Abstract
A simple approximate analytic expression is obtained for the diffusiophoretic mobility of a mercury drop with a thin electrical double layer and a zeta potential of arbitrary values in an electrolyte solution. The electrolyte is of the symmetrical type but may have different ionic drag coefficients for cations and anions. The obtained mobility expression involves Dukhin’s number for mercury drops, which is different from the usual Dukhin’s number for rigid particles. It is shown that the diffusiophoretic mobility plotted as a function of the drop zeta potential exhibits maxima due to the relaxation effect. It is also shown that in the limit of very high zeta potential, the diffusiophoretic mobility of a mercury drop tends to a non-zero limiting value. This limiting mobility value is found to be the same as that for a rigid sphere. That is, a liquid drop behaves like a rigid particle in this limit (solidification effect).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrokinetic phenomena in a suspension of mercury drops are quite different from those of rigid particles [1]. In particular, the electrophoretic mobility of a mercury drop of radius a surrounded by a thin electrical double layer in an electrolyte solution is considerably larger than that of a rigid particle of the same radius a and zeta potential by the order of κa (1/κ being the double layer thickness). The large mobility of mercury drops arises from their finite viscosity, which gives rise to a tangential frictional force acting on the surface smaller than that for rigid particles. The tangential component of the liquid flow velocity must be zero on the surface of a rigid particle but does not vanish on the drop surface. The tangential flow component decays from the electrophoretic velocity value U (just outside the double layer) to zero (at the drop surface) over the double-layer thickness 1/κ. The tangential frictional force on the surface is then of order of ηU/κ−1. For a mercury drop, on the other hand, the tangential flow component becomes zero at a certain point inside the drop, which is located at a distance of the order of a from the drop surface, i.e., decays over distances of order a. The tangential frictional force on the drop surface is then of order ηdU/a and hence the order of the ratio of the mobility of a mercury drop to that of a rigid particle becomes O(ηκa/ηd) ≈ O(κa). It should also be noticed that mercury drops are different from dielectric liquid drops in that mercury drops behave like an ideally polarizable conductor and the drop surface is always equipotential during electrophoresis. The tangential component of the Maxwell stress tensor is thus zero on the mercury drop surface.
For the case of diffusiophoresis, which is the motion of a particle in an applied electrolyte concentration gradient (for example, see Refs. [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17] for rigid particles, Refs. [11,12,13,14,15,16,17,18,19,20,21] for liquid drops, and Refs. [22,23,24,25,26,27] for soft particles), the same phenomenon due to the relaxation effect should be observed. In a previous paper [21], we derived an approximate analytic expression for the diffusiophoretic mobility of a mercury drop and indeed it exceeds that of a rigid particle by the order of κa. The expression derived in previous paper [21], however, can be applied for weakly charged mercury drops, since it neglects the relaxation effect, which becomes appreciable for higher zeta potential values. In the present paper, we treat mercury drops with thin electrical double layers (i.e., large κa) and zeta potential of arbitrary values, taking into account the relaxation effect and derive an approximate analytic mobility expression.
Theory
Consider a spherical mercury drop of radius a, viscosity ηd, and zeta potential ζ, moving with a diffusiophoretic velocity U in an aqueous liquid of viscosity η and relative permittivity εr containing a symmetrical electrolyte under a constant applied gradient of electrolyte concentration ∇n. The electrolyte is of the z:z symmetrical type with valence z but may have different ionic drag coefficients λ+ and λ- for cations and anions, respectively. Let n∞ be the bulk concentration of electrolytes in the absence of the applied electrolyte concentration gradient. We introduce a constant vector α proportional to ∇n, viz.,
where k is the Boltzmann constant, T is the absolute temperature, and e is the elementary electric charge. The symbol “e” refers to the elementary electric charge in Eqs. (1), (8), (10), (14), and (15). In other places “e” is the exponential constant.
The origin of the spherical polar coordinate system (r, θ, φ) is held fixed at the center of the mercury drop and the polar axis (θ = 0) is put parallel to α. For a spherical particle, U is parallel to α. The concentration gradient field α is assumed to be weak so that U is linear in α. The main assumptions are as follows. (i) The Reynolds number of the liquid flow is small enough to ignore inertial terms in the Navier–Stokes equation and the liquid can be regarded as incompressible. (ii) The equilibrium electric potential in the absence of the field α satisfies the Poisson-Boltzmann equation. (iii) No electrolyte ions can penetrate the drop surface. (iv) The drop surface remains spherical during diffusiophoresis. (v) The mercury drop behaves like an ideally polarizable conductor and the drop surface is always equipotential during diffusiophoresis.
