the 13 studies included in our study indicates a similar result in term of specificity, but lower sensitivity rate. Considering the false-positive result rate of 8% (28/368) [1,2,3,4,5,6,7,8,9,10,11,12,13,14, 17,18,19, 22] and false-negative result rate of 3% (28/802) [9,10,11,12,13,14,15, 17, 19, 22], the pooled sensitivity and specificity were 0.901 (95% CI 0.831–0.945) and 0.952 (95% CI 0.929–0.969), respectively. The reasons why patients with joint infection report normal levels of α-defensin in synovial fluid were not clarified in the studies included. One possible explanation for negative α-defensin results was the contamination of synovial fluid samples with debris or blood, as described in different papers [12, 19]. Furthermore, the presence of the sinus tract, which reduces the local accumulation of the pathogens, may be responsible of false-negative results. In fact, four patients in two studies [12, 17] with negative α-defensin levels presented with sinus tract communication with a joint.
Previous antibiotic administration and low-grade infections could be two others reasons for false-negative results. Antibiotic treatment does not appear to give false-negative results. Shahi et al. [20] performed a comparison between patients who were treated with antibiotics and those who were not treated before diagnostic workup. They found that the results of laboratory-based test α-defensin were not influenced by antibiotic administration. Due to some papers, detailed antibiotic administration was not well defined in all the papers we included. As a result, no results on antibiotic administration can be drawn in our meta-analysis; further studies are needed to clearly understand the role of antibiotics on the diagnostic accuracy of synovial fluid α-defensin.
Low-grade infection, generally sustained by less virulent bacteria such as P. acnes or coagulase negative Staphylococci, is a condition in which clinical and laboratory criteria may misdiagnose PJI [39, 40]. This can be attributed to the fact that organisms of low virulence rapidly adhere to implants, where they evade host defense, resulting in a weak immune response [41]. The dampened inflammation could explain the increase of false-negative results of serum and synovial fluid biomarkers in low-grade PJI [39, 40].
The ability of α-defensin to role-in PJI can be altered by the presence of systemic and local inflammatory diseases or by the presence of metallosis. False positives were reported in four studies, with detailed descriptions [9, 10, 16, 17]. Metallosis was responsible of seven false-positive results. Bingham et al. [19] reported on two patients with false-positive results. In those patients, other markers of inflammation, such as C-reactive protein (CRP), cell count, and erythrocyte sedimentation rate (ESR), were elevated as well. They theorized that aseptic inflammation might be responsible for elevated α-defensin levels. However, these confounding conditions did not explain all the false results reported; in particular, those cases included in studies in which the presence of inflammatory diseases or metallosis were considered exclusion criteria [9, 10, 12, 13].
Alpha-defensin testing is also prone to false-positive results in the setting of an adverse local tissue reaction (ALTR) secondary to metal wear debris from a metal on metal (MoM) bearing or secondary to corrosion at the head–neck junction in a metal on polyethylene (MoP) bearing total hip arthroplasty (THA). Okroj et al. [42] found that in patients with ALTR false-positive results of alpha-defensin and an increased synovial WBC counts were reported. The positivity of alpha-defensin assessment may be related to the local release of this peptide from increased dead white blood cells.
The positive likelihood ratio and negative likelihood ratio of α-defensin were 18.846 (95% CI 11.887–29.880) and 0.069 (95% CI 0.36–0.132), respectively. This finding demonstrated that a positive or negative result for α-defensin indicates an increased or decreased probability of PJI. Moreover, the DOR and AUC in our study support this finding. In our analysis, α-defensin had a high diagnostic utility with elevate discriminatory test performance between patients with and without a PJI, as demonstrated by a DOR of > 1 and an AUC of 0.94 (95% CI 0.92–0.94).
Furthermore, we performed a comparison of the diagnostic accuracy of the laboratory-based test and the lateral flow test kit to diagnose TKA and THA infections. Both assays had high diagnostic accuracy, but laboratory assessment of synovial fluid α-defensin reported higher diagnostic indices for diagnosis PJI. Even lower results in terms of the pooled sensitivity and specificity were found for the qualitative test. There were no statistical differences between the two accuracy values.
With 13.5% (27 cases) of false-negative results, the pooled sensitivity of qualitative test was 83%, 6–12 points higher than that of recent meta-analyses [27, 28]. These results are lower than those described by laboratory-based test, in which only five (3%) false-negative results were reported with a sensitivity of 97%. Low virulence coagulase negative species contributed to four of the false-negative results cases in lateral flow group [9, 12].
The diagnosis for these patients was reported in five papers [9, 12, 14, 16, 17]. The majority of patients were deemed to have periprosthetic joint infection based on major criteria (four patients had a draining sinus and eight patients had the phenotypically same microorganism cultured twice) and only two patients fulfilled three minor criteria.
