Abstract
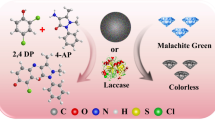
Seeking simple, green, and inexpensive preparation methods and materials of enzyme mimics has always been highly desirable. In this paper, a nanocomposite with laccase activity was successfully fabricated by using a simple self-association method with copper and tannic acid. Characterizations through SEM, XRD, FTIR, and XPS showed that the Cu-TA hybrid composites had good spherical in shape with a diameter of about 20 nm and three different valence Cu ions (Cu2+, Cu+, and Cu0). The catalytic activity was displayed against typical oxidation substrate of laccases, 2,4-DP. Compared to natural laccases, Cu-TA composites were able to better tolerate changes of pH, temperature, ionic strength, and storage conditions. The Cu-TA composites could remove malachite green (MG) from aqueous solutions effectively. The removal efficiency of MG was optimized using a series of batch tests with single-factor experiment design. Under the optimum conditions, the removal efficiency of MG still retained about 90% after 3 times cycles. Therefore, the Cu-TA composites, as a laccases-like nanozyme, have excellent developing potential in wastewater treatment due to the low-cost of TA and simplicity of preparation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laccases are a class of multi-copper-containing oxidase that can effectively oxidize a wide range of organic substrates with the simultaneous reduction of oxygen to water without harsh conditions. Because of their low substrate specificity and high availability, laccases have drawn wide attention in many areas of research, such as synthetic catalysis, sensors, biomedicine, and environmental remediation [1,2,3,4,5]. However, as a natural enzyme, the practical application of laccases is often hampered by their intrinsic drawbacks, such as a narrow range of operational pH and temperature, time-consuming purification, and high cost [6, 7]. To mitigate the existing challenges and boost the exploitation of laccases, many concerted approaches are being pursued and envisaged. Among them, nanomaterial-based artificial enzymes (nanozymes) attracted much attention due to their high activity, facile preparation, low production costs, and good stability against stringent conditions. For example, Liang and Zhang et al. [8, 9] have separately prepared a nanozyme by using nucleotides to coordinate with copper ions, which exhibited excellent laccase-like activity and can convert a diverse range of phenol containing substrates such as hydroquinone, naphthol, catechol, epinephrine, and o-phenylenediamine (OPD). Particularly, as compared with natural laccases, the Cu/nucleotides nanozyme is much more stable at extreme pH, high salt, high temperature, and for long-term storage. Wang et al. prepared a nanozyme with laccase-like activity through the coordination of Cu+/Cu2+ with a cysteine (Cys)-histidine (His) dipeptide, which can efficiently degrade chlorophenols and bisphenols compared with natural laccases [10]. Ren et al. also produced a copper-containing carbon dots that possess intrinsic laccase-like activities and can catalyze the oxidation of the laccase substrate under high-salt conditions or in a broad pH range 3.0–13.5 [11]. Therefore, artificial nanozyme is a promising alternative to natural laccases.
The pollution of organic dyes in aquatic environment has been a serious global problem because of the serious negative consequences on the quality of ecosystems [12]. Malachite green (MG) is a highly water-soluble triarylmethane dye that is used widely for dyeing cotton, wool, silk, paper, and leather [13]. MG was already considered to be a priority chemical for carcinogenicity by the US Food and Drug Administration [14] because it could bind DNA to form complexes through intercalation and electrostatic interactions. Hence, elimination of MG, as other organic dyes, from water environments has attracted growing attention. The adsorption and degradation are two main ways that have been proposed for eliminating organic dyes from aqueous solution [12, 15]. Enzymatic biodegradation of dyes is an environment-friendly process. Many studies have shown that natural laccases can successfully degrade MG [16, 17]. However, the large-scale application of natural laccases was restricted due to its high production cost, easy inactivation, poor reusability, and low storage stability [18]. In order to offset those shortcomings, laccase-like artificial nanozyme is becoming a potent alternative.
The availability, environmental friendliness, low-cost, and remarkable effect of organic ligands can have an important impact on the widely application of laccase-mimicking nanozyme. Despite of that the reported laccase-mimicking nanozyme have shown good catalytic performance, the application of this nanozyme is limited by the high cost and hazard of the organic ligands. For wider applications, other common raw materials are needed to seek. By contrast, tannic acid (TA) with abundant phenolic hydroxyl groups, a water-soluble natural polyphenol obtained from the ubiquitous plants, may be a potential candidate. It has been applied in pharmaceutical field, food additive, and other numerous industry [19]. Additionally, TA is already an FDA-approved compound [20]. TA possesses strong metal chelating ability, especially with metal ions Ce3+, Cu2+, and Mn2+ [21, 22]. In addition, TA has an effective antioxidant function, but it also exhibits a stronger pro-oxidant action in the presence of Cu (II) [19]. These two properties of TA appear to be related to the position and number of hydroxyls on the molecule. Given that TA is pure natural, easy-gained, inexpensive, and environment friendly, studies on the nanozymes of TA, as an organic ligand, will be very useful for the future application of laccase-mimicking nanozyme.
