Abstract
The general expression is derived for the diffusiophoretic velocity of a large spherical colloidal particle of radius a in a concentration gradient of general electrolytes of Debye-Hückel parameter κ. On the basis of this expression, simple approximate analytic expressions for the diffusiophoretic velocity correct to the order of (1/κa)0 are derived, which can be applied for large particles with κa ≥ 50 at arbitrary values of the particle zeta potential with negligible errors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Our understanding of diffusiophoresis, that is, the motion of charged colloidal particles in an electrolyte concentration gradient, has been advanced by a lot of theoretical studies [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. In particular, the readers should refer to a review article by Keh [8]. Experimentally observed diffusiophoretic mobility of latex particles was in good agreement theoretical results [25]. In a previous paper [24], we derived a general expression for the diffusiophoretic mobility of a spherical particle of radius a in a solution of symmetrical electrolytes of Debye-Hückel parameter κ. On the basis of this general expression, we obtained an approximate diffusiophoretic velocity expression correct to the order of 1/κa, which is found to be applicable for κa ≥ 20 at arbitrary values of the particle zeta potential. The obtained expression takes a much simpler form than those previously obtained. The leading-order term of the expression is correct to the order of (1/κa)0, which is applicable for κa ≥ 50 with negligible errors. In the present paper, we extend the previous theory for the case of symmetrical electrolytes to the diffusiophoresis of large spherical particles in a solution of general electrolytes and obtain approximate diffusiophoretic velocity expressions correct to the order of (1/κa)0. Our theory is based on the standard Poisson-Boltzmann theory on the electrical diffuse double layer around a colloidal particle. A comprehensive review of recent advances in the theory of diffuse double layer accounting for the effects of structural details of the ions and the solvent was given by Bohinc et al. [26]. The analytic expressions for the diffusiophoretic velocity of colloidal particles derived in the present paper can thus be applied to the case where the above effects may be neglected.
Theory
Consider a spherical particle of radius a moving with diffusiophoretic velocity U in an aqueous liquid of viscosity η and relative permittivity εr containing a general electrolyte under a constant applied gradient of electrolyte concentration. We suppose that the electrolyte consists of N ionic species with valence zi and drag coefficient λi (i = 1, 2, …, N). Let \({n}_{i}^{\infty }\) be the bulk concentration (number density) of the i th ionic species in the absence of the applied electrolyte concentration gradient. The electroneutrality condition is given by \(\sum_{i=1}^{N}{z}_{i}{n}_{i}^{\infty }=0\). Let ni(r) be the concentration (number density) of the i th ionic species at position r. The concentration gradient for the i th ionic species is expressed as ∇ni in the region beyond the electrical double layer around the particle. We treat the case where all the ionic species have the same relative concentration gradient ∇ni /\({n}_{i}^{\infty }\) and introduce a constant vector a proportional to ∇noi:
where e is the elementary electric charge, k is the Boltzmann constant, and T is the absolute temperature. The origin of the spherical polar coordinate system (r, θ, ϕ) is held fixed at the center of the particle and the polar axis (θ = 0) is put parallel to a. For a spherical particle, U is parallel to a. We treat the case where the following conditions are satisfied: (i) The electrolyte concentration gradient field a is weak so that U is linear in a, where a =|a| and U is the magnitude with sign of U (positive (negative) values of U correspond to migration toward higher (lower) electrolyte concentration). (ii) In the absence of a, the particle has a uniform surface potential, which is regarded as the particle zeta potential z at r = a, where r =|r|. (iii) The Reynolds number of the liquid flow is small enough to ignore inertial terms in the Navier–Stokes equation and the liquid can be regarded as incompressible. (iv) Electrolyte ions cannot penetrate the particle surface. (v) The liquid flow velocity relative to the particle is zero at the particle surface.
The Navier-Stoke equation for a steady incompressible liquid flow velocity u(r) = (ur(r), uθ(r), 0) at low Reynolds numbers and the continuity equation for u(r) are given by
where p(r) is the pressure, ψ(r) is the electric potential, and ρel(r) is the charge density given by
The velocity vi(r) = (vir(r), viθ(r), 0) of the i th ionic species, which is given by
satisfies the following continuity condition:
where
is the electrochemical potential of the i th ionic species and \({\mu }_{\mathrm{o}}^{i}\) is a constant terms of μi(r).
