Abstract
We derive an approximate analytic expression for the diffusiophoretic mobility of a cylindrical colloidal particle oriented perpendicularly to an applied electrolyte concentration gradient field in a symmetrical electrolyte solution. This expression, which is correct to the third order of the particle zeta potential, is applicable for particles with low and moderate zeta potentials at arbitrary values of the electrical double layer thickness. This is an improvement of the mobility formula derived by Keh and Wei (2002), which is correct to the second order of the particle zeta potential. We also calculate the average diffusiophoretic mobility of a cylinder oriented at an arbitrary angle between its axis and the applied electrolyte concentration gradient field by combining the obtained mobility expression with the previously obtained mobility expression for a cylinder oriented parallel to the applied electrolyte concentration gradient field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There have been a lot of theoretical studies on diffusiophoresis of particles of various types such as rigid spheres [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] (see in particular Ref. [6] for weakly charged spheres with arbitrary electrical double layer thickness, Ref. [14] for moderately charged spheres with arbitrary electrical double layer thickness, Ref. [4, 5, 12] for highly charged spheres, and Ref. [8, 11, 13] for spheres in a general electrolyte solution), rigid cylinders [16,17,18], particles in an electrolyte with finite ion size [19, 20], dielectric liquid drops [21,22,23], conducting drops or mercury drops [24,25,26], and soft particles (polymer-coated particles) [27,28,29,30,31,32,33]. The diffusioosmosis in fibrous porous media is closely related to the diffusiophoresis of cylindrical particles. Keh and his coworkers have developed the general theory of diffusioosmosis [34,35,36,37]. Keh and Wei [34] presented an analytic formula for the transverse diffusiophoretic mobility of a weakly charged cylindrical particle oriented perpendicular to the applied electrolyte concentration gradient field. This expression is correct to the second power of the zeta potential of the particle. In the present paper, we make corrections to the third powers of zeta potentials in their formula [34] and derive an analytic diffusiophoretic mobility expression for a cylinder in a symmetrical electrolyte solution applicable for low to moderate particle zeta potentials. We also calculate the diffusiophoretic mobility of a cylinder oriented at an arbitrary angle between its axis and the applied electrolyte concentration gradient field by combining the obtained transverse mobility expression with a previously obtained expression for the tangential diffusiophoretic mobility of a cylinder oriented parallel to the applied electrolyte concentration gradient field [18].
Theory
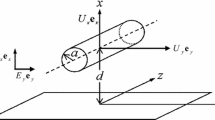
Consider an infinitely long cylinder of radius a and zeta potential ζ moving with a diffusiophoretic velocity U in an aqueous liquid of relative permittivity εr and viscosity η containing a symmetrical electrolyte of valence Z under an applied constant electrolyte concentration gradient. The ionic drag coefficient of electrolyte cations λ+ and that of electrolyte anions λ− may be different. We take a cylindrical coordinate system (r, θ, z) with its origin fixed at the center of the cylinder, where the z-axis coincides with the cylinder axis (Fig. 1). Let n+(r) be the concentration (number density) of cations at position r, n−(r) be that of anions, and n(r) be their concentrations beyond the electrical double layer around the cylinder, where n+(r) = n−(r) = n(r). Let the applied electrolyte concentration gradient be expressed in terms of n(r) as ∇n. We denote by n∞ the bulk concentration of electrolytes in the absence of ∇n. The following vector α proportional to ∇n is introduced:
where k is the Boltzmann constant, T is the absolute temperature, and e is the elementary electric charge. We assume that the field α is weak so that U is linear in α and the Reynolds number of the liquid flow is low so that inertial terms in the Navier–Stokes equation can be neglected, and the liquid can be regarded as incompressible. We also assume that no electrolyte ions can penetrate the particle surface.
We consider first the cylinder oriented perpendicularly to α and denote the diffusiophoretic velocity U by \({{\varvec{U}}}_{\perp }\). The z-axis, which coincides with the cylinder axis, is put perpendicular to α (Fig. 1).