Furthermore, the pooled specificity of qualitative assay and laboratory-based test were 94% and 96%, reporting only 16 and 9 false-positive results, respectively. Metallosis was responsible for two false results in the α-defensin group and five negative results in the laboratory group. Both assays reported high diagnostic accuracy, as confirmed by a DOR of > 1, an AUC of > 0.9, a positive likelihood of > 10, and a negative likelihood of < 0.1. Statistically significant differences between the two assays were seen. Laboratory-based test showed higher DOR, AUC, and PLR than that of the lateral flow test.
The strengths and potential limitations of this study should be acknowledged. This study is the first metanalysis on the utility of α-defensin in hip and knee prosthetic infections. We adopted stringent eligibility criteria that led to the exclusion of studies that assessed results of α-defensin in patients with prosthetic infection that were different from TKA and THA. We only included studies on hip and knee failed prothesis using MSIS and modified MSIS as diagnostic reference standard, which has not been considered in previous reviews. Another strength of this study is the increased number of included studies on lateral flow test compared with that of recent metanalyses. We analyzed seven papers in contrast to the four and three that were described by Ahmad et al. and Erikson et al., respectively.
This study has a few drawbacks. First, this meta-analysis was performed on cohort or retrospective studies because of the lack of randomized controlled trials on the diagnostic accuracy of the two methods to detect α-defensin in synovial fluid in patients with suspicious of prosthetic infection. Moreover, the different diagnostic criteria used to rule out PJI, in addition of the small number of patients included in the studies, may have contributed to the heterogeneity among studies that emerged for some outcomes assessed in the present meta-analysis. Furthermore, many studies do not include in their diagnostic workup for PJI some criteria included in MSIS and modified MSIS criteria. This disparity could alter the ability of these diagnostic criteria to distinguish between septic and aseptic loosening and, consequently, alter the accuracy of new diagnostic tests. Lastly, some confounders, such as chronic inflammatory disease, metallosis of patients included, and use concomitant antibiotic treatment, may be responsible for false results of the α-defensin evaluation and represent another limitation of this study. We know that the presence of rheumatic disease is one of the most important reasons for false-positive result, as is the presence of metallosis.
Conclusion
Detection of α-defensin is an accurate test that helps to diagnose hip and knee prosthetic infections. The diagnostic accuracy of the two α-defensin assessment methods is comparable. The qualitative assay is a valid, rapid, and more available diagnostic tool than laboratory-based test, particularly when ruling out PJI. Although the diagnostic accuracy of qualitative assessment of synovial fluid α-defensin has been increasing, with a higher number of false and negative results, the diagnostic accuracy is lower than α-defensin laboratory-based immunoassay.
References
Lavernia C, Lee DJ, Hernandez VH (2006) The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res 446:221–226
Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ (2010) The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468:45–51
Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ (2009) The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am 91:128–133
Hossain F, Patel S, Haddad FS (2010) Midterm assessment of causes and results of revision total knee arthroplasty. Clin Orthop Relat Res 468:1221–1228
Kim YH, Park JW, Kim JS, Kim DJ (2015) The outcome of infected total knee arthroplasty: culture-positive versus culture-negative. Arch Orthop Trauma Surg 135:1459–1467
Windisch C, Brodt S, Roehner E, Matziolis G (2017) C-reactive protein course during the first 5 days after total knee arthroplasty cannot predict early prosthetic joint infection. Arch Orthop Trauma Surg 137:1115–1119
Janz V, Wassilew GI, Kribus M, Trampuz A, Perka C (2015) Improved identification of polymicrobial infection in total knee arthroplasty through sonicate fluid cultures. Arch Orthop Trauma Surg 135:1453–1457
Balato G, Franceschini V, Ascione T, Lamberti A, Balboni F, Baldini A (2018) Diagnostic accuracy of synovial fluid, blood markers, and microbiological testing in chronic knee prosthetic infections. Arch Orthop Trauma Surg 138:165–171
Balato G, Franceschini V, Ascione T, Lamberti A, D'Amato M, Ensini A, Baldini A (2017) High performance of α-defensin lateral flow assay (Synovasure) in the diagnosis of chronic knee prosthetic infections. Knee Surg Sports Traumatol Arthrosc 26:1717–1722
Berger P, Van Cauter M, Driesen R, Neyt J, Cornu O, Bellemans J (2017) Diagnosis of prosthetic joint infection with alpha-defensin using a lateral flow device: a multicentre study. Bone Joint J. 99B:1176–1182
Suda AJ, Tinelli M, Beisemann ND, Weil Y, Khoury A, Bischel OE (2017) Diagnosis of periprosthetic joint infection using alpha-defensin test or multiplex-PCR: ideal diagnostic test still not found. Int Orthop 41:1307–1313
Gehrke T, Lausmann C, Citak M, Bonanzinga T, Frommelt L, Zahar A (2018) The accuracy of the alpha defensin lateral flow device for diagnosis of periprosthetic joint infection: comparison with a gold standard. J Bone Joint Surg Am. 100:42–48
Kasparek MF, Kasparek M, Boettner F, Faschingbauer M, Hahne J, Dominkus M (2016) Intraoperative diagnosis of periprosthetic joint infection using a novel alpha defensin lateral flow assay. J Arthroplasty 31:2871–2874