The deviations of ni(r), ψ(r), μi(r), and ρel(r) from their equilibrium values are small for a weak field a, so that we may write
where the quantities with superscript (0) refer to those at equilibrium in the absence of a.
We assume that the equilibrium concentration \({n}_{i}^{(0)}\left(r\right)\) obeys the Boltzmann distribution and the equilibrium electric potential y(0)(r) satisfies the Poisson-Boltzmann equation, viz.,
with
where y(r) is the scaled equilibrium electric potential, κ is the Debye-Hückel parameter, and εo is the permittivity of a vacuum.
The boundary conditions for ni(0)(r), ψ(0)(r),u(r), and δni(r) are given by
The ionic flows vi(r) induce the diffusion potential field, which nullifies the net electric current. The electric current density i(r) is given by
By substituting Eqs. (5), (8), and (10) into Eq. (22) and neglecting the products of the small quantities u, δni, and δμi, we obtain
which must be zero beyond the particle double layer. We thus find that (see Appendix)
with
where
is the scaled drag coefficient of i th ionic species. It follows from Eqs. (21) and (24) that the boundary condition for δμi(r) is given by
Finally, the boundary condition for vi(r) is given by
which follows from the condition (iv). In addition, we have the constraint that the net force acting on the particle must be zero.
By symmetry, we may write
where ϕi(r) and h(r) are functions of r. By substituting Eqs. (29) and (30) into Eqs. (2)–(6), the following equations for ϕi(r) and h(r) are obtained:
with
The boundary conditions, Eqs. (19), (20), (27), and (28) reduce to
Equations (31) and (32) subject to Eqs. (36)–(39) can be solved to give
and
By using Eq. (39), the magnitude (with sign) U of the diffusiophoretic velocity U is given by
From Eqs. (41) and (42), we find that
We note that the fundamental electrokinetic equations for the electrophoresis and diffusiophoresis problems are the same except for the boundary condition for ϕi(r) for r → ∞. Indeed, Eq. (43) is obtained from the expression for the electrophoretic velocity of a spherical particle in an applied electric field E [27,28,29] by replacing E with α. Thus, by applying the same approximation method as in Ref. [28], we can derive similar approximate formulas for the diffusiophoretic velocity U correct to order (1/κa)0 with the same accuracy as those derived for the electrophoresis problem [28]. We define the scaled diffusiophoretic mobility U* as
We give the results applicable for large particles with κa ≥ 50 at arbitrary values of ζ as follows.
with
and
where \(\stackrel{\sim }{\zeta }\) is the scale zeta potential and sgn(ζ) is + 1(-1) if ζ > 0 (ζ < 0).
Results and discussion
We have derived the general expression (45) for the scaled diffusiophoretic mobility U*. We give below explicit expressions for U* for the case of binary electrolytes.
(i) z+:z- binary electrolytes (z+ > 0 and z- < 0)
with
(ii) z:z symmetrical electrolytes (z+ = -z- = z > 0)
with
Note that Eqs. (56) and (57) differ from Eq. (54) in Ref. [23] by a factor of 1/z2, because of the different definitions of U* and a.
In the limit of κa → ∞, Eqs. (56) and (57) reduce to
which agrees with previously derived well-known expression [1,2,3]
(iii) 2:1 electrolytes
with
(iv) 1:2 electrolytes
with
Note that F corresponds to Dukhin’s number.
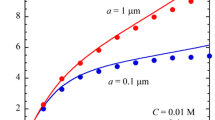
Figure 1 shows examples of the results of the calculation of the reduced diffusiophoretic velocity U* of a charged spherical particle of radius a calculated with Eqs. (56) and (57) as a function of the particle zeta potential ζ for m+ = 0.176 and m- = 0.169, which are, respectively, the values of m+ for K+ ions and m- for Cl− ions in an aqueous KCl solution at 25 °C. Figures 2, 3, respectively, show the results for MgCl2 with m+ = 0.122 and m- = 0.169 at 25 °C and those for LaCl3 with m+ = 0.0618 and m- = 0.169 at 25 °C. It is seen that there are maxima in the mobility curves plotted as a function of the particle zeta potential ζ. This is caused by the relaxation effect, which becomes appreciable for higher zeta potential values as in the electrophoresis problem. We also note that U* reaches a nonzero finite value as the magnitude of the zeta potential tends to infinity. This is a kind of counterion condensation effect, as in the case of electrophoresis [30, 31]. The limiting values for several types of electrolytes can be obtained from the above expressions for U* with the result that.