In order to remove malachite green (MG) from aqueous solution, we fabricated a laccase-mimicking nanozyme composed of copper ions and tannic acid. The catalytic activity, stability, and reaction condition of the laccase-like nanozymes were evaluated and compared to the natural laccases in this paper. Additionally, the removal performance of the nanozymes towards MG in aqueous solution was investigated under various effect factors, including contact time, initial nanozymes concentration, solution pH, and temperature. We reason that such fabricated nanozyme is of great significance for removal of organic dyes from aqueous solution.
Materials and methods
Chemicals and reagents
Tannic acid (TA), cupric sulfate pentahydrate (CuSO4·5H2O), sodium hydroxide (NaOH), anhydrous potassium dihydrogen phosphate (NaH2PO4), anhydrous sodium hydrogen phosphate (Na2HPO4), and sodium chloride (NaCl) were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. 2,4-Dichlorophenol (2,4-DP) and 4-Aminoantipyrine (4-AP) were purchased from Tianjin Heowns Biochem Ltd. Malachite Green (MG) was purchased from Shanghai Sangon Biotech. 1-Hydroxybenzotriazole (Hobt) was obtained from Shanghai Energy Chemical. Laccase (from Trametes versicolor, 3000 units·g−1) was obtained from Beijing Solarbio Science & Technology Co., Ltd. All chemicals used in the study were not further purified unless otherwise specified.
Preparation of Cu-TA Nanozyme
The Cu-TA nanozyme was prepared according to the following steps. Firstly, 0.6 mL TA (150 mM) was dripped into 30 mL CuSO4 aqueous solution (30 mM); then, the mixture was stirred for 15 min. After that, the mixture pH was adjusted to about 6.0 with by dropwise addition of appropriate amount of 0.1 M NaOH. After 12 h of incubation at room temperature, precipitates was washed four times with distilled water to remove unreacted components; the collected samples were stored in ultrapure water for further experiments.
Characterizations
Scanning electron microscopy (SEM, S-4800, Hitachi) was employed to examine the morphology of Cu-TA nanozymes. X-ray diffraction (XRD, D8-Focus, Bruker AXS) analysis was performed on CuKα radiation (40 kV, 40 mA) from 10 to 80° at 2θ with a scanning rate of 8° min−1 to reveal the crystal structure of the samples. Fourier transform infrared (FT-IR, Bio-Rad Excalibur FTS 300) was employed to examine the infrared spectra of the samples. Samples were prepared by KBr-discs technique, the wt ratio of samples to KBr was 1:200, and the spectra were collected in the range of 4000–400 cm−1. X-ray photoelectron spectroscopy (XPS, Thermo Scientific, K-Alpha) with Al Kα radiation was used to analyze the elemental composition and oxidation state of the samples.
Determination of laccase-like activity of Cu-TA nanozymes
The laccase-like activity of Cu-TA nanozymes was tested by using 2,4-dichlorophenol (2,4-DP) as the substrate together with 4-aminoantipyrine (4-AP) as a chromogenic ligand [8]. Each compound alone has no absorption in the visible region. 2,4-DP is the real substrate, and its laccase oxidation product reacts with 4-AP to produce a pink antipyrilquinoneimine adduct with an absorption peak at 510 nm [8]. In brief, 300 μL of 4-AP (1 g·L−1) and 300 μL of 2,4-DP (1 g·L−1) were mixed with 2.4 mL phosphate-buffered saline (PBS, 0.05 M, pH 6.0). And then, 100 μL of Cu-TA nanozymes aqueous dispersion or laccase solution (3 g·L−1) was added into the mixture for incubating 1 h at 25 °C. After the mixture was centrifuged for 5 min at 13,000 rpm, the absorbance of the supernatant was measured at 510 nm by using a UV–vis spectrophotometer (TU1810, Beijing Purkinje General Instrument Co., Ltd., China).
Studies on influence factors on the activity of Cu-TA nanozymes
To evaluate the effect of pH on the activity of Cu-TA nanozymes, the Cu-TA nanozymes were incubated in the PBS with different pH (from 3.0 to 9.0) for 8 h, respectively. After that, the residual activities of Cu-TA nanozymes were determined as Sect. 2.4. The enzyme activity at pH 6.0 was set as 100%. The activity of Cu-TA nanozymes at different pH conditions relative to its activity at pH 6.0 was defined as relative activity.
In order to explore the effect of temperature on the activity of Cu-TA nanozymes, the Cu-TA nanozymes was separately incubated at different temperature (25–85 °C) for 2 h. After that, the activities of Cu-TA nanozymes were analyzed. The activities before incubation were defined as 100%.
