Abstract
An algorithm is presented for the calculation of the diffusiophoretic mobility of a colloidal particle on the basis of the modified Poisson-Boltzmann equation taking into account the ion size effects through the Carnahan-Starling activity coefficients of electrolyte ions. The obtained mobility expression is applicable when the particle size is much larger than the Debye length so that the particle surface can be regarded as planar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffusiophoresis is a motion of colloidal particles in a concentration gradient of electrolytes. That is, the particle moves toward regions of higher or lower electrolyte concentrations depending on the particle zeta potential and the relative ionic mobilities. It is thus possible to evaluate the particle zeta potential from the relation between the particle diffusiophoretic mobility and the zeta potential as in the case of electrophoresis, which is a motion of colloidal particles in an externally applied electric field. The governing equations for diffusiophoresis are the same as those for electrophoresis with the only difference being the boundary conditions for the ionic electrochemical potentials. There are many theoretical studies on diffusiophoresis of different types of particles such as rigid particles [1,2,3,4,5,6,7,8,9,10,11,12,13,14], liquid drops [15,16,17], and soft particles [18,19,20,21,22,23].
The theory of the diffusiophoresis of colloidal particles in an electrolyte solution is usually based on the Poisson-Boltzmann equation for the electric potential distribution around the particles [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. The standard Poisson-Boltzmann equation, however, assumes that electrolyte ions are point charges by neglecting the effects of ionic size. There are many theoretical studies on the modified Poisson-Boltzmann equation, which considers the effect of ionic size by introducing the activity coefficients of electrolyte ions (see, e.g., Refs [24,25,26,27,28,29,30,31]). López-García et al. [24, 25, 27] provided the numerical results of the electrophoretic mobility of a spherical particle in an electrolyte solution considering the ionic size effect. Hoshyargar et al. [26], in particular, demonstrated a significant effect of finite ion size on diffusioosmosis, which is an electrokinetic phenomenon closely related to diffusiophoresis. In a previous paper [29], on the basis of the equation for the ionic activity coefficients given by Carnahan and Starling [32], which is the most accurate among existing theories, we presented a simple algorithm for the calculation of an approximate electrophoretic mobility of a charged spherical colloidal particle in an electrolyte solution.
In the present paper, we derive an algorithm for the calculation of the diffusiophoretic velocity of a large colloidal particle in an electrolyte solution based on the modified Poisson-Boltzmann equation [28, 29]. The obtained expression is applicable when the particle size is much larger than the Debye length so that the particle surface can be regarded as planar.
Modified Poisson-Boltzmann equation
Consider a large particle carrying zeta potential ζ in an aqueous liquid of viscosity η and relative permittivity εr containing a symmetrical electrolyte. The electrolyte is of the Z:Z symmetrical type with valence Z but may have different ionic drag coefficients λ+ and λ− for cations and anions, respectively. We treat the case where the particle size is large enough to regard the particle surface as a planar surface. We take an x-axis to be perpendicular to the particle surface with its origin on it and a z-axis to be parallel to the particle surface (Fig. 1).
