Abstract
To prepare a robust biocatalyst and enhance the removal of bisphenol A in wastewater, succinic anhydride was reacted with laccase to obtain succinic anhydride-modified laccase (SA-laccase) and then co-crystallized with Cu3(PO4)2 to form SA-laccase@Cu3(PO4)2 hybrid nanoflowers (hNFs). The activity of SA-laccase@Cu3(PO4)2 reached 5.27 U/mg, 1.86-, 2.88- and 2.15-fold those of bare laccase@Cu3(PO4)2, laccase@Ca3(PO4)2 and laccase@epoxy resin, respectively. Compared with free laccase, the obtained hNFs present enhanced activity and tolerance to pH and high temperature in the removal of BPA. Under the optimum conditions of pH 6.0 and 35 °C, BPA removal reached 93.2% using SA-laccase@Cu3(PO4)2 hNFs, which was 1.21-fold of that using free laccase. In addition, the obtained SA-laccase@Cu3(PO4)2 hNFs retained nearly 90% of their initial catalytic activity for BPA removal after 8 consecutive batch cycles. This efficient method for preparing immobilized laccase can also be further developed and improved to acquire green biocatalysts for removing persistent organic pollutants in wastewater.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, there has been increasing concern about the occurrence of endocrine-disrupting compounds (EDCs), which can affect the sexual development and reproduction of vertebrates by disrupting the function of hormone and endocrine systems [1]. Currently, many EDCs can be found in wastewater and landfill leachates [2, 3]; bisphenol A (BPA), a typical EDCs, is found in many products, such as food, beverage packaging, paper coatings and flame retardants because it is the main additive in polycarbonates, acrylic esters and epoxy resins, which are widely used in the industrial production of plastic food containers and metal can linings. With the extensive usage of BPA and its constant release into the environment, it has become a general toxic pollutant in various environmental media. BPA cannot be degraded in wastewater [4]; thus, this organic pollutant poses serious potential threats to natural ecosystem safety and human health [5].
Traditional BPA removal methods such as membrane technology, reverse osmosis technology and adsorption can only achieve phase transfer of pollutants, and secondary pollution problems remain unsolved. To date, an increasing number of clean technologies have been used to remove BPA in wastewater, such as photo removal [6], radiolysis [7], electrocatalysis [8, 9] and chemical oxidation [10]. However, there are also inherent problems in these methods, such as high cost, the requirement for specific equipment and the usage of highly concentrated chemical products that are strongly aggressive and oxidative. But the biological treatment of environmental hormones has advantages, such as a low processing cost, no secondary pollution and no use of strongly aggressive and oxidative chemicals.
Among the biological methods for removing EDCs, enzymatic treatment is safe and effective and has high substrate specificity, a short reaction time, and mild reaction conditions [11, 12]. In addition, immobilized enzymes present several distinct advantages [13, 14]; as recyclable enzyme catalysts, they have highly efficient catalytic activity and higher stability than free enzymes under severe catalytic conditions. Among the strategies for obtaining immobilized enzyme preparations, enzyme-inorganic hybrid nanoflowers (hNFs) are one of the most convenient strategies because of their facile preparation procedure and enhanced activity and stability [15, 16], which are inspired by biomineralization and contribute to green development for chemical enterprises [17]. The inorganic metal ions used for preparing hNFs have been expanded from Cu2+ to Zn2+, Co2+, Ca2+, Mn2+, etc. [18,19,20,21].
Laccase (EC 1.10.3.2) as a copper oxidase, catalyze the one-electron oxidation of electron-rich substrates such as phenols and aromatic amines by creating a free radical with an accompanying reduction of oxygen to water [22, 23]. They are widely present in insects, bacteria, fungi, lichens and higher plants [24]. Laccase has attracted increasing attention because of its immense biotechnological potential and versatility in oxidizing various substrates [25, 26]. In recent years, laccase has been used to degrade diverse groups of biological compounds, including EDCs [27, 28], emerging/(re)-emerging contaminants (ECs) [29], tetracycline [30] and other drugs. Free laccase application in the bio removal of xenobiotics has the disadvantages of a high production cost, poor stability and nonreusability. According to these characteristics, laccase treatment is economically unacceptable [31]. However, some immobilized laccases having good reusability and storage stability [32,33,34] and could be used to tackle the limitation of large-scale application to some extent [35] if the catalytic activity of laccase immobilized via traditional immobilization methods, such as adsorption, entrapment, covalent binding and cross-linking, can be elevated [36, 37]. Recently, there has been an increasing number of reports that the activity of laccase can be improved after suitable chemical modification [38,39,40], which may be due to the induction and enrichment of substrate to the enzyme [39].
In this work, taking into account the compatibility between Cu2+ and laccase, SA-laccase@Cu3(PO4)2 hNFs were prepared by cocrystallizing laccase, Cu2+ and PO43− after chemical modification of laccase using succinic anhydride (SA). The obtained chemically modified laccase@Cu3(PO4)2 hNFs were then used to catalyze the removal of BPA, a typical EDC. The tolerance to pH and temperature was examined, and the stability of the modified hNFs was carefully assessed by reuse several times.
Materials and methods
Materials
Laccase (≥ 0.5 U/mg) was purchased from Nanjing Duly Biotech Co., Ltd (Nanjing, China). Bisphenol A (2,2-bis(4-hydroxyphenyl) propane, BPA) (≥ 99.0%) and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, ≥ 98.0%) were obtained from Angie Chemical (Shanghai, China). SA (analytically pure), copper sulfate pentahydrate (analytically pure) and all other chemicals were of analytical grade.