Concerning the effect of ionic strength on the activity of Cu-TA nanozymes, the experiments were performed in different concentrations of NaCl solutions (0, 0.05, 0.1, 0.2, 0.3 M). The activity of Cu-TA nanozymes in zero concentration of NaCl solution was set as 100%.
To test the storage stability of Cu-TA nanozymes, the Cu-TA nanozymes were stored in purified water at 4 °C and 25 ± 2 °C for 10 days, respectively. The catalytic activity was measured every other day according to the method in “Determination of laccase-like activity of Cu-TA nanozymes.” The initial activity was set as 100%.
Removal experiments of MG
A single-factor experiment design in batch style tests was carried out to explore the effects of the individual conditions, including the initial MG concentrations, dosage of Cu-TA nanozymes, ionic strength (NaCl concentrations), pH, and temperature of the reaction, on the removal efficiency of MG. One factor was changed, while the other factors were fixed. A PBS solution (0.05 M) of MG was employed throughout the experiments by adding 50 mL of MG solution of desired concentration (from 10 to 100 mg·L−1) at pH 3.0–9.0 with a certain amount of Cu-TA nanozymes (15 to 60 mg·mL−1) to 150-mL flasks. Mixtures were then placed into an incubator shaker at 25–85 °C and stirred at 120 rpm for 60 min. The supernatant was obtained by centrifuging at 13,000 rpm for 5 min, and the concentrations of MG in the supernatant were measured by a UV–vis spectrophotometry at 617 nm [4]. The removal efficiency of MG was calculated using the Eq. (1):
where C0 and Ce (mg·mL−1) are the initial and final MG concentrations in the supernatant, respectively. All measurements were detected in triplicate.
Hobt was chosen as a redox mediator in the degradation reaction of MG by Cu-TA nanozymes. The concentration of Hobt was optimized in a PBS solution (0.05 M) of 25 mg·L−1 MG with various concentrations of Hobt ranging from 0.0 to 0.4 mM at pH 6.0 and 25 °C over a duration of 60 min.
In order to verify the removal effect of MG resulted from the enzymatic activity of Cu-TA nanozymes rather than possible adsorption by individual TA molecules or individual Cu ions, the removal efficiency of MG by individual TA molecules or individual Cu ions were pre-studied. The removal experiments were carried out under that mentioned above. The results revealed that the removal efficiency of MG by single TA or by single Cu ions was only 10% and 3%, respectively. Therefore, it is impossible that the efficient removal of MG from solution based on the adsorption mechanism by single TA molecules or by single Cu ions.
Statistical analysis
A value of p < 0.05 was considered in the statistical determination. All independent experiments were performed three times under same condition to calculate mean values and standard deviations. All collected data was expressed as mean ± SD. SPSS version 13.0 was used for statistical analysis.
Results and discussion
Characterizations of Cu-TA nanozymes
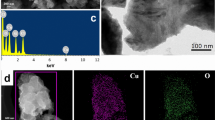
Morphologies of Cu-TA nanozymes were investigated via SEM. As shown in Fig. 1A, the SEM image shows that Cu-TA composites are spherical in shape with a diameter of about 20 nm. The high surface area is conducive for mass transfer, which consequently improved reaction efficiency. A possible mechanism of complexation between Cu2+ and TA could be explained by ion exchange [23]. The Cu2+ could attach to adjacent hydroxyl and oxyl groups of TA. This hydroxyl and oxyl groups of TA give one or two electrons to Cu2+; thus, it leads to the chelate formation by releasing two hydrogen ions into solution. The TA, which contains a lot of pyrogallol groups, can be offered a unique combination for the complexation of metal ions. The complexation mechanism between metal ions and TA is dependent on the pH in reaction system [24].
Characterizations of Cu-TA nanozymes. A SEM image of Cu-TA nanozymes; B XRD image of Cu-TA nanozyme (a), TA (b), Cu(OH)2 (c), CuO (d), and CuO2 (e); C FT-IR spectra of TA (a) and Cu-TA nanozymes (b); D XPS images of Cu-TA nanozymes. Fully scanned spectrum (a), Cu 2p spectrum (b), and Cu LM2 spectrum (c) (grey: baseline, blue: peak of Cu2+, green: peak of Cu+, pink: peak of Cu0)
XRD characterization was performed to further investigate the crystal structure of Cu-TA nanozymes. It shows that Cu-TA nanozymes have different absorption peaks (in Fig. 1B) compared to other Cu-based materials (i.e., Cu2O, CuO, and Cu(OH)2), which indicates that Cu-TA nanozyme is a unique crystal with amorphous structure. It suggests that the laccase activity of Cu-TA nanozymes is not only dependent on the crystallization of copper ions, but related to TA.