We have previously shown that the general expression for the diffusiophoretic velocity U of a colloidal particle in an electrolyte concentration gradient field α is obtained from the expression for its electrophoretic velocity UE in an applied electric field E by replacing E with α. Indeed, the fundamental equations take the same form for these two velocities [15, 16, 21]. The only difference is the far-field boundary condition for the deviations δμ±(r) of the ionic electrochemical potentials μ±(r) caused by α, where μ+(r) and μ-(r) are, respectively, the electrochemical potentials of cations and anions. The diffusiophoretic velocity U of a mercury drop can thus be derived from the corresponding expression for the electrophoretic mobility [1, 21] with the result that:
with
where r =|r| is the radial distance from the drop center, m+ and m- are, respectively, the scaled ionic drag coefficients of cations and anions, ψ(0)(r) is the equilibrium electric potential at position r outside the mercury drop in the absence of the field α, y(r) is its scaled quantity, and εo is the permittivity of a vacuum. The functions h(r) and ϕ±(r) are, respectively, related to the liquid fluid velocity u(r) outside the drop (r > a) and the deviations δμ±(r) of the ionic electrochemical potentials μ±(r) due to α as
and
where α =|α|. We now introduce the diffusiophoretic mobility U* defined by
On the basis of the same approximation method as used for deriving the electrophoretic mobility of a mercury drop [1], we finally obtain the following expression for U*, which is correct to the order O((1/κa)0) and thus applicable for large κa (κa ≥ 30):
with
where \(\stackrel{\sim }{\zeta }\) is the scaled zeta potential, κ is the Debye-Hückel parameter and D is Dukhin’s number for liquid drops [1].
Results and discussion
Equation (13) is the required large κa approximate expression for the diffusiophoretic mobility U* of a spherical mercury drop of radius a in an electrolyte concentration gradient field α. Equation (14), which is applicable for arbitrary zeta potentials, takes into account the relaxation effect though Dukhin’s parameter D defined by Eq. (16),
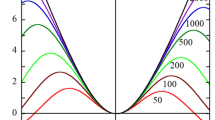
Figure 1 shows some examples of the results of the calculation via Eq. (13) for U* of a mercury drop in an aqueous KCl solution at 25 ℃ \({m_+=0.176,m_-=0.169,\beta=-0.02,\eta=0.89\;\mathrm{mPa {\cdot}s}}\). In Fig. 1, U* is plotted as a function of \(\widetilde\zeta\) for various values of κa, showing how strongly U* depends on κa. It is seen that U* exhibits two maxima due to the relaxation effect as in the case of electrophoresis [1]. The values of \(\widetilde\zeta\) at which U* exhibit maxima are \(\widetilde\zeta\) \(\approx\) \(\pm3.5,\mathrm i.\mathrm e.,\zeta\approx\pm88\) mV, which are easily experimentally accessible.
Scaled diffusiophoretic mobility U* of a spherical mercury drop of radius a, viscosity ηd = 1.525mPa·s, and zeta potential z in an aqueous KCl solution at 25℃ (m+ = 0.176, m- = 0.169, β= -0.02, η = 0.89mPa·s) as a function of the scaled zeta potential at several values of κa in comparison with that for a rigid sphere (ηd \(\rightarrow\infty\)) of radius a. Calculated via Eq. (13) for a mercury drop (solid lines) and via Eqs. (17) and (18) for a rigid sphere (dashed lines). Dotted lines represent results calculated via the low zeta potential approximation Eq. (24)
Figure 1 compares the results of U* for a mercury drop and those or a rigid sphere, showing a remarkable difference between mercury drops (solid lines) and rigid particles (dashed lines) in that the diffusiophoretic mobility of mercury drops much exceeds that of rigid particles for large κa as in the case of electrophoresis [1]. The large κa approximate expression for a rigid sphere is given by [15, 16]
with
where F is Dukhin’s number for rigid particles. By comparing Eq. (13), (17) and (18), we find that
That is, the diffusiophoretic mobility of a mercury drop is considerably larger than that of a rigid sphere for large κa, as is seen in Fig. 1.
As is also seen in Fig. 1 , in the limit of very high ζ, Eq. (13) for U* of a liquid drop (solid lines in Fig. 1) and Eqs. (17) and (18) for a rigid sphere (dashed lines in Fig. 1) tend to the same limiting value given by
Namely, in the limit of very high zeta potential ζ, a liquid drop behaves like a rigid particle. This solidification effect at high ζ occurs generally for electrokinetic phenomena including electrophoresis of mercury drops [1].
Finally, we compare the present result Eq. (13) and a low-zeta-potential mobility expression correct to the order of ζ2 [21], which is given by
with
and
where En(ka) is the n th order exponential integral of order n defined by
In the limit of large κa, Eq. (24) tends to
which agrees with the low zeta potential limiting form of Eq. (13). As is seen in Fig. 1 , the results obtained by Eq. (24) (dotted lines) agrees quite well with the results by Eq. (13) (solid lines) for |\(\stackrel{\sim }{\zeta }\)|≤ 2, implying that Eq. (24) is a good approximation for |\(\stackrel{\sim }{\zeta }\)|≤ 2 with negligible errors.
Concluding remarks
We have derived a simple approximate analytic expression, Eq. (13), for the diffusiophoretic mobility U* of a mercury drop applicable for arbitrary zeta potential values and large κa. Equation (13) takes into account the relaxation effect through Dukhin’s number D. It is shown that the diffusiophoretic mobility U* plotted as a function of the drop zeta potential exhibits maxima due to the relaxation effect. It is also shown that in the limit of very high zeta potential, the diffusiophoretic mobility of a mercury drop tends to a non-zero limiting value given by Eqs. (22) and (23). This limiting mobility value is found to be the same as that for a rigid sphere as in the case of electrophoresis (solidification effect).