Preparation and characterization of SA-laccase@Cu3(PO4)2 hNFs
The 0.25 g laccase was fully dissolved in 4 mL of PBS (0.01 M, pH 7.4) and then centrifuged (10,000 rpm, 10 min) to remove the enzyme protectant, and a 0.02 mg/mL laccase solution was obtained. A total of 150 μL of 0.1 M SA solution was added to the laccase solution for reaction with the enzyme at 4 °C for 3 h, and then the reaction mixture was dialyzed against PBS (0.01 M, pH 7.4) at 4 °C for 5 h. At the end of dialysis, SA laccase (SA-laccase) was collected from the dialysis bag and dispersed in a clean Erlenmeyer flask. Then, 150 μL of 0.12 M CuSO4 solution was added to the mixture and coassembled with the chemically modified enzyme protein to form SA-laccase@Cu3(PO4)2 hNFs at room temperature for 24 h. During this period, copper phosphate crystals were gradually formed, and laccase was immobilized on the copper phosphate crystals to generate SA-laccase@Cu3(PO4)2 hNFs (Fig. 1). The supernatant was removed by centrifugation (4000 rpm, 5 min) to obtain SA-laccase@Cu3(PO4)2 hNFs, and the protein concentration was determined by Bradford dye assay using BSA as a standard [41].
The centrifuged hNFs pellet was washed three times with ultrapure water and lyophilized for subsequent tests. Scanning electron microscopy (SEM, S4800, Hitachi High Technologies Corporation) was used to observe the morphology of the hNFs, and secondary electron images were acquired by a field emission scanning electron microscope at 3 kV and a current of 5 mA. Powder X-ray diffraction (XRD, Bruker D8 Advance X-ray diffractometer) was also used to examine and confirm the crystal structures and components of the hNFs. Fourier transform infrared (FT-IR) spectra of the hNFs were recorded using an FT-IR spectrophotometer (Thermo Nicolet iS5) from 4000 to 500 cm−1 with samples dispersed in KBr pellets, and the mathematical data were analyzed using software. After correction for baseline variation, deconvolution of the peak was performed to further examine the change in secondary structure. As an important tool for cellular protein imaging, LSCM, was utilized to characterize SA-laccase@Cu3(PO4)2 hybrid nanoflowers which was labeled with FITC at the excitation wavelength of 490 nm and 420 nm. Additionally, thermogravimetric analysis (TGA) (STA 449 F3, NETZSCH-Gerätebau GmbH, Germany) was carried out at a scan rate of 5 °C/min in a nitrogen atmosphere.
Kinetic experiments
To determinine the Vmax and KM of laccase prepartions, initial reaction rate measurements were carried out at 37 °C in citric acid/sodium hydroxide buffer (50 mM, pH 4.0), using ABTS (0.01–2.00 mmol/L) as substrate. The oxidation of ABTS was monitored at 420 nm by computing the initial slope of the absorbance change over a 3 min period immediately after reaction initiation by the addition of 0.1U laccase. Depending on the obtained data, the kinetic parameters Vmax and KM were calculated as Michaelis–Menten equation as flowed,
where V0 is the initial rate and [S] is the substrate concentration.
Activity assay of laccase preparations
The laccase activity was determined by measuring the change in absorbance at 420 nm with a UV–visible spectrophotometer using ABTS as a substrate. ABTS has a maximum molar absorption coefficient (εmax) at this wavelength, and its value is 3.6 × 104 M−1 cm−1 [42]. One laccase activity unit (U) is defined as the amount of enzyme required to oxidize 1 μmol of ABTS per minute [25]. Free laccase was mixed with ABTS in citric acid/sodium hydroxide buffer (50 mmol/L, pH 4.0). The final concentration of ABTS was 0.4 mmol/L. The solution was incubated for 3 min at 37 °C with shaking at 220 rpm. Then, the absorbance at 420 nm was measured by a UV–visible spectrophotometer and was used to calculate enzyme activity. The calculation formula (2) of laccase activity is listed as follows:
where A is the absorbance, V is the reaction volume, εmax is the maximum molar absorption coefficient, b is the absorption layer thickness, t is the reaction time, and m is the weight of laccase.
When measuring the activity of immobilized laccase, the carrier and ABTS were mixed in the same buffer solution. The final concentration of ABTS and the system environmental conditions were consistent with those of free laccase. This method was used to determine the oxidation rate of laccase oxidizing ABTS to generate ABTS·+ radicals, and the scheme for the mechanism of enzyme activity determination is listed as follows (Fig. 2).
Removing BPA in wastewater using laccase preparations
To evaluate the BPA removal using free laccase, SA-laccase, laccase@Cu3(PO4)2 and SA-laccase@Cu3(PO4)2 hNFs, different types of laccase preparations were performed in 50-mL centrifuge tubes containing 5 mg/L BPA and 0.1 U/mL laccase activity in PBS (0.01 M, pH 6.0). The centrifuge tubes containing the reaction mixture were incubated at 35 °C with shaking at 125 rpm to make the biocatalytic reaction sufficient and homogeneous. In the period of the enzymatic reaction, to determine the content of residual BPA in the aqueous solution, samples were collected every 2 h from the mixture, and BPA was extracted with adequate dichloromethane. Then, the organic phase was evaporated with a rotary evaporator, and the residual BPA was dissolved in an equal volume of acetonitrile and analyzed using high-performance liquid chromatography (HPLC, Agilent 1260) at a wavelength of 275 nm. To determine the adsorption of BPA on the hNFs, SA-laccase@Cu3(PO4)2 hNFs was inactivated at 80 °C until the enzyme activity is < 5% of the initial and utilized to catalyze the removal of BPA as control.
The HPLC instrument was equipped with a C18 reversed-phase column (5 μm × 4.6 mm × 250 mm), its column temperature was 35 °C, the flow rate was 1 mL/min, the mobile phase was the mixture of acetonitrile and water (50:50, v/v), and the injection volume was 10 μL. The removal efficiency (%) of BPA was calculated according to the peak areas before and after enzyme-catalyzed treatment. The equation is as follows:
where \({\rm{Area}}_{0}\) and \({\rm{Area}}_{t}\) represent the peak areas before and after the reaction of BPA [43].