To investigate the chemical bonding, FT-IR spectra of TA and Cu-TA composites are presented in Fig. 1C. The peaks at 3370 cm−1 and 1317 cm−1 in curve “a” can be attributed to Ar-OH and C-O groups in the TA molecule, respectively. The appearance of a broad band at 1708 cm−1 in curve “b” indicates the presence of C = O groups in Cu-TA, corresponding to the 1716 cm−1 of TA in curve “a.” Additionally, the vibration band at pink frame in TA spectra can be compared to the band at green frame in Cu-TA nanozymes; the stretching C = C groups of aromatic rings were observed at 1614 cm−1, 1535 cm−1, and 1449 cm−1 from the TA spectra, which exhibits a shifted and weakened absorption in curve “b.” Thus, we speculate that the benzene of TA is partially oxidized to a benzoquinone structure, while Cu2+ is partially reduced to Cu+ and Cu0 during the formation of Cu-TA complex.
XPS was employed to analyze the content and oxidation state of Cu ions, and the results were shown in Fig. 1D. The full scan spectrum in Fig. 1D (a) shows that the main elements in Cu-TA nanozymes are Cu, C, and O, while the peaks of C 1 s and O 1 s should be due to the TA. High-resolution spectrum of Cu 2p and Cu LM2 were shown in Fig. 1D (b) and (c). The main peaks at 934.9 eV and 954.8 eV belong to the Cu 2p3/2 and Cu 2p1/2 electrons of Cu2+. The lower binding energy peaks at 932.5 eV (2p3/2) and 952.4 eV (2p1/2) indicate the existence of Cu+. Cu LM2 Auger spectra of Cu-TA (Fig. 1D (c)) were further analyzed to explore the oxidation state of Cu. The peak at 570.8 eV is attributed to Cu+; the peak fitting at 566.4 eV is suggested the presence of Cu0 atoms. These revealed that Cu-TA nanozymes contain three different valence Cu ions (Cu2+, Cu+, and Cu0). The XPS spectra analysis is consistent with FT-IR, which indicated that redox reaction occurred in the synthetic process of Cu-TA nanozymes, resulting in the reduction of partial Cu2+ to Cu+ and Cu0.
Laccase-like activity of Cu-TA nanozymes
Laccase-like activity of Cu-TA nanozymes was confirmed by using 2,4-DP as the substrates. Its oxidation product reacts with 4-AP to generate pink product with absorption at 510 nm. The reaction equation of 2,4-DP and 4-AAP catalyzed by Cu-TA nanozyme or laccase were shown in Fig. 2A. Figure 2B represents the photos of chromogenic product.
Furthermore, the optimal pH condition for activity of Cu-TA nanozymes was investigated. It showed the changes of absorbance of the colored products with the different pH in reaction solutions. Higher absorbance signifies formation of more colored products, which indicates that the activity of Cu-TA nanozymes is stronger. As shown in Fig. 3, it should be clear that the activity of Cu-TA nanozymes was inhibited when pH < 4 or pH > 7, while higher catalytic activity appeared in the range of pH 4–7. One possible explanation is that the coordination ability of TA to copper is varied with different pH environments. TA possesses abundant hydroxyl groups as binding sites to metal ions. At pH < 4, the concentration of H+ is relatively high. Hydrogen ion is most likely to compete with copper ions for TA binding in nanozymes, which leads to partial protonation of TA. Consequently, the chelation reaction between copper and TA will be weakened. Under alkaline environment, the hydroxyl ions (OH−) in aqueous solution are readily to react with copper in nanozymes to form insoluble Cu(OH)2, consequently, affecting the catalytic ability of Cu-TA nanozymes to substrates. Also, natural laccases has good catalytic activity in the range of pH 5–7 [25]. Considering that the comparability of Cu-TA nanozymes to natural laccases and simplicity of experimental manipulation, the PBS with pH 6.0 was employed in subsequent experiments.
Assessment of stability of Cu-TA nanozyme
The storage stability of bio-catalysts is an important factor for their practical application. The storage stability of Cu-TA nanozymes and natrual laccases was evaluated by setting at 4 °C and room temperature for 10 days, respectively. Compared to initial activity, the natural laccases have lost over 80% for under room temperature and 50% for at 4 °C after 10 days of storage, respectively. In contrast, Cu-TA nanozymes still maintained 100% of its original activity in the same storage conditions (Fig. 4). Actually, even after storage for 30 days, Cu-TA nanozymes still retained 100% of its original activity whether at 4 °C or at room temperature (data not shown). Apparently, Cu-TA nanozymes presented better storage stability than natural laccases. The changes of the protein conformation may be the major reason for loss of the enzymatic activity of natural laccases [26]. The better storage stability of Cu-TA nanozymes was gained due to the coordination of Cu2+ and tannic acid. There were not noticeable changes in the Cu-TA nanozyme morphology, size, and structure during 30 days of storage at room temperature (data not shown).