Consider first the equilibrium situation where there is no electrolyte concentration gradient. Let n+(x) and n−(x), which depend on only x, be the concentrations (number densities) of electrolyte cations and anions, respectively, and n∞ be their concentration beyond the electrical double layer around the particle, where n+(∞) = n−(∞) = n∞. The equilibrium electric potential ψ(x) and the space charge density ρel(x), which also depend only on x, obey the Poisson equation:
with
where εo is the permittivity of a vacuum and e is the elementary electric charge. We take into account steric interactions among ions of the finite size by introducing an ionic activity coefficient. We assume that the activity coefficients of cations and anions have the same value γ(x). The electrochemical potential μ+(x) of cations and that of anions μ−(x) are thus given by
where k is Boltzmann’s constant and T is the absolute temperature. The electrochemical potentials μ±(x) must take the same value as those in the bulk solution phase (where ψ(∞) = 0), viz.,
with \({\gamma }^{\infty }=\gamma (\infty )\). By equating \({\mu }_{\pm }(x)={\mu }_{\pm }(\infty )\), we obtain
where
is the scaled electric potential. Thus, Eq. (2) gives
so that Eq. (1) becomes the following modified Poisson-Boltzmann equation:
where
is the Debye-Hückel parameter and 1/κ is the Debye length. When γ(x) = 1, Eq. (8) tends back to the standard Poisson-Boltzmann equation without taking into account the ion size effect, viz.,
We now assume that cations and anions have the same radius a. This assumption is particularly valid for diffusiophoresis in a KCl solution, in which the hydrated radii of the anions and cations can be considered equal to 0.33 nm. For details, the readers should refer to the work by Ganjizade et al. [33, 34]. We introduce the volume fraction ϕ+(x) of cations and that of anions ϕ−(x) at position x. Then, we have
The total ion volume fraction ϕ(x) at position x is thus given by
Let the total ion volume fraction in the bulk solution phase be ϕB ≡ ϕ(∞). Then from Eq. (12), we obtain
so that Eq. (12) becomes
By substituting Eq. (5) into Eq. (14), we obtain
The modified Poisson-Boltzmann Eq. (8) becomes by using Eq. (15)
Now we employ the expressions for γ(x) derived by Carnahan and Starling [32], viz.,
and
Then, Eq. (15) becomes
which is a transcendental equation for ϕ(x) for given values of ϕB and y(x). Since ϕ(x) thus obtained is a function of y(x), we rewrite ϕ(x) as ϕ(y).
Equation (16) is subject to the following boundary conditions:
where \(\stackrel{\sim }{\zeta }\) is the scaled zeta potential defined by
Electrokinetic equations
Now consider the case where the electrolyte concentration gradient ∇n∞ (0, 0, dn∞/dz) is applied so that n∞ becomes a function of z and the particle is moving with a diffusiophoretic velocity U. We treat the case where the applied electrolyte concentration gradient ∇n∞(0, 0, dn∞/dz), the diffusiophoretic velocity U(0, 0, U), and thus the liquid flow u(r) are parallel to the z-axis, as shown in Fig. 1. We introduce a constant vector α(0, 0, α) proportional to ∇n, viz.,
We assume that the liquid velocity u(r) = (0, 0, u(x, z)) at position r satisfies the following Navier–Stokes equation:
where p(r) is the pressure. The boundary conditions for u(r) are
The boundary condition for the electric potential ψ(r) far from the particle at x → ∞ can be derived as follows. The ionic flows caused by ∇n∞ induce a macroscopic electric field, i.e., the diffusion potential field E(0, 0, E), which nullifies the net electric current and hence ψ(r) tends to − Ez as x → ∞. The electric current density i(r) at position r is given by
with
where are the velocities of cations and anions, respectively. From the condition that i(r) must be zero beyond the electrical double layer around the particle (x → ∞), we find that
and
where β is defined by
and α is the z-component of α given by (see Eq. (23))
Following Prieve [2], from the x-component of Eq. (24), we obtain
with
where y′ is an integral variable. Substituting Eq. (34) back into Eq. (24) and solving its z-component, we finally obtain the following expression for the scaled diffusiophoretic mobility U*:
where we have defined U* as
Results and discussion
The principal result of the present paper is Eq. (35) for the diffusiophoretic mobility U* of a large colloidal particle in an electrolyte solution based on the modified Poisson-Boltzmann equation (Eq. (16)) by taking into account the ion size effect through the Carnahan-Starling activity coefficient [32]. In the limiting case of ϕB → 0, Eq. (35) tends to the following electrophoretic mobility expression based on the standard Poisson-Boltzmann approach [1,2,3]:
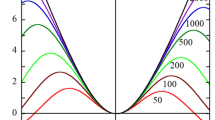
Figure 2 shows some examples of the exact results (solid lines) of the calculation of the scaled diffusiophoretic mobility U* in a KCl solution (β = − 0.02) obtained via Eq. (35) as a function of the scale zeta potential \(\stackrel{\sim }{\zeta }\) for several values of the total ion volume fraction ϕB. It is seen that the ionic size effect gives rise to an increase in the magnitude of U*. This is because the ionic size effect leads to a decrease in the ionic shielding effect so that the magnitude of U* increases. Figure 2 also shows results (dotted lines) obtained via an approximate expression for U* given by
Scaled diffusiophoretic mobility U* in a KCl solution (β = − 0.02) as a function of the scaled zeta potential \(\stackrel{\sim }{\zeta }\) for several values of the total ion volume fraction ϕB. Solid curves represent exact results calculated with Eq. (35), which is based on the modified Poisson-Boltzmann equation (Eq. 16)). The curve with ϕB = 0 corresponds to the result obtained via the standard Poisson-Boltzmann equation (Eq. 10). Dotted curves represent approximate results calculated with Eq. (38)
with
Equation (38) can be derived from the following approximate form valid at low values of ϕ and low to moderate values of y(x) for the Carnahan-Starling activity coefficient (Eq. 17) [28, 29]:
or equivalently (see Eq. (15))
which is the first-order approximation for ϕ(x) obtained by expanding Eq. (19) in a power series of ϕ(x) and ϕB.