Optimal reaction pH and temperature of laccase and SA-laccase@Cu3(PO4)2 hNFs for removing BPA
The optimal reaction pH and temperature for removing BPA using free laccase- and SA-laccase@Cu3(PO4)2 hNF-catalyzed reactions were determined by measuring BPA removal after reaction at certain temperatures and pH values under successive shaking for a certain time. A series of pH 5.0–8.4 phosphate buffers (0.01 M) were prepared for the BPA removal reaction. A reaction mixture containing 0.1 U/mL laccase and 5 mg/L BPA was added to buffers of different pH values. The reaction was performed at 30 °C with shaking at 125 rpm. At the end of the reaction, the reaction solution was treated, and the residual BPA was extracted using the above method and analyzed by HPLC. The maximum removal of BPA at the optimal pH was 100%, and the relative removal was calculated as the ratio of removal to the maximum at each pH. To assess the optimal reaction temperature for removing BPA, the removal of BPA was carried out at different temperatures (20 °C, 25 °C, 30 °C, 35 °C, and 40 °C). SA-laccase@Cu3(PO4)2 hNFs, laccase@Cu3(PO4)2 and free laccase (0.1 U/mL) were stirred with 5 mg/L BPA at 150 rpm. The removal results were used to compare the biocatalytic activity of free and immobilized laccase at different temperatures, which was used to determine the optimal temperature for removing BPA.
Reuse stability of SA-laccase@Cu3(PO4)2 hNFs for removing BPA
To evaluate the reuse stability of SA-laccase@Cu3(PO4)2 hNFs in the biocatalytic removal of BPA in wastewater, the hNF precipitate was recycled for BPA removal through successive recycling experiments under the same conditions mentioned above. In each batch, the reaction mixture containing 5 mg/L BPA and an equal amount of SA-laccase@Cu3(PO4)2 hNFs was incubated at 35 °C with shaking at 125 rpm. At the end of the removal reaction, the laccase@Cu3(PO4)2 hNFs were centrifuged at 4 °C (4000 rpm, 5 min) and reused for the next batch, and the content of residual BPA in the supernatant was measured using HPLC. The relative removal (%) was normalized to the BPA removal in the first batch, which was taken as 100%.
Results and discussion
Laccase immobilization
To evaluate the effect of chemical modification using SA on the catalytic activity of the immobilized laccase preparation, different methods were used to produce immobilized laccase, and the activities of all samples were examined using a consistent procedure. As shown in Table 1, SA-laccase@Cu3(PO4)2 hNFs gave the highest catalytic activity among the enzyme preparations, 1.86-, 2.88- and 2.15-fold those of bare laccase@Cu3(PO4)2, laccase@Ca3(PO4)2 and laccase@epoxy resin, respectively. This result indicates that laccase may have a better affinity for copper phosphate, which is biocompatible with calcium phosphate [44, 45], which may result from the intrinsic nature of laccase as a copper oxidase. Copper ions constitute the active sites of laccase and act as mediators to transfer electrons from substrates to oxygen. In addition, the Ksp of calcium phosphate is 2.07 × 10–29, nearly 108-fold that of copper phosphate in aqueous solution, 1.40 × 10–37 [46], which means that Cu2+ can more easily form insoluble phosphate precipitate than Ca2+. This ease of precipitation would induce the leaching of Cu2+ from laccase and destroy the protein structure when laccase cocrystallizes with calcium phosphate to produce laccase@Ca3(PO4)2 hNFs, which would decrease the catalytic activity of laccase in the hNFs.
When laccase was chemically modified using SA to form SA-laccase, carboxyl groups were further enriched on the surface of the enzyme. As the pH increased, the increasing negative charges induced the copper ions to approach the laccase, which increased the number of biomimetic nucleation centers of copper phosphate crystals. Having copper ions near laccase would improve structural stability and be helpful for transferring electrons for catalytic oxidation.
Characterization of SA-laccase@Cu3(PO4)2 hNFs
SEM and LSCM
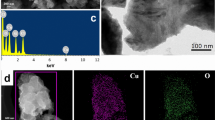
The obtained SA-laccase@Cu3(PO4)2 hNFs were characterized by SEM, and Fig. 3 shows the shape and morphology of SA-laccase@Cu3(PO4)2 when they were formed at 24 h (a–c), 36 h (d–f), 48 h (g–i) and 72 h (j–l). Because the lower concentration of protein decreases the number of nucleation sites, hNFs can grow with greater size than that in a higher concentration of protein [15]. After 24 h (Fig. 3a–c), large agglomerates of protein molecules and primary crystals were formed and anisotropic growth results in the formation of a branched flower-like structure. However, after 36 h (Fig. 3d–f), 48 h (Fig. 3g–i) and 72 h (Fig. 3j–l), we observed a nearly complete flower-like structure. The layered nanoflower structure may be achieved by the strong coordination and electrostatic interaction of Cu2+ and laccase. After the nucleation centers [47] of copper phosphate crystals were formed, laccase was confined in the copper phosphate crystals. This confinement can be further proved using LSCM characterization profiles of the SA-laccase@Cu3(PO4)2 hybrid nanoflowers shown in Fig. 4. As an important tool for cellular protein imaging, LSCM can characterize the sites and morphology of protein. Labeled with FITC, laccase proteins appear green and red when the excitation is carried out at the wavelength of 490 nm and 420 nm, respectively. The LSCM results indicated that enzymes were uniformly distributed in supports, which facilitates the diffusion of substrate to the catalytic sites [48].
The inorganic crystal embeds the enzyme in a hard “coat” and restrains it from unfolding its peptide chains. Thus, the mimetic macromolecular crowding [49, 50] from this confinement efficiently stabilizes the laccase structure and enhances the refolding of protein to its native state, which improves the enzyme activity [15, 21]. This process is also consistent with reported lipase@Zn3(PO4)2 hNFs and our papain@Cu3(PO4)2 hNFs. Furthermore, the nanometer pores greatly increase the surface area of the SA-laccase@Cu3(PO4)2 hNFs, which causes more exposure of laccase active centers and less mass transfer limitation and improves the affinity of the enzyme and substrate [51]. Hence, the high specific surface area of these nanocatalysts can provide a greater contact area and more active sites for the target reaction [52], which would be beneficial to increase laccase catalytic activity and the removal of organic pollutants such as BPA.