The thermal stability of enzyme is another important influence factor for its application. The effects of temperature on the laccase-like activities of Cu-TA nanozymes were studied in the temperature range of 25–85 °C for 2 h of incubation. It can be seen from Fig. 5 that the natural laccases retained 80% of its original activity after incubation for 2 h at 55 °C, and yet it has plummeted after this temperature point. Incubating for 2 h at 85 °C, almost all of the activities of natural laccases were lost. In contrast, the temperature below 65 °C has not significant influence on the catalytic activities of Cu-TA nanozymes. However, the catalytic activity of Cu-TA nanozymes increased gradually with increase of temperature after 65 °C and reached 120% of its initial activity at 85 °C. This results indicated that the hot tolerance of Cu-TA nanozymes was significantly higher than that of natrual laccases. The good thermostability of Cu-TA nanozymes may be due to the high heat resistance of tannic acid that starts to decompose at around 190 °C [27]. Tannic acid complexation with copper ions is possible benefits for further improving the thermal properties of Cu-TA nanozymes.
Removal of MG
Laccase is a class of oxidoreductases that catalyze the transfer of electrons from reductants to oxidants. Based on the laccase action mechanism, the addition of an extra compound which typically called “mediator” can significantly improve its catalytic function [28]. Laccase mediators involve artificial and natural compounds. Among them, ABTS (2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)) and Hobt (N-hydroxybenzotriazole) are the two typical mediators in artificial class [29]. Hobt was selected as the mediator in the next removal experiments of MG because the solution color of ABTS can interfere with the detection results of MG by colorimetric method. In order to examine the effect of Hobt concentration on removal of MG, 25 mg·L−1 of MG solution with pH 6.0 was performed in the presence of 30 mg·L−1 Cu-TA nanozymes and Hobt at concentrations from 0 to 0.4 mM under room temperature. The obtained results in Fig. 6 showed that the removal efficiency of MG increases from 70 to 90% with Hobt addition concentration from zero to 0.3 mM within 30 min. However, there was no longer a significant increase in the removal efficiency when the Hobt concentration was increased to 0.4 mM. Thus, Hobt concentration of 0.3 mM was selected as optimum concentration for the removal study of MG.
Effect of pH
The effects of solution pH on the removal efficiency of MG at initial concentration of 25 mg·L−1 are shown in Fig. 7. It could be seen that when the pH was 3.0 at room temperature, the removal efficiency of MG was only about 70% after 30 min reaction. As the pH was increased to the range of 4.0 to 7.0, the removal efficiency increased to > 90% within 30-min reaction. Then as the pH was continuously increased to > 8.0, the removal efficiency decrease to < 85%. Therefore, the pH range of 4.0 to 7.0 can be considered as optimum pH of Cu-TA nanozymes for removal of MG, which is superior to the optimum pH profile of natural laccases [25]. The initial pH of the reaction system was a crucial parameter for the removal of MG, which could impact the active sites and redox potential of Cu-TA nanozymes during the reaction process. This may be due to that the presence of excess protons and hydroxyl groups in reaction solution would affect the interaction of the substrate (MG) and the nanozymes, besides the optimal pH condition of Cu-TA nanozymes. The pKa for MG is 6.9 [30], so MG molecules bear a negative net charge at pH > 6.9 and a positive net charge when pH < 6.9. The calculated pKa value of TA showed three, the first at pH 5.6, the second at pH 6.9, and the third at pH 8.1 due to the dissociation of different function groups and position [31]. This means that all of the carboxylic and phenolic hydroxyl groups on TA could be protonated when the solution pH < 5.6, resulting in positive net charge on the surface of nanozymes. Consequently, the electrostatic repulsion impeded the affinity between nanozymes and positively charged MG. When the solution pH > 5.6, the carboxylic groups on TA firstly deprotonated, which would lead to a net charge reversal of nanozymes from positive to negative charge. Meanwhile, the electrostatic interaction between nanozymes and positively charged MG turned to attraction from repulsion. When the solution pH value rise to the second pKa of TA (= 6.9), a progressive deprotonation of the phenolic groups on TA would occur, leading to the increase in the intensity of negative charge on nanozymes. Much stronger electrostatic attraction between nanozymes and MG molecules could contribute to the high efficiency of MG removal. If the solution pH value is above 6.9 that is also the pKa of MG, the charge state of MG molecules can also turn to negative charge from positively charged molecules. So, both of nanozymes and MG molecules will be negatively charged, which lead to decrease in the affinity between TA and MG. This may be a reason for the reduced removal efficiency of MG by Cu-TA nanozymes when reaction solution is alkaline condition. At lower pH values (< 4.0), the TA are less accessible for MG molecules due to the protonation of the phenolic hydroxyl groups on the TA at higher H+ concentration. At moderate pH values (4.0–6.0), linked H+ is released from the phenolic-OH and the adsorbed amount of MG is increased. In this pH range, it is believed that hydrogen bond formation is the major mechanism for the binding interaction between Cu-TA nanozymes and MG.