The approximate results (dotted lines) agree well with the exact results (solid lines). The maximum error in Fig. 2 is found to be about 6%.
Conclusion
We have derived an expression (35) for the diffusiophoretic mobility U* of a large colloidal particle in a symmetrical electrolyte solution based on the modified Poisson-Boltzmann equation (Eq. (8) or Eq. (16)), which takes into account the effects of ionic size on the basis of ionic activity coefficient given by Carnahan and Starling [32]. The obtained expression is applicable when the particle size is much larger than Debye length 1/κ. It has been shown that U* increases with increasing total ionic volume fraction ϕB. We have also derived an approximate expression for U* (Eq. (38)), which is in good agreement with the exact results (Eq. (35)).
References
Derjaguin BV, Dukhin SS, Korotkova AA (1961) Diffusiophoresis in electrolyte solutions and its role in the mechanism of film formation of cationic latex by ionic deposition. Kolloidyni Zh 23:53–58
Prieve DC (1982) Migration of a colloidal particle in a gradient of electrolyte concentration. Adv Colloid Interface Sci 16:321–335
Prieve DC, Anderson JL, Ebel JP, Lowell ME (1984) Motion of a particle generated by chemical gradients. Part 2. Electrolytes J Fluid Mech 148:247–269
Prieve DC, Roman R (1987) Diffusiophoresis of a rigid sphere through a viscous electrolyte solution. J Chem Soc Faraday Trans II 83:1287–1306
Anderson JL (1989) Colloid transport by interfacial forces. Ann Rev Fluid Mech 21:61–99
Pawar Y, Solomentsev YE, Anderson JL (1993) Polarization effects on diffusiophoresis in electrolyte gradients. J Colloid Interface Sci 155:488–498
Keh HJ, Chen SB (1993) Diffusiophoresis and electrophoresis of colloidal cylinders. Langmuir 9:1142–1149
Keh HJ (2016) Diffusiophoresis of charged particles and diffusioosmosis of electrolyte solutions. Curr Opin Colloid Interface Sci 24:13–22
Gupta A, Rallabandi B, Howard A. Stone HA (2019) Diffusiophoretic and diffusioosmotic velocities for mixtures of valence-asymmetric electrolytes. Phys Rev Fluids 4:043702
Gupta A, Shim S, Stone HA (2020) Diffusiophoresis: from dilute to concentrated electrolytes. Soft Matter 16:6975–6984
Wilson JL, Shim S, Yu YE, Gupta A, Stone HA (2020) Diffusiophoresis in multivalent electrolytes. Langmuir 36:7014–7020
Keh HJ, Wei YK (2000) Diffusiophoretic mobility of spherical particles at low potential and arbitrary double-layer thickness. Langmuir 16:5289–5294
Ohshima H (2021) Approximate analytic expressions for the diffusiophoretic velocity of a spherical colloidal particle. Electrophoresis. https://doi.org/10.1002/elps.202100178
Ohshima H (2021) Diffusiophoretic velocity of a large spherical colloidal particle in a solution of general electrolytes. Colloid Polym Sci 299:1877–1884
Lou J, Lee E (2008) Diffusiophoresis of concentrated suspensions of liquid drops. J Phys Chem C 112:12455–12462
Yang F, Shin S, Stone HA (2018) Diffusiophoresis of a charged drop. J Fluid Mech 852:37–59
Wu Y, Jian E, Fan L, Tseng J, Wan R, Lee E (2021) Diffusiophoresis of a highly charged dielectric fluid droplet. Phys Fluids 33:122005