FT-IR
To investigate the effect of chemical modification using SA and copper phosphate interactions on the structure of laccase, the secondary structures of the laccase before and after immobilization were evaluated using FT-IR spectroscopy. Figure 5 presents the FT-IR spectra of the free laccase (a), SA-laccase (b), laccase@Cu3(PO4)2 hNFs (c) and SA-laccase@Cu3(PO4)2 hNFs (d), which were recorded and analyzed in the range of 4000–400 cm−1. Amide I band (1700–1600 cm−1) is the most sensitive spectral region and correlated closely to the protein secondary structural components, which is due almost entirely to the C=O stretch vibrations of the peptide linkages (approximately 80%). In contrast, amide II band, derives mainly from in-plane N–H bending (40–60% of the potential energy) and from the C–N stretching vibration (18–40%), and present much less protein conformational sensitivity than the counterpart in amide I [53, 54]. In curves a, b, and d, there are characteristic peaks of amide I at 1640 cm−1. However, no characteristic peaks for amide I were found in the FT-IR spectrum of laccase@Cu3(PO4)2 hNFs (c), which indicates the distinct change in the secondary structure of laccase in Cu3(PO4)2 crystals. This change in laccase secondary structure is consistent with the content change of α-helices, β-sheets and random coils [55], which can be identified in the shifted amide I band, as shown in Fig. 5c.
Figure 6 shows the fractions of α-helix, β-sheet, turn and random protein structures in laccase and SA-laccase@Cu3(PO4)2 hNFs determined with FT-IR measurements. α-helical content decreased from 17 to 14%, β-sheets increased from 37 to 41% and β-turns also increased from 26 to 29% and random decreased from 19 to 16%. Low α-helical contents of 11% for laccase and β-sheet contents of 37% are consistent with that in the previous report [54]. Thus, the mathematical data analysis and deconvolution of the peak results further suggest that no apparent and significant change occurs to the secondary structure of laccase when laccase was chemical modified using SA and then confined in Cu3(PO4)2 hNFs.
XRD
To analyze the crystal structure of immobilized laccase, three samples, Cu3(PO4)2, laccase@Cu3(PO4)2 hNFs and SA-laccase@Cu3(PO4)2 hNFs, were characterized by XRD. In Fig. 7, the XRD patterns of SA-laccase@Cu3(PO4)2 and laccase@Cu3(PO4)2 display peaks at 2θ = 13.2°, 22.0°, 29.4°, 34.1°, 37.3°, 41.9°, and 53.6°, and all the peak positions were well matched, which indicates that the main component of the nanoflowers was Cu3(PO4)6·14H2O. The strong peaks indicate that the nanoparticles show good crystallinity.
The results show that coordination with laccase changes the crystal structure of Cu3(PO4)2. Complexation and electrostatic interactions with the carboxyl groups of succinic acid on the laccase protein surface may alter the interaction direction, facilitate the stabilization of the protein structure and further protect against refolding activity.
TGA
The TGA data for SA-laccase@Cu3(PO4)2 hNFs and Cu3(PO4)2 are shown in Fig. 8. The initial weight loss of SA-laccase@Cu3(PO4)2 hNFs and Cu3(PO4)2 from 25 to 150 °C is associated with the loss of adsorbed water [56, 57]. The SA-laccase@Cu3(PO4)2 hNFs showed weight loss up above 150 °C and this was mainly due to the loss of low molecular weight compounds. Further increase in temperature to 300 °C resulted in higher weight loss in immobilized forms and this corresponds to the degradation of proteins and structurally bound water molecules. The highest decrease in weight loss was observed above 300 °C and this was due to the complete degradation of organic compounds [56, 58], which means the presence of enzyme protein in the hNFs.
Effect of pH and temperature on the removal of BPA
To explore the effect of pH on the removal of BPA catalyzed using free laccase and SA-laccase@Cu3(PO4)2 hNFs, the removal of BPA was carried out in aqueous solutions of different pH values. As shown in Fig. 9, similar to most laccases [59], when using both free laccase and hNFs, the highest BPA removal was achieved at pH 6.0. Additionally, BPA removal using free laccase was reduced significantly as the pH in the solution shifted from the optimum value, and only 10% of the optimal activity remained when the pH reached 8.0. However, even when the pH was 8.4, the relative BPA removal using hNFs was still 85%. As the structure and function of laccase are now understood more clearly, it is known that lysine residues are not part of the active site of laccase [60, 61]. The reaction between SA and the lysine residues of laccase, which introduces carboxyl groups with an anionic charge into the enzyme molecule, enhances the coordination ability of laccase with copper ions. This reaction also shifts the optimum pH to less acidic values [62]. For these reasons, compared with native laccase, the modified laccase exhibits remarkably higher pH stability, catalytic efficiency and substrate affinity [63]. This result suggests that chemical modification using SA can efficiently stabilize the enzyme structure [64], which possibly results from the confinement of the laccase molecule in the support [65, 66].
Figure 10 demonstrates that both laccase and SA-laccase@Cu3(PO4)2 hNFs present optimal catalytic activities for the BPA removal reaction at 35 °C. Compared with free laccase, SA-laccase@Cu3(PO4)2 hNFs have a wider suitable temperature range for biocatalyzing BPA removal. Most importantly, at low temperature, SA-laccase@Cu3(PO4)2 hNFs have a higher removal efficiency, 1.63-fold that of free laccase (52.6%), for removing BPA. This higher efficiency may have resulted from the increased number of Cu2+ ions interacting with SA-laccase by complexation and electrostatic attraction. Multicopper oxidases (MCOs) catalyze the reduction of O2 to H2O by transferring 4e− from the substrate [67]. The increased Cu2+ ions on the laccase protein surface in suitable directions favored and facilitated electron transport from the substrate [22, 23], which promoted the catalytic removal of BPA at low temperature.