Effect of initial MG concentration
As shown in Fig. 8, initial MG concentration is a significant parameter for removal of MG. When a specified amount of Cu-TA nanozyme (30 mg·L−1) was added into a series of MG concentrations (10, 25, 50, 75 mg·L−1) in 0.05 M PBS buffer (pH 6.0), the removal efficiency and rate of MG were markedly different with initial MG concentration. The removal efficiency of MG with the initial concentration of 10 mg·L−1 was the highest within 30 min, reaching 97%. The removal efficiency decreases as the initial MG concentration increases. The removal efficiency decreased from 97 to 60% with the increase of MG concentration to 75 mg·L−1. Although the removal efficiency and rate were markedly decreased when the concentration of MG was raised to 50 mg·L−1 or 75 mg·L−1, the performance of Cu-TA nanozymes was still much higher than that of natural laccases. For instance, to attain 95% removal efficiency for 50 mg·L−1 MG concentration, the reaction between the laccases immobilized on magnetic grapheme oxide and MG would last at least 3 h [32]. Even if the MG concentration was only 20 mg·L−1, 90% removal efficiency of MG by laccase-biotitania biocatalysts also needed 6 h [17]. Therefore, compared with the reported literatures, Cu-TA nanozyme is an ideal substitute for laccase application in the removal of pollutants.
Effect of Cu-TA nanozymes dosage
The catalyst concentration is an important factor in the catalytic reactions since it strongly influences the transformation efficiency of substrates. The effect of Cu-TA nanozyme dosage on the removal of MG was evaluated by using the reaction solution containing 25 mg·L−1 MG and 0.3 mM Hobt at pH 6.0. Figure 9 shows that the increase in the Cu-TA nanozyme concentration from 15 to 45 mg·L−1 increases the removal efficiency of MG from 55% to about 97% within 30 min. The increase in Cu-TA nanozymes concentration supplied larger amount of active sites of Cu ions, which is beneficial to the removal of MG. However, as the Cu-TA nanozyme concentration increases to 60 mg·L−1, only the rate of MG removal has been improved. Hence, in terms of MG removal efficiency and the cost-effectiveness of the process, 30–45 mg·L−1 Cu-TA nanozyme dosage would be seen as an optimum value for 25 mg·L−1 MG concentration.
Effect of ionic strength
Usually, there is a high concentration of salt ions in wastewater, which will affect the removal efficiency of pollutants. The influence of ionic strength on the removal efficiency of MG was determined by incubating them in solutions containing a range of NaCl levels (0–0.3 M) at pH 6.0 and ambient temperature. The removal efficiency of MG increased appreciably as the NaCl concentration raised, which indicated that Cu-TA nanozymes is unlike natural laccases that is susceptible to the ionic strength in reaction system [8]. Our results showed in Fig. 10 are consistent with those of reported study on a related system, which showed that the catalytic activity of Cu-based nanozymes increased with increasing ionic strength [9, 10]. The increase in the removal efficiency of MG may be explained from two aspects. One is from Na+ ions. The addition of Na+ may have altered the structural organization of water and screened the electrostatic repulsion between the Cu-TA nanozymes and MG molecules, thereby strengthening the hydrophobic attraction between the two molecules [33]. The studies by Wang et al. [10] have revealed that the existence of NaCl promoted the adsorption of 2,4-DP on CH-Cu nanozymes and thus enhances the catalytic performance. Another is from Cl− ions. Some studies have shown that Cl− ions could accelerate the copper-based Fenton reaction [34, 35]. Cu (II), serving as Fenton catalyst, reacted with oxygen or peroxide to produce hydroxyl radical, prompting redox reaction process.
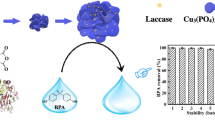
Reusability of Cu-TA nanozymes
The reusability of an enzyme mimic is critical for its potential application in industry. The reusability of Cu-TA nanozymes was examined by applying consecutive cycles of MG removal. The experiments were carried out at room temperature by using the optimum conditions; i.e., the reaction solution (pH 6.0) consisted of 25 mg·L−1 MG, 45 mg·L−1 Cu-TA nanozymes, and 0.3 mM Hobt. As can be seen in Fig. 11, the removal efficiency of the first three recycling declined about 10%, while the subsequent three recycling was rapidly reduced to 50%. The decrease in removal efficiency of MG with cycle times may be attributed to that the adsorption sites (phenolic groups in TA) of Cu-TA nanozymes were occupied by MG molecules during continuous catalytic operations. On the other hand, the repeated washing and separating operations could also lead to the loss of Cu ions and hydrolysis of TA on the Cu-TA nanozymes.