Sigmund IK, Holinka J, Gamper J, Staats K, Böhler C, Kubista B, Windhager R (2017) Qualitative α defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty. Bone Joint J 99B:66–72
Renz N, Yermak K, Perka C, Trampuz A (2018) Alpha defensin lateral flow test for diagnosis of periprosthetic joint infection: not a screening but a confirmatory test. J Bone Joint Surg Am 100:742–750
Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J (2014) Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am 96:1439–1445
Bonanzinga T, Zahar A, Dütsch M, Lausmann C, Kendoff D, Gehrke T (2017) How reliable is the alpha-defensin immunoassay test for diagnosing periprosthetic joint infection? A prospective study. Clin Orthop Relat Res 475:408–415
Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA (2016) α-Defensin accuracy to diagnose periprosthetic joint infection-best available test? J Arthroplasty 31:456–460
Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B (2014) The alpha defensin-biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res 472:4006–4009
Shahi A, Parvizi J, Kazarian GS, Higuera C, Frangiamore S, Bingham J, Beauchamp C, Valle CD, Deirmengian C (2016) The alpha-defensin test for periprosthetic joint infections is not affected by prior antibiotic administration. Clin Orthop Relat Res 474:1610–1615
Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE Jr (2015) The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res 473:2229–2235
Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Booth RE Jr, Parvizi J (2015) The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res 473:198–203
Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J (2014) Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res 472:3254–3262
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N (2018) The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 33:1309–1314
Suen K, Keeka M, Ailabouni R, Tran P (2018) Synovasure 'quick test' is not as accurate as the laboratory-based α-defensin immunoassay: a systematic review and meta-analysis. Bone Joint J 100B:66–72
Eriksson HK, Nordström J, Gabrysch K, Hailer NP, Lazarinis S (2018) Does the alpha-defensin immunoassay or the lateral flow test have better diagnostic value for periprosthetic joint infection? A systematic review. Clin Orthop Relat Res 476:1065–1072
Ahmad SS, Hirschmann MT, Becker R, Shaker A, Ateschrang A, Keel MJB, Albers CE, Buetikofer L, Maqungo S, Stöckle U, Kohl S (2018) A meta-analysis of synovial biomarkers in periprosthetic joint infection: Synovasure™ is less effective than the ELISA-based alpha-defensin test. Knee Surg Sports Traumatol Arthrosc 26:3039–3047
Di Donato SL, Balato G, Mariconda M, Baldini A (2017) Quantitative vs qualitative assessment of alpha-defensin in periprosthetic joint infection: a systematic review and meta-analysis. PROSPERO CRD42017077276. https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017077276
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–34
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58:982–990
Shamseer L, Moher D, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647
Doebler P, Holling H, Böhning D (2012) A mixed model approach to meta-analysis of diagnostic studies with binary test outcome. Psychol Methods 17:418
Ahmad SS, Shaker A, Saffarini M, Chen AF, Hirschmann MT, Kohl S (2016) Accuracy of diagnostic tests for prosthetic joint infection: a systematic review. Knee Surg Sports Traumatol Arthrosc 24:3064–3074
Alijanipour P, Bakhshi H, Parvizi J (2013) Diagnosis of periprosthetic joint infection: the threshold for serological markers. Clin Orthop Relat Res 471:3186–3195
Ascione T, Pagliano P, Balato G, Mariconda M, Rotondo R, Esposito S (2016) Oral therapy, microbiological findings, and comorbidity influence the outcome of prosthetic joint infections undergoing 2-stage exchange. J Arthroplasty 32:2239–2243
Lee YS, Koo KH, Kim HJ, Tian S, Kim TY, Maltenfort MG, Chen AF (2017) Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 99:2077–2084
Yuan J, Yan Y, Zhang J, Wang B, Feng J (2017) Diagnostic accuracy of alpha-defensin in periprosthetic joint infection: a systematic review and meta-analysis. Int Orthop 41:2447–2455
Akgün D, Müller M, Perka C, Winkler T (2018) The serum level of C-reactive protein alone cannot be used for the diagnosis of prosthetic joint infections, especially in those caused by organisms of low virulence. Bone Joint J 100:1482–1486
Pérez-Prieto D, Portillo ME, Puig-Verdié L et al (2017) C-reactive protein may misdiagnose prosthetic joint infections, particularly chronic and low-grade infections. Int Orthop 41:1315–1319
Zimmerli W, Moser C (2012) Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 65:158–168
Okroj KT, Calkins TE, Kayupov E (2018) The alpha-defensin test for diagnosing periprosthetic joint infection in the setting of an adverse local tissue reaction secondary to a failed metal-on-metal bearing or corrosion at the head-neck junction. J Arthroplasty 33:1896–1898
Funding
The authors received no funding for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
One author of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. The others authors have no conflict of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balato, G., de Matteo, V., Ascione, T. et al. Laboratory-based versus qualitative assessment of α-defensin in periprosthetic hip and knee infections: a systematic review and meta-analysis. Arch Orthop Trauma Surg 140, 293–301 (2020). https://doi.org/10.1007/s00402-019-03232-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-019-03232-5