Huang PY, Keh HJ (2012) Diffusiophoresis of a spherical soft particle in electrolyte gradients. J Phys Chem B 116:7575–7589
Tseng S, Chung Y-C, Hsu J-P (2015) Diffusiophoresis of a soft, pH-regulated particle in a solution containing multiple ionic species. J Colloid Interface Sci 438:196–293
Majee PS, Bhattacharyya S (2021) Impact of ion partitioning and double layer polarization on diffusiophoresis of a pH-regulated nanogel. Meccanica 56:1989–2004
Wu Y, Lee YF, Chang WC, Fan L, Jian E, Tseng J, Lee E (2021) Diffusiophoresis of a highly charged soft particle in electrolyte solutions induced by diffusion potential. Phys Fluids 33:012014
Lee YF, Chang WC, Wu Y, Fan L, Lee E (2021) Diffusiophoresis of a highly charged soft particle in electrolyte solutions induced by diffusion potential. Langmuir 37:1480–1492
Ohshima H (2022) Diffusiophoretic velocity of a spherical soft particle. Colloid Polym Sci. https://doi.org/10.1007/s00396-021-04933-3
López-García JJ, Horno J, Grosse C (2012) Equilibrium properties of charged spherical colloidal particles suspended in aqueous electrolytes: finite ion size and effective ion permittivity effects. J Colloid Interface Sci 380:213–221
López-García JJ, Horno J, Grosse C (2015) Influence of steric interactions on the dielectric and electrokinetic properties in colloid suspensions. J Colloid Interface Sci 458:273–283
Hoshyargar V, Ashrafizadeh SN, Sadeghi A (2015) Drastic alteration of diffusioosmosis due to steric effects. Phys Chem Chem Phys 17:29193
López-García JJ, Horno J, Grosse C (2016) Ion size effects on the dielectric and electrokinetic properties in aqueous colloidal suspensions. Curr Opinion in Colloid Interface 24:23–31
Ohshima H (2016) An approximate analytic solution to the modified Poisson-Boltzmann equation. Effects of ionic size. Colloid Polym Sci 194:2121–2125
Ohshima H (2017) A simple algorithm for the calculation of an approximate electrophoretic mobility of a spherical colloidal particle based on the modified Poisson-Boltzmann equation. Colloid Polym Sci 295:543–548
Koranlou A, Ashrafizadeh SN, Sadeghi A (2019) Enhanced electrokinetic energy harvesting from soft nanochannels by the inclusion of ionic size. J Phys D Appl Phys 52:155502
Carrique F, Ruiz-Reina E, Arroyo FJ, Delgado AV (2020) Influence of ion size effects on the electrokinetics of aqueous salt-free colloids in alternating electric fields. Phys Rev E 102:032614
Carnahan NF, Starling KE (1969) Equation of state for nonattracting rigid spheres. J Chem Phys 51:635–636
Ganjizade A, Ashrafizadeh SN, Sadeghi A (2017) Effect of ion partitioning on the electrostatics of soft particles with a volumetrically charged core. Electrochem Commun 84:19–23
Ganjizade A, Sadeghi A, Ashrafizadeh SN (2018) Effect of ion partitioning on electrostatics of soft particles with volumetrically charged inner core coated with pH-regulated polyelectrolyte layer. Colloids Surfaces B: Biointerfaces 170:129–135
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ohshima, H. Ion size effect on the diffusiophoretic mobility of a large colloidal particle. Colloid Polym Sci 300, 1229–1234 (2022). https://doi.org/10.1007/s00396-022-04954-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-022-04954-6