It has previously been shown [12] that one can obtain the general expression for the diffusiophoretic velocity U of a colloidal particle in an electrolyte concentration gradient field α from the expression for its electrophoretic velocity UE in an applied electric field E by replacing E with α, since the governing electrokinetic equations take essentially the same form for U and UE [12]. The only difference is the far-field boundary condition for the deviations δμ±(r) of the electrochemical potentials of ions μ±(r) caused by α and E, where μ+(r) and μ−(r) are, respectively, the electrochemical potentials of cations and anions. The transverse diffusiophoretic velocity \({{\varvec{U}}}_{\perp }\) in a transverse field α can thus be derived from the corresponding expression for UE [38, 39], viz.,
with
and
where
are the scaled ionic drag coefficients of cations and anions. The equilibrium electric potential ψ(0)(r) is assumed to satisfy the cylindrical Poisson-Boltzmann equation, viz.,
with
where y(r) is the scaled equilibrium electric potential, κ is the Debye-Hückel parameter, and εo is the permittivity of a vacuum. Equation (9) is subject to the following boundary conditions:
The liquid fluid velocity u(r) and the deviations δμ±(r) of the ionic electrochemical potentials μ±(r) due to α are related to h(r) and ϕ±(r), respectively, as
where α is |α|. Let us define the scaled diffusiophoretic mobility \({U}_{\perp }^{*}\) as
We expand y(r) in powers of ζ, which is the solution to Eq. (9) [39], viz.,
with
where
is the scaled zeta potential and In(κa) and Kn(κa) are, respectively, the nth order modified Bessel functions of the first and second kinds.
We first derive an expression for \({U}_{\perp }^{*}\) correct to the order of ζ2. It follows from Eqs. (3), (4), (6), and (17) that the following approximate form for G(r) correct to the order of ζ2, which involves only y1(r), can be obtained:
By combining Eq. (2) with Eq. (21) for G(r) and Eq. (18) for y1(r), we obtain an approximate expression for U⊥* which is correct to the order of ζ2, viz.,
where
and
Equation (22) agrees with the result of Keh and Wei [34]. Note that f1(κa) and f2(κa) are equivalent to Θ1(κa) and Θ2(κa), respectively, in their paper (Eqs. (35a) and (35b) in Ref. [34]). Equation (22) for U⊥* consists of two terms. The first term is the electrophoresis component and the second term is the chemiphoresis component. Note also that f1(κa) given by Eq. (23) corresponds to Henry’s function for the transverse electrophoretic mobility of a cylinder oriented perpendicular to an applied electric field [38, 39].
Next let us derive an expression for U⊥* correct to the order of ζ3. This term is related to the electrophoresis component, and thus, it is proportional to β. By using the same method employed to obtain the transverse electrophoretic mobility of a cylinder of radius a correct to the order of ζ3 [39], by using Eq. (17), we finally obtain the following expression for \({U}_{\perp }^{*}\) correct to the order of ζ3:
with
and
where
and
The first and second terms on the right-hand side of Eq. (25) are equivalent to Eq. (22), and the last term is the correction term of the order of ζ3 to Eq. (22). The above functions f1(κa), f2(κa), f3(κa), and f4(κka) defined by Eqs. (23), (24), (26), and (27) in the present paper, respectively, correspond to the functions f1(κa)–f4(κa) defined in the previous paper [39] but they differ from each other by a factor of 2/3 due to different definitions in the present paper and the previous paper [39].
In the large κa limit (κa → ∞), Eq. (25) becomes
which agrees with the result for a particle with a planar surface [1, 2]. In the small κa limit (κa → 0), on the other hand, Eq. (25) becomes
It is thus seen that there are no contributions of f3(κa) and f4(κa) in the limits of κa → ∞ and κa → 0.
We next consider the tangential diffusiophoretic mobility \({U}_{\parallel }^{*}\) of a cylindrical particle oriented parallel to α. It can be shown [18] that \({U}_{\parallel }^{*}\) can be expressed as
where
Note that Eq. (32) is already correct to the order of \({\widetilde{\zeta }}^{3}\), since the next-order correction term to Eq. (32) is of the order of \({\widetilde{\zeta }}^{4}\). It is to be noted that \({U}_{\parallel }^{*}\) depends on κa unlike the case of electrophoresis, where the tangential electrophoretic mobility of a cylinder does not depend on κa [38, 39].
In the limit of κa → ∞, f(κa) tends to 1 so that Eq. (32) tends to Eq. (30), viz.,
In the opposite limit of κa → 0, f(κa) tends to 0 so that Eq. (32) tends to
It is thus seen that in the limit of κa → ∞, \({U}_{\perp }^{*}\) and \({U}_{\parallel }^{*}\) tend to the same limiting value (Eqs. (30) and (34)), while in the opposite limit of κa → 0, the limiting value of \({U}_{\parallel }^{*}\) (Eq. (35)) is twice that of \({U}_{\perp }^{*}\) (Eq. (31)).