References
Ohshima H, Healy TW, White LR (1984) Electrokinetic phenomena in a dilute suspension of charged mercury drops. J Chem Soc Faraday Trans II 80:1643–1667
Derjaguin BV, Dukhin SS, Korotkova AA (1961) Diffusiophoresis in electrolyte solutions and its role in the mechanism of film formation of cationic latex by ionic deposition. Kolloidyni Zh 23:53–58
Prieve DC (1982) Migration of a colloidal particle in a gradient of electrolyte concentration. Adv Colloid Interface Sci 16:321–335
Prieve DC, Anderson JL, Ebel JP, Lowell ME (1984) Motion of a particle generated by chemical gradients. Part 2. Electrolytes J Fluid Mech 148:247–269
Prieve DC, Roman R (1987) Diffusiophoresis of a rigid sphere through a viscous electrolyte solution. J Chem Soc Faraday Trans II 83:1287–1306
Anderson JL (1989) Colloid transport by interfacial forces. Ann Rev Fluid Mech 21:61–99
Pawar Y, Solomentsev YE, Anderson JL (1993) Polarization effects on diffusiophoresis in electrolyte gradients. J Colloid Interface Sci 155:488–498
Keh HJ, Chen SB (1993) Diffusiophoresis and electrophoresis of colloidal cylinders. Langmuir 9:1142–1149
Keh HJ, Wei YK (2000) Diffusiophoretic mobility of spherical particles at low potential and arbitrary double-layer thickness. Langmuir 16:5289–5294
Hoshyargar V, Ashrafizadeh SN, Sadeghi A (2015) Drastic alteration of diffusioosmosis due to steric effects. Phys Chem Chem Phys 17:29193
Keh HJ (2016) Diffusiophoresis of charged particles and diffusioosmosis of electrolyte solutions. Curr Opin Colloid Interface Sci 24:13–22
Gupta A, Rallabandi B, Howard A. Stone HA (2019) Diffusiophoretic and diffusioosmotic velocities for mixtures of valence-asymmetric electrolytes. Phys Rev Fluids 4:043702
Gupta A, Shim S, Stone HA (2020) Diffusiophoresis: from dilute to concentrated electrolytes. Soft Matter 16:6975–6984
Wilson JL, Shim S, Yu YE, Gupta A, Stone HA (2020) Diffusiophoresis in multivalent electrolytes. Langmuir 36:7014–7020
Ohshima H (2021) Approximate analytic expressions for the diffusiophoretic velocity of a spherical colloidal particle. Electrophoresis. https://doi.org/10.1002/elps.202100178
Ohshima H (2021) Diffusiophoretic velocity of a large spherical colloidal particle in a solution of general electrolytes. Colloid Polym Sci 299:1877–1884
Ohshima H (2022) Ion-size effect on the diffusiophoretic mobility of a large colloidal particle. Colloid Polym Sci. https://doi.org/10.1007/s00396-022-04954-6
Lou J, Lee E (2008) Diffusiophoresis of concentrated suspensions of liquid drops. J Phys Chem C 112:12455–12462
Yang F, Shin S, Stone HA (2018) Diffusiophoresis of a charged drop. J Fluid Mech 852:37–59
Wu Y, Jian E, Fan L, Tseng J, Wan R, Lee E (2021) Diffusiophoresis of a highly charged dielectric fluid droplet. Phys Fluids 33:122005
Ohshima H (2022) Diffusiophoresis of a mercury drop. Colloid Polym Sci. https://doi.org/10.1007/s00396-022-04964-4
Huang PY (2012) Keh HJ (2012) Diffusiophoresis of a spherical soft particle in electrolyte gradients. J Phys Chem B 116:7575–7589
Tseng S, Chung Y-C, Hsu J-P (2015) Diffusiophoresis of a soft, pH-regulated particle in a solution containing multiple ionic species. J Colloid Interface Sci 438:196–293
Majee PS, Bhattacharyya S (2021) Impact of ion partitioning and double layer polarization on diffusiophoresis of a pH-regulated nanogel. Meccanica 56:1989–2004
Wu Y, Lee YF, Chang WC, Fan L, Jian E, Tseng J, Lee E (2021) Diffusiophoresis of a highly charged soft particle in electrolyte solutions induced by diffusion potential. Phys Fluids 33:012014
Lee YF, Chang WC, Wu Y, Fan L, Lee E (2021) Diffusiophoresis of a highly charged soft particle in electrolyte solutions. Langmuir 37:1480–1492
Ohshima H (2022) Diffusiophoretic velocity of a spherical soft particle. Colloid Polym Sci 300:153–157
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ohshima, H. Relaxation effect on the diffusiophoretic mobility of a mercury drop. Colloid Polym Sci 300, 593–597 (2022). https://doi.org/10.1007/s00396-022-04967-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-022-04967-1