Conclusions
In this study, Cu-TA composites with laccases activity were successfully prepared using a simple self-association method. Characterizations via SEM, XRD, FTIR, and XPS showed that the Cu-TA hybrid composites had good spherical in shape with a diameter of about 20 nm and three different valence Cu ions (Cu2+, Cu+, and Cu0). The catalytic activity was displayed against typical oxidation substrate of laccases, 2,4-DP. Compared to natural laccases, Cu-TA composites were able to better tolerate changes of pH, temperature, ionic strength, and storage conditions. The Cu-TA composites could effectively removal of MG from aqueous solutions. The removal efficiency of MG was optimized using a series of batch tests with single-factor experiment design. Under the optimum conditions, the removal efficiency of MG still retained about 90% after 3 times cycles. Therefore, the Cu-TA composites, as a laccases-like nanozyme, have excellent developing potential in wastewater treatment due to the low-cost of TA and simplicity of preparation.
References
Chaurasia PK, Bharati SL, Sarma C (2016) Laccases in pharmaceutical chemistry: a comprehensive appraisal. Mini-Rev Org Chem 13:430–451
Kudanga T, Nemadziva B, Le Roes-Hill M (2017) Laccase catalysis for the synthesis of bioactive compounds. Appl Microbiol Biotechnol 101:13–33
Mohit E, Tabarzad M, Faramarzi MA (2020) Biomedical and pharmaceutical-related applications of laccases. Curr Protein Pept Sci 21:78–98
Sun T, Fu M, Xing J, Ge Z (2020) Magnetic nanoparticles encapsulated laccase nanoflowers: evaluation of enzymatic activity and reusability for degradation of malachite green. Water Sci Technol 81:29–39
Timur S, Pazarlioglu N, Pilloton R, Telefoncu A (2004) Thick film sensors based on laccases from different sources immobilized in polyaniline matrix. Sensors Actuators B Chem 97:132–136
Martinez AT, Ruiz-Duenas FJ, Camarero S, Serrano A, Linde D, Lund H, Vind J, Tovborg M, Herold-Majumdar OM, Hofrichter M, Liers C, Ullrich R, Scheibner K, Sannia G, Piscitelli A, Pezzella C, Sener ME, Kilic S, van Berkel WJH, Guallar V, Lucas MF, Zuhse R, Ludwig R, Hollmann F, Fernandez-Fueyo E, Record E, Faulds CB, Tortajada M, Winckelmann I, Rasmussen J-A, Gelo-Pujic M, Gutierre A, del Rio JC, Rencoret J, Alcalde M (2017) Oxidoreductases on their way to industrial biotransformations. Biotechnol Adv 35:815–831
Yang J, Li W, Ng TB, Deng X, Lin J, Ye X (2017) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol 8
Liang H, Lin F, Zhang Z, Liu B, Jiang S, Yuan Q, Liu J (2017) Multicopper laccase mimicking nanozymes with nucleotides as ligands. ACS Appl Mater Interfaces 9:1352–1360
Zhang S, Lin F, Yuan Q, Liu J, Li Y, Liang H (2020) Robust magnetic laccase-mimicking nanozyme for oxidizing o-phenylenediamine and removing phenolic pollutants. J Environ Sci 88:103–111
Wang J, Huang R, Qi W, Su R, Binks BP, He Z (2019) Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants. Appl Catal B Environ 254:452–462
Ren X, Liu J, Ren J, Tang F, Meng X (2015) One-pot synthesis of active copper-containing carbon dots with laccase-like activities. Nanoscale 7:19641–19646
Bulgariu L, Belen Escudero L, Bello OS, Iqbal M, Nisar J, Adegoke KA, Alakhras F, Kornaros M, Anastopoulos I (2019) The utilization of leaf-based adsorbents for dyes removal: A review. J Mol Liq 276:728–747
Mall ID, Srivastava VC, Agarwal NK, Mishra IM (2005) Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids and Surfaces a-Physicochemical and Engineering Aspects 264:17–28
Shang N, Ding M, Dai M, Si H, Li S, Zhao G (2019) Biodegradation of malachite green by an endophytic bacterium Klebsiella aerogenes S27 involving a novel oxidoreductase. Appl Microbiol Biotechnol 103:2141–2153
Thung W-E, Ong S-A, Ho L-N, Wong Y-S, Ridwan F, Lehl HK, Oon Y-L, Oon Y-S (2018) Biodegradation of Acid Orange 7 in a combined anaerobic-aerobic up-flow membrane-less microbial fuel cell: mechanism of biodegradation and electron transfer. Chem Eng J 336:397–405