Effect of reaction time on the removal of BPA using laccase and SA-laccase@Cu3(PO4)2 hNFs
To evaluate the catalytic activity of free laccase, laccase@Cu3(PO4)2 and SA-laccase@Cu3(PO4)2 hNFs, we compared the BPA removal under the optimal conditions obtained before (i.e., 0.1 U/mL laccase activity, 5 mg/L BPA, 35 °C and pH 6.0 in PBS buffer). Samples were taken from the reaction mixtures every two-hour period and analyzed by HPLC. As shown in Fig. 11, the BPA removal by SA-laccase@Cu3(PO4)2 hNFs reached 77.1% at 2 h and 93.2% after 8 h, while that using free laccase was only 77.5% after 8 h. It could be interpreted that the rate of BPA removal using SA-laccase@Cu3(PO4)2 hNFs was fourfold than that using free laccase in the initial period. Approximately 93.2% of the BPA was removed by SA-laccase@Cu3(PO4)2 hNFs, a rate 1.21-fold that of using free laccase. A total of 85.99% of BPA was removed when the catalyst was prepared without SA, which was a 1.11-fold higher compared to the removal rate obtained using free laccase. The results show that immobilized laccase shows better catalytic activity than free laccase and that SA modification can appropriately enhance enzyme stability and catalytic activity.
In this work, ABTS was used as a substrate to examine the kinetic properties of free laccase and SA-laccase@Cu3(PO4)2 (Fig. 12). KM and Vmax were 0.24 mmol/L and 1.69 mmol/min for free enzyme, 0.35 mmol/L and 2.81 mmol/min for SA-laccase@Cu3(PO4)2, respectively. This increase in KM of the SA-laccase@Cu3(PO4)2 indicated a lower affinity for its substrate, which was attributed to some factors such as diffusional limits, steric effects, or loss of enzyme flexibility that is necessary for optimal substrate binding [68, 69]. Vmax value was enhanced after a confinement of laccase in hybrid nanoflowers, which indicates a higher BPA removal rate than that using free laccase. Therefore, chemical modification and confinement in SA-laccase@Cu3(PO4)2 give rise to a 14% growth of the Vmax/KM ratio. This might be due to favorable interactions between lysine, Cu2+ ions and laccase, which maintains its active form and results in the increased enzymatic efficiency [70, 71].
In the previous report [72], laccase immobilized in hydrogel microparticles could remove just 93% of BPA in solution after 24 h of a long time reaction. Thus, in the obtained SA-laccase@Cu3(PO4)2 hNFs, electrostatic interactions in suitable directions and the biocompatibility of copper ions with laccase protein may sustain the structural stability of laccase and support the high catalytic activity of laccase in hNFs for BPA removal. In addition, the BPA removal reached 93.2% using SA-laccase@Cu3(PO4)2 hybrid nanoflowers in the present work, which was 1.24 folds of activated sludge (average removal 74.7%), 1.34 folds of anaerobic-anoxic–oxic or anoxic–oxic (average removal 69.9%), 1.22 folds of oxidation ditch (average removal 76.0%), and 1.60 folds of aerated lagoon/facultative lagoon/stabilization lagoon (average removal 58.3%), respectively [73].
Possible removal process of BPA by SA-laccase@Cu3(PO4)2 hNFs
We evaluated the adsorption of BPA on the SA-laccase@Cu3(PO4)2 hNFs to give an in-depth discussion of the removal mechanism of BPA using SA-laccase@Cu3(PO4)2 hNFs. In the proposed growth process of SA-laccase@Cu3(PO4)2, the protein induces the nucleation of copper phosphate crystals to form a scaffold for the petals and serves as a ‘glue’ to bind the petals together. Without the proteins, large crystals, but no nanoflowers, are formed. This was also verified by the loss of the flower structure and scattered petals [15] after the high-temperature calcination (350 °C) of nanoflowers made with NF-2. An early study evaluated the thermal stability of laccase nanoflowers that were modified by acid anhydride, and the residual activities after incubating the nanoflowers at 80 °C for 30 min were measured. The results showed that after incubation at 80 °C, the hNFs retained 42% activity [74].
Based on that research, we just used 80 °C as the heat inactivation temperature. Upon extending the time to 1.5 h, the enzyme activity was less than 5% of the previous value. When laccase was inactivated in the reaction mixture, only approximately 18.13% of the BPA was removed, which may be partially attributed to the adsorption of BPA on the surface of the hNFs (Fig. 13). The results show that SA-laccase@Cu3(PO4)2 hNFs have a certain degree of adsorption for BPA. According to control experiments with inactivated hNFs, BPA adsorption on hNFs was < 20%; therefore, laccase was still the most significant factor responsible for BPA removal (more than 80%). BPA is a neutral molecule below pH 7.0 and starts to deprotonate above pH 7.0 [75]. The protonated phenolic group is not a particularly good ligand for metal cations, but once deprotonated, an oxygen center is generated that possesses a high charge density, a so-called "hard" ligand. Although the pKa value of most phenols is in the region of 9.0–10.0, in the presence of suitable cations, such as iron(III) or copper(II), the proton is displaced at much lower pH values, e.g., 5.0–8.0. Aliphatic alcohols do not share this property, as the resulting oxygen anion is not stabilized by the typical mesomeric effect of phenols [76]. In our research, the chelation of Cu(II) may also contribute to the removal of BPA.