For a cylindrical particle oriented at an arbitrary angle between its axis and the applied electrolyte concentration gradient field a, its diffusiophoretic mobility \({U}_{\mathrm{av}}^{*}\) averaged over a random distribution of orientation is given by [40]:
Results and discussion
The main result of this paper is Eq. (25) for the transverse diffusiophoretic mobility \({U}_{\perp }^{*}\) of a cylinder oriented perpendicular to an applied electrolyte concentration gradient, which is correct to the order of ζ3. Equation (25) is an improvement of Eq. (22) correct to the order of ζ2.
Since Eqs. (23), (24), (26), (27), and (33) for f1(κa)–f4(κa) and f (κa) are not very convenient for the practical use, since they involve modified Bessel functions and numerical integrations, we derive simpler approximate formula for f1(ka), f3(ka), and f4(ka) without involving modified Bessel functions and numerical integrations on the basis of the same approximation method as used in the electrophoresis problem [38, 39]. As for f2(κa), Keh and Wei [34] derived an excellent approximate expression (Eq. (38)). Thus, we find that
Also, we find that f(κa) given by Eq. (33) can be approximated with negligible errors as
By using the above equations, Eq. (25) becomes
and Eq. (32) becomes
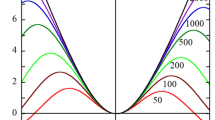
Figure 2a, b shows some results of \({U}_{\perp }^{*}\) calculated via Eq. (25) (or Eq. (42) with negligible errors) for a cylinder of radius a in an aqueous KCl solution (m+ = 0.176, m− = 0.169, β = − 0.02) (Fig. 2a) and NaCl solution (m+ = 0.258, m− = 0.169, β = − 0.2) (Fig. 2b) at 25 °C (solid curves) in comparison with those obtained via Eq. (22) (dotted curves, Keh and Wei [34]). The contribution of the correction term of order ζ3 is related to the electrophoresis component of diffusiophoresis and becomes more significant as β becomes larger. Indeed, it is seen from Fig. 2a, b that this correction contribution is larger for NaCl (β = − 0.2) than for KCl (β = − 0.02). We also see that this contribution is large for moderate values of κa (0.1 < κa < 100) and becomes small for large κa (> 100) or small κa (< 0.1), vanishing in the limit of κa → ∞ (thin double layer limit) or κa → 0 (thick double layer limit). Note that in these two limiting cases the solid and dotted curves coincide with each other.
Scaled diffusiophoretic mobility \({U}_{\perp }^{*}\) of a cylindrical colloidal particle of radius a in an aqueous electrolyte solution at 25 °C as a function of the scaled zeta potential \(\widetilde{\zeta }\) at several values of κa. Calculated via Eq. (25) (or Eq. (42) with negligible errors) (solid curves) in comparison with the results for \({U}_{\perp }^{*}\) correct to the order of ζ.2 obtained from Eq. (22) (Keh and Wei [8]) (dotted curves). Results for KCl (m+ = 0.176, m− = 0.169, β = − 0.02) (a) and NaCl (m+ = 0.258, m− = 0.169, β = − 0. 2) (b)
In Fig. 3a, b, we plot the scaled average electrophoretic mobility given by Eq. (36) of a cylinder of radius a and zeta potential ζ as a function of scaled zeta potential \(\widetilde{\zeta }\) (solid curves) in comparison with the scaled diffusiophoretic mobility \({U}_{\mathrm{sp}}^{*}\) of a sphere of the same radius a and zeta potential ζ as the cylinder, which is correct to the order of ζ3 (dotted curves) [14]. It is seen that the curve for Usp* lies between those for \({U}_{\parallel }^{*}\) and \({U}_{\perp }^{*}\) and is close to that of \({U}_{\mathrm{av}}^{*}\).
Scaled average diffusiophoretic mobility Uav* of a cylindrical colloidal particle of radius a in an aqueous electrolyte solution at 25 °C as a function of the scaled zeta potential \(\widetilde{\zeta }\) at κa = 10. Calculated via Eqs. (25) and (32) (or Eqs. (42) and (43) with negligible errors) (solid curves) in comparison with the results for the diffusiophoretic mobility Usp* for a spherical particle [14] (dotted curves). Results for KCl (m+ = 0.176, m− = 0.169, β = −0.02) (a) and NaCl (m+ = 0.258, m− = 0.169, β = −0. 2) (b)
It has been shown [1, 2] that in the case of diffusiophoresis of a sphere, the chemiphoresis component causes the particle to move toward higher electrolyte concentrations, while the electrophoresis component moves the particle toward higher or lower electrolyte concentrations depending on the sign of the particle zeta potential, which may cause a sign reversal of the diffusiophoretic mobility in some cases. Figures 2 and 3 demonstrate that a cylinder exhibits the same diffusiophoretic behavior as a sphere. That is, for electrolytes with almost equal ion mobilities such as KCl (β = −0.02, Figs. 2a and 3a), the electrophoretic contribution becomes negligible relative to chemiphoresis and almost only chemiphoresis component moves the cylinder toward higher electrolyte concentrations (positive diffusiophoretic mobility). In the case of a cylinder with large κa in NaCl (β = −0. 2, Figs. 2b and 3b), however, a particle of low to moderate positive zeta potential migrates toward lower electrolyte concentrations, while a particle of high positive zeta potential can migrate toward higher electrolyte concentrations.