(i) z:z symmetrical electrolytes
(ii) 2:1 electrolytes
(iii) 1:2 electrolytes
Concluding remarks
We have derived the general expression for the diffusiophoretic mobility U* of a spherical colloidal particle of radius a in a concentration gradient of general electrolyte (Eq. (43)). On the basis of this expression, we have derived simple approximate analytic expressions for U correct up to the order of (1/κa)0 (Eq. (45)) applicable for large particles with κa ≥ 50 at arbitrary zeta potential with negligible errors. Explicit expressions for U* for particles in z+:z-, z:z, 2:1, and 1:2 electrolyte solutions are given (Eqs. (49), (56), (57), (62), (63), (67), and (68)).
Abbreviations
- a :
-
Particle radius
- a :
-
Electrolyte concentration gradient vector
- β :
-
Parameter relating to the diffusion potential field defined by Eq. (25)
- ∇n i :
-
Concentration gradient of the i th ionic species
- e :
-
Elementary electric charge
- ε o :
-
Permittivity of a vacuum
- ε r :
-
Relative permittivity of an electrolyte solution
- ϕ i(r):
-
Function relating to the electrochemical potential of the i th ionic species
- h(r):
-
Function relating to the liquid flow velocity u(r)
- η :
-
Viscosity of an electrolyte solution
- i :
-
Electric current density
- k :
-
Boltzmann’s constant
- \(\kappa\) :
-
Debye-Hückel parameter
- λ i :
-
Drag coefficient of the i th ionic species
- m i :
-
Scaled drag coefficient of the i th ionic species
- μ i(r):
-
Electrochemical potential of the i th ionic species at position r
- n i(r):
-
Concentration (number density) of the i th ionic species at position r
- n i ∞ :
-
Bulk concentration (number density) of i th ionic species in the absence of the applied electrolyte concentration gradient
- p(r):
-
Pressure at position r
- ρ el(r):
-
Space charge density at position r
- T :
-
Absolute temperature
- u :
-
Liquid flow velocity
- U :
-
Diffusiophoretic velocity
- U :
-
Magnitude with sign of U
- U*:
-
Scaled diffusiophoretic mobility
- v i(r):
-
Velocity of the i th ionic species at position r
- y :
-
Scaled equilibrium electric potential
- ψ(r):
-
Electric potential at position r
- ψ (0)(r):
-
Equilibrium electric potential at position r
- z i :
-
Valence of i th ionic species
- ζ :
-
Zeta potential
- \(\stackrel{\sim }{\zeta }\) :
-
Scaled zeta potential
References
Derjaguin BV, Dukhin SS, Korotkova AA (1961) Diffusiophoresis in electrolyte solutions and its role in the mechanism of film formation of cationic latex by ionic deposition. Kolloidyni Zh 23:53–58
Prieve DC (1982) Migration of a colloidal particle in a gradient of electrolyte concentration. Adv Colloid Interface Sci 16:321–335
Prieve DC, Anderson JL, Ebel JP, Lowell ME (1984) Motion of a particle generated by chemical gradients. Part 2. Electrolytes J Fluid Mech 148:247–269
Prieve DC, Roman R (1987) Diffusiophoresis of a rigid sphere through a viscous electrolyte solution. J Chem Soc Faraday Trans II 83:1287–1306
Anderson JL (1989) Colloid transport by interfacial forces. Ann Rev Fluid Mech 21:61–99
Pawar Y, Solomentsev YE, Anderson JL (1993) Polarization effects on diffusiophoresis in electrolyte gradients. J Colloid Interface Sci 155:488–498
Keh HJ, Chen SB (1993) Diffusiophoresis and electrophoresis of colloidal cylinders. Langmuir 9:1142–1149
Keh HJ (2016) Diffusiophoresis of charged particles and diffusioosmosis of electrolyte solutions. Curr Opin Colloid Interface Sci 24:13–22
Keh HJ, Wei YK (2000) Diffusiophoretic mobility of spherical particles at low potential and arbitrary double-layer thickness. Langmuir 16:5289–5294
Tseng S, Su C-Y, Hsu J-P (2016) Diffusiophoresis of a charged, rigid sphere in a Carreau fluid. J Colloid Interface Sci 465:54–57
Hsu J-P, Hsieh S-H, Tseng S (2017) Diffusiophoresis of a pH-regulated polyelectrolyte in a pH-regulated nanochannel. Sensors Actuators B: Chemical 252:1132–1139