Reuse stability of SA-laccase@Cu3(PO4)2 hNFs in the removal of BPA
Since stability is an essential parameter to be considered in large-scale applications, we assessed the reuse stability of SA-laccase@Cu3(PO4)2 hNFs by consecutive cycles of BPA removal; BPA was quantitatively removed for 2 h in every batch. As indicated in Fig. 12, BPA removal using SA-laccase@Cu3(PO4)2 hNFs was reduced only slightly in consecutive batches. However, the removal rate still reached 90% after 8 batches of reuse, which suggests the high reuse stability of the obtained SA-laccase hNFs. This high reuse stability possibly results from the coordination and electrostatic interactions between Cu2+ and SA-laccase in a suitable direction. Moreover, confinement in the Cu3(PO4)2 crystal reduces the conformational flexibility of the laccase protein and increases the rigidity of the enzyme, which efficiently decreases the possibility and tendency of the refolding of SA-laccase protein. This high reuse stability of SA-laccase@Cu3(PO4)2 hNFs can support low-cost biocatalysis for BPA removal.
Conclusion
In summary, chemical modification using SA enriched the carboxyl groups and negative charges on the laccase protein surface, which induced coordination and electrostatic interactions in appropriate directions. These interactions as well as confinement in the Cu3(PO4)2 hNFs sustained SA-laccase protein stability and supported high activity and stability in removing BPA. In addition, the copper ions in the hNFs coated the SA-laccase protein surface, efficiently bridging electron transport and enhancing the catalytic removal of BPA in wastewater at low temperature. This immobilization method inspired by the biocompatibility of laccase and copper ions and the enzymatic reaction mechanism significantly improved laccase immobilization and its catalytic removal of BPA in wastewater.
References
Rahman MF, Yanful EK, Jasim SY (2009) Occurrences of endocrine disrupting compounds and pharmaceuticals in the aquatic environment and their removal from drinking water: challenges in the context of the developing world. Desalination 248(1–3):578–585
Loffredo E, Castellana G, Senesi N (2014) Decontamination of a municipal landfill leachate from endocrine disruptors using a combined sorption/bioremoval approach. Environ Sci Pollut Res 21(4):2654–2662
Joseph L, Zaib Q, Khan IA, Berge ND, Park YG, Saleh NB, Yoon Y (2011) Removal of bisphenol A and 17 alpha-ethinyl estradiol from landfill leachate using single-walled carbon nanotubes. Water Res 45(13):4056–4068
De Freitas EN, Bubna GA, Brugnari T, Kato CG, Nolli M, Rauen TG, Moreira R, Peralta RA, Bracht A, De Souza CGM, Peralta RM (2017) Removal of bisphenol A by laccases from Pleurotus ostreatus and Pleurotus pulmonarius and evaluation of ecotoxicity of degradation products. Chem Eng J 330:1361–1369
Dekant W, Voelkel W (2008) Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol 228(1):114–134
Wang GH, Qi PR, Xue XF, Wu F, Deng NS (2007) Photodegradation of bisphenol Z by UV irradiation in the presence of beta-cyclodextrin. Chemosphere 67(4):762–769
Chen PJ, Linden KG, Hinton DE, Kashiwada S, Rosenfeldt EJ, Kullman SW (2006) Biological assessment of bisphenol A degradation in water following direct photolysis and UV advanced oxidation. Chemosphere 65(7):1094–1102
Deng B, Li YT, Tan WH, Wang ZX, Yu ZW, Xing SY, Lin H, Zhang H (2018) Degradation of bisphenol A by electro-enhanced heterogeneous activation of peroxydisulfate using Mn-Zn ferrite from spent alkaline Zn-Mn batteries. Chemosphere 204:178–185
Zhang YM, Zhang DD, Zhou LC, Zhao YL, Chen J, Chen Z, Wang F (2018) Polypyrrole/reduced graphene oxide aerogel particle electrodes for high-efficiency electro-catalytic synergistic removal of Cr(VI) and bisphenol A. Chem Eng J 336:690–700
Chan YY, Yue YN, Li YX, Webster RD (2013) Electrochemical/chemical oxidation of bisphenol A in a four-electron/two-proton process in aprotic organic solvents. Electrochim Acta 112:287–294
Sharma B, Dangi AK, Shukla P (2018) Contemporary enzyme based technologies for bioremediation: a review. J Environ Manag 210:10–22
Villegas LGC, Mashhadi N, Chen M, Mukherjee D, Taylor KE, Biswas N (2016) A short review of techniques for phenol removal from wastewater. Curr Pollut Rep 2(3):157–167
Bernal C, Rodriguez K, Martinez R (2018) Integrating enzyme immobilization and protein engineering: an alternative path for the development of novel and improved industrial biocatalysts. Biotechnol Adv 36(5):1470–1480
Valencia D, Guillen M, Furst M, Lopez-Santin J, Alvaro G (2018) An immobilized and highly stabilized self-sufficient monooxygenase as biocatalyst for oxidative biotransformations. J Chem Technol Biotechnol 93(4):985–993
Ge J, Lei JD, Zare RN (2012) Protein-inorganic hybrid nanoflowers. Nat Nanotechnol 7(7):428–432
Yu JY, Chen XX, Jiang M, Wang AM, Yang LL, Pei XL, Zhang PF, Wu SG (2018) Efficient promiscuous Knoevenagel condensation catalyzed by papain confined in Cu3(PO4)2 nanoflowers. RSC Adv 8(5):2357–2364
Chen YT, Hu SY, Chen DJ, Zhai HX, Bao ST, Lv TB (2019) An evaluation method of green development for chemical enterprises. Sustainability 11(22):6491
Cui JD, Jia SR (2017) Organic–inorganic hybrid nanoflowers: a novel host platform for immobilizing biomolecules. Coord Chem Rev 352:249–263