Evel and others [41] measured the diffusiophoretic mobility of spherical latex particles. As for cylindrical particles, McMullen and others [42] recently performed diffusiophoresis measurement of DNA cylinders. The present theory is thus expected to apply for such systems.
Concluding remarks
Keh and Wei [34] derived an analytic expression for the transverse diffusiophoretic mobility of a cylinder in a symmetrical electrolyte solution correct to the order of ζ2. In the present paper, we have provided the next-order correction terms to the mobility expression by Keh and Wei [34] and derived Eq. (25) as well as its simpler approximate expression (Eq. (42)). These equations, which are correct to the order of ζ3, are applicable for any values of κa at low to moderate values of ζ. The contribution of the correction terms becomes larger as the magnitude of β becomes larger and becomes zero in the limits of κa → ∞ and κa → 0. We also present an approximate expression (Eq. (43)) for the tangential diffusiophoretic mobility and calculate the average diffusiophoretic mobility of a cylinder oriented at an arbitrary angle between its axis and the applied electrolyte concentration gradient field using Eq. (36).
References
Derjaguin BV, Korotkova DSS, AA, (1961) Diffusiophoresis in electrolyte solutions and its role in the mechanism of film formation of cationic latex by ionic deposition. Kolloidn Zh 23:53–58
Prieve DC (1982) Migration of a colloidal particle in a gradient of electrolyte concentration. Adv Colloid Interface Sci 16:321–335
Prieve DC, Anderson JL, Ebel JP, Lowell ME (1984) Motion of a particle generated by chemical gradients. Part 2. Electrolytes J Fluid Mech 148:247–269
Prieve DC, Roman R (1987) Diffusiophoresis of a rigid sphere through a viscous electrolyte solution. J Chem Soc Faraday Trans II 83:1287–1306
Pawar Y, Solomentsev YE, Anderson JL (1993) Polarization effects on diffusiophoresis in electrolyte gradients. J Colloid Interface Sci 155:488–498
Keh HJ, Wei YK (2000) Diffusiophoretic mobility of spherical particles at low potential and arbitrary double-layer thickness. Langmuir 16:5289–5294
Keh HJ (2016) Diffusiophoresis of charged particles and diffusioosmosis of electrolyte solutions. Curr Opin Colloid Interface Sci 24:13–22
Gupta A, Rallabandi B, Howard A, Stone HA (2019) Diffusiophoretic and diffusioosmotic velocities for mixtures of valence-asymmetric electrolytes. Phys Rev Fluids 4:043702
Gupta A, Shim S, Stone HA (2020) Diffusiophoresis: from dilute to concentrated electrolytes. Soft Matter 16:6975–6984
Shin S (2020) Diffusiophoretic separation of colloids in microfluidic flows. Phys Fluids 32:101302
Wilson JL, Shim S, Yu YE, Gupta A, Stone HA (2020) Diffusiophoresis in multivalent electrolytes. Langmuir 36:7014–7020
Ohshima H (2022) Approximate analytic expressions for the diffusiophoretic velocity of a spherical colloidal particle. Electrophoresis 43:752–756
Ohshima H (2021) Diffusiophoretic velocity of a large spherical colloidal particle in a solution of general electrolytes. Colloid Polym Sci 299:1877–1884
Ohshima H (2022) Diffusiophoresis of a moderately charged spherical colloidal particle. Electrophoresis. https://doi.org/10.1002/elps.202200035
Shim S (2022) Diffusiophoresis, diffusioosmosis, and microfluidics: surface-flow-driven phenomena in the presence of flow. Chem Rev 122(7):6986–7009
Keh HJ, Chen SB (1993) Diffusiophoresis and electrophoresis of colloidal cylinders. Langmuir 9:1142–1149
Joo SW, Lee SY, Liu J, Qian S (2010) Diffusiophoresis of an elongated cylindrical nanoparticle along the axis of a nanopore. Chem Phys Chem 11:3281–3290