Siroosi M, Amoozegar MA, Khajeh K, Dabirmanesh B (2018) Decolorization of dyes by a novel sodium azide-resistant spore laccase from a halotolerant bacterium, Bacillus safensis sp strain S31. Water Sci Technol 77:2867–2875
Zhang X, Wang M, Lin L, Xiao G, Tang Z, Zhu X (2018) Synthesis of novel laccase-biotitania biocatalysts for malachite green decolorization. J Biosci Bioeng 126:69–77
Li C, Lou Y, Wan Y, Wang W, Yao J, Zhang B (2013) Laccase immobilized onto poly(GMA-MAA) microspheres for p-benzenediol removal from wastewater. Water Sci Technol 67:2287–2293
Khan NS, Ahmad A, Hadi SM (2000) Anti-oxidant, pro-oxidant properties of tannic acid and its binding to DNA. Chem Biol Interact 125:177–189
Shin M, Kim K, Shim W, Yang JW, Lee H (2016) Tannic Acid as a Degradable Mucoadhesive Compound. ACS Biomater Sci Eng 2:687–696
Guo J, Ping Y, Ejima H, Alt K, Meissner M, Richardson JJ, Yan Y, Peter K, von Elverfeldt D, Hagemeyer CE, Caruso F (2014) Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angewandte Chemie-International Edition 53:5546–5551
Sileika TS, Barrett DG, Zhang R, Lau KHA, Messersmith PB (2013) Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angewandte Chemie-International Edition 52:10766–10770
Cakar S, Ozacar M (2019) The pH dependent tannic acid and Fe-tannic acid complex dye for dye sensitized solar cell applications. Journal of Photochemistry and Photobiology a-Chemistry 371:282–291
Rahim MA, Ejima H, Cho KL, Kempe K, Muellner M, Best JP, Caruso F (2014) Coordination-driven multistep assembly of metal-polyphenol films and capsules. Chem Mater 26:1645–1653
Tinoco R, Pickard MA, Vazquez-Duhalt R (2001) Kinetic differences of purified laccases from six Pleurotus ostreatus strains. Lett Appl Microbiol 32:331–335
Li H, Hou J, Duan L, Ji C, Zhang Y, Chen V (2017) Graphene oxide-enzyme hybrid nanoflowers for efficient water soluble dye removal. J Hazard Mater 338:93–101
Nam S, Easson MW, Condon BD, Hillyer MB, Sun L, Xia Z, Nagarajan R (2019) A reinforced thermal barrier coat of a Na-tannic acid complex from the view of thermal kinetics. RSC Adv 9:10914–10926
Nguyen LN, Hai FI, Price WE, Leusch FDL, Roddick F, McAdam EJ, Magram SF, Nghiem LD (2014) Continuous biotransformation of bisphenol A and diclofenac by laccase in an enzymatic membrane reactor. Int Biodeterior Biodegradation 95:25–32
Canas AI, Camarero S (2010) Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705
Li YM, Miao X, Wei ZG, Cui J, Li SY, Han RM, Zhang Y, Wei W (2016) Iron-Tannic acid nanocomplexes: facile synthesis and application for removal of methylene blue from aqueous solution. Dig J Nanomater Biostruct 11:1045–1061
Ghigo G, Berto S, Minella M, Vione D, Alladio E, Nurchi VM, Lachowicz J, Daniele PG (2018) New insights into the protogenic and spectroscopic properties of commercial tannic acid: the role of gallic acid impurities. New J Chem 42:7703–7712
Chen J, Leng J, Yang X, Liao L, Liu L, Xiao A (2017) Enhanced performance of magnetic graphene oxide-immobilized laccase and its application for the decolorization of dyes. Molecules 22
Wu J, Deng X, Tian B, Wang L, Xie B (2008) Interactions between oat beta-glucan and calcofluor characterized by spectroscopic method. J Agric Food Chem 56:1131–1137
Shan Z, Lu M, Wang L, MacDonald B, MacInnis J, Mkandawire M, Zhang X, Oakes KD (2016) Chloride accelerated Fenton chemistry for the ultrasensitive and selective colorimetric detection of copper. Chem Commun 52:2087–2090
Wang L, Miao Y, Lu M, Shan Z, Lu S, Hou J, Yang Q, Liang X, Zhou T, Curry D, Oakes K, Zhang X (2017) Chloride-accelerated Cu-Fenton chemistry for biofilm removal. Chem Commun 53:5862–5865
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Disclaimer
The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, Z., Wu, B., Sun, T. et al. Laccase-like nanozymes fabricated by copper and tannic acid for removing malachite green from aqueous solution. Colloid Polym Sci 299, 1533–1542 (2021). https://doi.org/10.1007/s00396-021-04867-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-021-04867-w