Lei ZX, Gao CL, Chen L, He YT, Ma WD, Lin ZA (2018) Recent advances in biomolecule immobilization based on self-assembly: organic–inorganic hybrid nanoflowers and metal–organic frameworks as novel substrates. J Mater Chem B 6(11):1581–1594
Li ZX, Zhang YF, Su YC, Ouyang PK, Ge J, Liu Z (2014) Spatial co-localization of multi-enzymes by inorganic nanocrystal–protein complexes. Chem Commun 50(83):12465–12468
Zhang BL, Li PT, Zhang HP, Wang H, Li XJ, Tian L, Ali N, Ali Z, Zhang QY (2016) Preparation of lipase/Zn-3(PO4)(2) hybrid nanoflower and its catalytic performance as an immobilized enzyme. Chem Eng J 291:287–297
Brenna O, Bianchi E (1994) Immobilised laccase for phenolic removal in must and wine. Biotechnol Lett 16(1):35–40
Hublik S (2000) Characterization and immobilization of the laccase from Pleurotus ostreatus and its use for the continuous elimination of phenolic pollutants. Enzyme Microb Technol 27(3–5):330–336
Guzik U, Hupert-Kocurek K, Wojcieszynska D (2014) Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 19(7):8995–9018
Brugnari T, Pereira MG, Bubna GA, De Freitas EN, Contato AG, Correa RCG, Castoldi R, De Souza CGM, Polizeli M, Bracht A, Peralta RM (2018) A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci Total Environ 634:1346–1351
Mate DM, Alcalde M (2017) Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol 10(6):1457–1467
Barrios-Estrada C, Rostro-Alanis MD, Munoz-Gutierrez BD, Iqbal HMN, Kannan S, Parra-Saldivar R (2018) Emergent contaminants: endocrine disruptors and their laccase-assisted degradation—a review. Sci Total Environ 612:1516–1531
Li X, Lv PF, Yao YX, Feng Q, Mensah A, Li DW, Wei QF (2020) A novel single-enzymatic biofuel cell based on highly flexible conductive bacterial cellulose electrode utilizing pollutants as fuel. Chem Eng J 379:122316
Bilal M, Rasheed T, Nabeel F, Iqbal HMN, Zhao YP (2019) Hazardous contaminants in the environment and their laccase-assisted degradation—a review. J Environ Manag 234:253–264
Taheran M, Naghdi M, Brar SK, Knystautas EJ, Verma M, Surampalli RY (2017) Degradation of chlortetracycline using immobilized laccase on Polyacrylonitrile-biochar composite nanofibrous membrane. Sci Total Environ 605:315–321
Bilal M, Iqbal HMN, Barcelo D (2019) Mitigation of bisphenol A using an array of laccase-based robust bio-catalytic cues—a review. Sci Total Environ 689:160–177
Fu MH, Xing JF, Ge ZQ (2019) Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. Sci Total Environ 651:2857–2865
Lassouane F, Ait-Amar H, Amrani S, Rodriguez-Couto S (2019) A promising laccase immobilization approach for bisphenol A removal from aqueous solutions. Bioresour Technol 271:360–367
Zdarta J, Antecka K, Frankowski R, Zgola-Grzeskowiak A, Ehrlich H, Jesionowski T (2018) The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci Total Environ 615:784–795
Fernandez-Fernandez M, Sanroman MA, Moldes D (2013) Recent developments and applications of immobilized laccase. Biotechnol Adv 31(8):1808–1825
D'souza S (1999) Immobilized enzymes in bioprocess. Curr Sci 77:69–79
Tischer W, Wedekind F (1999) Immobilized enzymes: methods and applications. In: Fessner WD et al (eds) Biocatalysis-from discovery to application topics in current chemistry, vol 200. Springer, Berlin, Heidelberg
Ardao I, Magnin D, Agathos SN (2015) Bioinspired production of magnetic laccase-biotitania particles for the removal of endocrine disrupting chemicals. Biotechnol Bioeng 112(10):1986–1996
Chen Y, Wan J, Wu Q, Ma Y (2017) Chemical modification of laccase from Aspergillus oryzae and its application in OCC pulp. BioResources 12(1):673–683
Ren D, Zhang Y, Xu Q, Wang C, Wang T, Huang S, Zhang S (2013) Effects of chemical modification on laccase stability and degradation of indole. J Pure Appl Microbiol 7:765–770
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Mota TR, Kato CG, Peralta RA, Bracht A, De Morais GR, Baesso ML, De Souza CGM, Peralta RM (2015) Decolourization of Congo red by Ganoderma lucidum laccase: evaluation of degradation products and toxicity. Water Air Soil Pollut 226(10):1–11
Kimura Y, Takahashi A, Kashiwada A, Yamada K (2016) Removal of bisphenol A and its derivatives from aqueous medium through laccase-catalyzed treatment enhanced by addition of polyethylene glycol. Environ Technol 37(14):1733–1744
Ke C, Fan Y, Chen Y, Xu L, Yan Y (2016) A new lipase-inorganic hybrid nanoflower with enhanced enzyme activity. RSC Adv 6(23):19413–19416
Wang A, Chen X, Yu J, Li N, Li H, Yin Y, Xie T, Wu SG (2020) Green preparation of lipase@Ca3(PO4)2 hybrid nanoflowers using bone waste from food production for efficient synthesis of clindamycin palmitate. J Ind Eng Chem 89:383–391
Speight JG, Lange N (2005) Lange’s handbook of chemistry, 16th edn. McGraw-Hill, New York
Habraken W, Tao JH, Brylka LJ, Friedrich H, Bertinetti L, Schenk AS, Verch A, Dmitrovic V, Bomans PHH, Frederik PM, Laven J, Van Der Schoot P, Aichmayer B, De With G, Deyoreo JJ, Sommerdijk N (2013) Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat Commun 4:1–12
Amirkhani L, Moghaddas J, Jafarizadeh-Malmiri H (2016) Candida rugosa lipase immobilization on magnetic silica aerogel nanodispersion. RSC Adv 6(15):12676–12687
Guin D, Gruebele M (2019) Weak chemical interactions that drive protein evolution: crowding, sticking, and quinary structure in folding and function. Chem Rev 119(18):10691–10717