Chiu HC, Keh HJ (2017) Diffusiophoresis of a charged particle in a microtube. Electrophoresis 38:2468–2478
Chiu HC, Keh HJ (2017) Electrophoresis and diffusiophoresis of a colloidal sphere with double-layer polarization in a concentric charged cavity. Microfluid Nanofluid 21:45
Chiu YC, Keh HJ (2018) Diffusiophoresis of a charged porous particle in a charged cavity. J Phys Chem B 122:9803–9814
Chiu YC, Keh HJ (2018) Diffusiophoresis of a charged particle in a charged cavity with arbitrary electric double layer thickness. Microfluidics Nanofluidics 22:84
Yeh YZ, Keh HJ (2018) Diffusiophoresis of a charged porous shell in electrolyte gradients. Colloid Polym Sci 296:451–459
Gupta A, Rallabandi B, Howard A, Stone HA (2019) Diffusiophoretic and diffusioosmotic velocities for mixtures of valence-asymmetric electrolytes. Phys Rev Fluids 4:043702
Gupta A, Shim S, Stone HA (2020) Diffusiophoresis: from dilute to concentrated electrolytes. Soft Matter 16:6975–6984
Lin WC, Keh HJ (2020) Diffusiophoresis in suspensions of charged soft particles. Colloids Interfaces 4:30
Sin S (2020) Diffusiophoretic separation of colloids in microfluidic flows. Phys Fluids 32:101302
Wu Y, Chang W-C, Fan L, Jian E, Tseng J, Lee E (2021) Diffusiophoresis of a highly charged soft particle in electrolyte solutions induced by diffusion potential Phys. Fluids 33:012014
Wu Y, Lee E (2021) Diffusiophoresis of a highly charged soft particle normal to a conducting plane. Electrophoresis. https://doi.org/10.1002/elps.202100052
Majee PS, Bhattacharyya S (2021) Impact of ion partitioning and double layer polarization on diffusiophoresis of a pH-regulated nanogel. Meccanica 56:1989–2004
Ohshima H (2021) Approximate analytic expressions for the diffusiophoretic velocity of a spherical colloidal particle. Electrophoresis. https://doi.org/10.1002/elps.202100178
Ebel JP, Anderson JL, Prieve DC (1988) Diffusiophoresis of latex particles in electrolyte gradients. Langmuir 4:396–406
Bohinc K, Bossa GV, May S (2017) Incorporation of ion and solvent structure into mean-field modeling of the electric double layer. Adv Colloid Interface Sci 249:220–233
Ohshima H, Healy TW, White LR (1983) Approximate analytic expressions for the electrophoretic mobility of spherical colloidal particles and the conductivity of their dilute suspensions. J Chem Soc Faraday Trans II 79:1613–1628
Ohshima H (2005) Approximate expression for the electrophoretic mobility of a spherical colloidal particle in a solution of general electrolytes. Colloids Surf A: Physicochem Eng Asp 267:50–55
Ohshima H (2006) Theory of colloid and interfacial electric phenomena. Elsevier, Amsterdam
Ohshima H (2003) On the limiting electrophoretic mobility of a highly charged colloidal particle in an electrolyte solution. J Colloid Interface Sci 263:337–340
Ohshima H (2016) On the maximum of the magnitude of the electrophoretic mobility of a spherical colloidal particle in an electrolyte solution. Colloid Polym Sci 294:13–17
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Equation (24) can be derived from Eq. (23) as follows. Since beyond the particle double layer, \({\rho }_{\mathrm{el}}^{\left(0\right)}\left(r\right)\)→0 and \({n}_{i}^{\left(0\right)}\left(r\right)\) → \({n}_{i}^{\infty }\), we obtain from Eq. (23)
From Eqs. (7) and (10), we have
By substituting Eq. (79) into Eq. (78) and using Eq. (21), we obtain Eq. (24). Here, it must be noted that unlike the electrophoresis problem, in the diffusiophoresis problem dni does not tend to zero but to a nonzero value given by Eq. (21).
Rights and permissions
About this article
Cite this article
Ohshima, H. Diffusiophoretic velocity of a large spherical colloidal particle in a solution of general electrolytes. Colloid Polym Sci 299, 1877–1884 (2021). https://doi.org/10.1007/s00396-021-04898-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-021-04898-3