Ohshima H (2022) Diffusiophoresis of a cylindrical colloidal particle oriented parallel to an electrolyte concentration gradient field. Electrophoresis. https://doi.org/10.1002/elps.202200127
Hoshyargar V, Ashrafizadeh SN, Sadeghi A (2015) Drastic alteration of diffusioosmosis due to steric effects. Phys Chem Chem Phys 17:29193
Ohshima H (2022) Ion size effect on the diffusiophoretic mobility of a large colloidal particle. Colloid Polym Sci. https://doi.org/10.1007/s00396-022-04954-6
Lou J, Lee E (2008) Diffusiophoresis of concentrated suspensions of liquid drops. J Phys Chem C 112:12455–12462
Yang F, Shin S, Stone HA (2018) Diffusiophoresis of a charged drop. J Fluid Mech 852:37–59
Wu Y, Jian E, Fan L, Tseng J, Wan R, Lee E (2021) Diffusiophoresis of a highly charged dielectric fluid droplet. Phys Fluids 33:122005
Ohshima H (2022) Diffusiophoresis of a mercury drop. Colloid Polym Sci 300:583–586
Ohshima H (2022) Relaxation effect on the diffusiophoretic mobility of a mercury drop. Colloid Polym Sci 300:593–597
Fan L, Lee E (2022) Diffusiophoresis of a highly charged conducting fluid droplet. Phys Fluids 34:062013
Huang PY, Keh HJ (2012) Diffusiophoresis of a spherical soft particle in electrolyte gradients. J Phys Chem B 116:7575–7589
Tseng S, Chung YC, Hsu JP (2015) Diffusiophoresis of a soft, pH-regulated particle in a solution containing multiple ionic species. J Colloid Interface Sci 438:196–293
Majee PS, Bhattacharyya S (2021) Impact of ion partitioning and double layer polarization on diffusiophoresis of a pH-regulated nanogel. Meccanica 56:1989–2004
Wu Y, Lee YF, Chang WC, Fan L, Jian E, Tseng J, Lee E (2021) Diffusiophoresis of a highly charged soft particle in electrolyte solutions induced by diffusion potential. Phys Fluids 33:012014
Lee YF, Chang WC, Wu Y, Fan L, Lee E (2021) Diffusiophoresis of a highly charged soft particle in electrolyte solutions. Langmuir 37:1480–1492
Ohshima H (2022) Diffusiophoretic velocity of a spherical soft particle. Colloid Polym Sci 300:153–157
Ohshima H (2022) Diffusiophoresis of a soft particle as a model for biological cells. Colloids Interfaces 6(2):24. https://doi.org/10.3390/colloids6020024
Keh HJ, Wei YK (2002) Osmosis through a fibrous medium caused by transverse electrolyte concentration gradients. Langmuir 18:10475–10485
Wei YK, Keh HJ (2003) Theory of electrokinetic phenomena in fibrous porous media caused by gradients of electrolyte concentration. Colloids Surf A: Physicochem Eng Asp 222:301–310
Keh HJ, Hsu LY (2008) Diffusioosmosis of electrolyte solutions in fibrous porous media Microfluid Nanofluid 5:347–356
Hsu LY, Keh HJ (2009) Diffusioosmosis of electrolyte solutions around a circular cylinder at arbitrary zeta potential and double-layer thickness. Ind Eng Chem Res 48:2443–2450
Ohshima H (1996) Henry’s function for electrophoresis of a cylindrical colloidal particle. J Colloid Interface Sci 180:299–301
Ohshima H (2015) Approximate analytic expression for the electrophoretic mobility of moderately charged cylindrical colloidal particles. Langmuir 31:13633–13638
de Keizer A, van der Drift WPJT, Overbeek JThG (1975) Electrophoresis of randomly oriented cylindrical Particles. Biophys Chem 3:107–108
Ebel JP, Anderson JL, Prieve DC (1988) Diffusiophoresis of latex particles in electrolyte gradients. Langmuir 4:396–406
McMullen A, Araujo G, Winter M, Stein D (2019) Osmotically driven and detected DNA translocations. Sci Rep 9:15065
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ohshima, H. Diffusiophoresis of a moderately charged cylindrical colloidal particle. Colloid Polym Sci 301, 127–133 (2023). https://doi.org/10.1007/s00396-022-05047-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-022-05047-0