Jiang M, Guo Z (2007) Effects of macromolecular crowding on the intrinsic catalytic efficiency and structure of enterobactin-specific isochorismate synthase. J Am Chem Soc 129(4):730–731
Lee SW, Cheon SA, Kim MI, Park TJ (2015) Organic–inorganic hybrid nanoflowers: types, characteristics, and future prospects. J Nanobiotechnol 13:54
Rong J, Zhang T, Qiu FX, Rong XS, Zhu XL, Zhang XY (2016) Preparation of hierarchical micro/nanostructured Bi2S3-WO3 composites for enhanced photocatalytic performance. J Alloys Compd 685:812–819
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39(8):549–559
Marschelke C, Mueller M, Koepke D, Matura A, Sallat M, Synytska A (2019) Hairy particles with immobilized enzymes: impact of particle topology on the catalytic activity. ACS Appl Mater Interfaces 11(1):1645–1654
Xie M, Liu Y (2003) Studies on amide III infrared bands for the secondary structure determination of proteins. Chem J Chin Univ 24(2):226–231
Wang Z, Ren D, Yu H, Jiang S, Zhang S, Zhang X (2020) Study on improving the stability of adsorption-encapsulation immobilized Laccase@ZIF-67. Biotechnol Rep (Amst) 28:e00553–e00553
Kadam AA, Shinde SK, Ghodake GS, Saratale GD, Saratale RG, Sharma B, Hyun S, Sung J-S (2020) Chitosan-grafted halloysite nanotubes-Fe3O4 composite for laccase-immobilization and sulfamethoxazole-degradation. Polymers 12(10):2221
Muthuvelu KS, Rajarathinam R, Selvaraj RN, Rajendren VB (2020) A novel method for improving laccase activity by immobilization onto copper ferrite nanoparticles for lignin degradation. Int J Biol Macromol 152:1098–1107
Ramirez-Cavazos LI, Junghanns C, Ornelas-Soto N, Cardenas-Chavez DL, Hernandez-Luna C, Demarche P, Enaud E, Garcia-Morales R, Agathos SN, Parra R (2014) Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J Mol Catal B Enzym 108:32–42
Piontek K, Antorini M, Choinowski T (2002) Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-angstrom resolution containing a full complement of coppers. J Biol Chem 277(40):37663–37669
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30(2):215–242
Khaparde SS, Singhal RS (2001) Chemically modified papain for applications in detergent formulations. Bioresour Technol 78(1):1–4
Xiong Y, Gao J, Zheng J, Deng N (2011) Effects of succinic anhydride modification on laccase stability and phenolics removal efficiency. Chin J Catal 32(10):1584–1591
Yang HL, Chen Y, Xin Y, Zhang L, Zhang YR, Wang W (2013) Chemically modified sepharose as support for the immobilization of cholesterol oxidase. J Microbiol Biotechnol 23(9):1212–1220
Arıca MY, Bayramoǧlu G, Bıçak N (2004) Characterisation of tyrosinase immobilised onto spacer-arm attached glycidyl methacrylate-based reactive microbeads. Process Biochem 39(12):2007–2017
Bayramoglu G, Arica MY (2008) Enzymatic removal of phenol and p-chlorophenol in enzyme reactor: horseradish peroxidase immobilized on magnetic beads. J Hazard Mater 156(1–3):148–155
Yoon J, Liboiron BD, Sarangi R, Hodgson KO, Hedman B, Solomona EI (2007) The two oxidized forms of the trinuclear Cu cluster in the multicopper oxidases and mechanism for the decay of the native intermediate. Proc Natl Acad Sci USA 104(34):13609–13614
Lin J, Liu Y, Chen S, Le X, Zhou X, Zhao Z, Ou Y, Yang J (2016) Reversible immobilization of laccase onto metal-ion-chelated magnetic microspheres for bisphenol A removal. Int J Biol Macromol 84:189–199
Li D-F, Ding H-C, Zhou T (2013) Covalent immobilization of mixed proteases, trypsin and chymotrypsin, onto modified polyvinyl chloride microspheres. J Agric Food Chem 61(44):10447–10453
Maurya SS, Nadar SS, Rathod VK (2020) Dual activity of laccase-lysine hybrid organic–inorganic nanoflowers for dye decolourization. Environ Technol Innov 19:100798
Cui J, Zhao Y, Liu R, Zhong C, Jia S (2016) Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci Rep 6:1–13
Piao MY, Zou DL, Yang YS, Ren XH, Qin CY, Piao YX (2019) Multi-functional laccase immobilized hydrogel microparticles for efficient removal of bisphenol A. Materials 12(5):704
Wang H, Liu Z-H, Zhang J, Huang R-P, Yin H, Dang Z, Wu P-X, Liu Y (2019) Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci Total Environ 692:107–116
Zhu P, Wang Y, Li G, Liu K, Liu Y, He J, Lei J (2019) Preparation and application of a chemically modified laccase and copper phosphate hybrid flower-like biocatalyst. Biochem Eng J 144:235–243
Bhatnagar A, Anastopoulos L (2017) Adsorptive removal of bisphenol A (BPA) from aqueous solution: a review. Chemosphere 168:885–902
Hider RC, Liu ZD, Khodr HH (2001) Metal chelation of polyphenols. Methods Enzymol 335:190–203
Acknowledgements
This study was supported by the National Natural Science Foundation of China (22078079, 81730108), the Natural Science Foundation of Zhejiang Province (LY18B060009), the National Innovation and Entrepreneurship Training Program for Undergraduate (201810346008), the “Star and light” Project for Talent Students in Hangzhou Normal University (2019) and Research Plan for Sprout Talents in University in Zhejiang Province (2020R427071).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, H., He, P., Yin, Y. et al. Succinic anhydride-based chemical modification making laccase@Cu3(PO4)2 hybrid nanoflowers robust in removing bisphenol A in wastewater. Bioprocess Biosyst Eng 44, 2061–2073 (2021). https://doi.org/10.1007/s00449-021-02583-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02583-x