Abstract
Biodegradable N-maleyl gelatin (N-MAGEL) was synthesized with gelatin (GEL) and maleic anhydride (MAH) by acylation. With Bis-acrylamide (Bis) cross-linker, a series of cross-linked poly (N-isopropylacrylamide-co-N-maleyl gelatin) [P(NIPAAm-co-N-MAGEL)] hydrogels were prepared, and their lower critical solution temperature (LCST), water content, swelling/deswelling kinetics, drug delivery behaviors, and enzymatic degradation properties were investigated. As weight ratios of N-MAGEL/NIPAAm in hydrogel were increasing, water content increased, and it had no significant influence on LCST of hydrogel in spite of the weight ratios reaching 30:70. In the swelling/deswelling kinetics, hydrogels with higher weight ratios of N-MAGEL/NIPAAm exhibited faster swelling rate and slower deswelling rate. Drug delivery for bovine serum albumin (BSA) research indicated that increasing weight ratios of N-MAGEL/NIPAAm in hydrogels contribute to raising release time and cumulative release ratios. Lastly, hydrogels with higher weight ratios of N-MAGEL/NIPAAm degraded rapidly in phosphate-buffered saline (PBS) containing papain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels are three-dimensional networks of hydrophilic polymer which may absorb from 10 to 20 % up to thousands of times their dry weight in water without dissolving or losing their structural integrity [1, 2]. Intelligent hydrogels do not only have the characteristic of traditional hydrogels but also have the capability to respond to small external stimulus changes, such as temperature, pH, photo field, and antigen. Temperature- and pH-responsive hydrogels have attracted significant attention among the intelligent hydrogels [3]. As a typical thermoresponsive hydrogel, poly(N-isopropylacrylamide) (PNIPAAm) hydrogel undergoes a rapidly reversible hydrophilic/hydrophobic phase transition when temperature is higher than the lower critical solution temperature (LCST, 32 °C) in aqueous solutions. At the same time, the hydrogel often exhibits a volume phase transition from a swollen state to a collapsed state, called volume phase transition temperature (VPTT, 32 °C). Because of the benefits from the thermoresponsive properties, P(PNIPAAm) hydrogels are of increasing interest in biomedical field, such as drug delivery system and tissue engineering. However, difficulty to degrade in vivo, poor biocompatibility, and cytotoxicity limited the potential materials for further application. Yueqin Yu [3–5] and her research team dedicated to solving these problems simply and effectively by adding biodegradable materials in hydrogels. In the past decade, they developed various biodegradable materials as cross-linkers applied in synthesis of hydrogels. For example, they prepared a thermoresponsive P(NIPAAm) hydrogel cross-linked with acryloyloxyethyl-aminopolysuccinimide (AEA-PSI), and the results showed the cross-linking agent promoted degradation of hydrogel. In their work, only a small amount of biodegradable material was introduced in hydrogels causing the biocompatibility of hydrogel hardly to improve. The purpose of this study was to prepare hydrogel with fine biodegradability and biocompatibility by increasing the content of natural polymer in hydrogel.
Gelatin is a well-known biocompatible and biodegradable natural polymer [6], which is composed of kinds of amino acid with amido bond. Gelatin is obtained from collagen which is abounded in skin, bones, and connective tissue. It has better biocompatibility and without cytotoxicity compared with other natural polymers because all sorts of organisms’ protein are composed of amino acid. Similar to chitosan [3], the free amino groups in branch chain of gelatin make it possible to introduce in other compound by acylation reaction. These advantages make gelatin widely used in food engineering, tissue engineering, and so on. However, weakness in mechanical strength and rapid dissolution in aqueous environments limit gelatin practical applications. In order to improve the chemical stability and mechanical properties of gelatin, some simple methods including chemical cross-linking and blending gelatin with a natural polymer were introduced. Cui et al. [7] prepared a novel biomaterial composed of chitosan and gelatin cross-linked by genipin; this IPN hydrogel was expected to be applied in controlled drug delivery system.
In this study, gelatin obtained from scale and fish skin was introduced in P(NIPAAm) hydrogel after being modified by maleic anhydride (MAH). N-maleyl gelatin (N-MAGEL) was applied as an additive to offer biocompatibility and degradability for hydrogel, and a series of hydrogels with different NIPAAm/N-MAGEL weight ratios and using Bis-acrylamide (Bis) as cross-linker were prepared. Their thermoresponsive behaviors, water contents, and swelling/deswelling kinetics were investigated. In addition, their drug delivery behaviors were evaluated with bovine serum albumin (BSA) as model medicine, and phosphate-buffered solution (PBS) containing papain was used to evaluate degradability.
Experimental sections
Materials
Gelatin (gel strength 202 bloom, Qinghai Chem.), papain (from papaya latex, 0.5–2 units/mg, Sigma Chem.), N-isopropylacrylamide (NIPAAm, Aladdin Chem.), Bis (analytic ultrapure grade), N,N,N′,N′-tetramethylethylenediamine (TEMED, analytic ultrapure grade), ammonium persulfate (APS, analytic ultrapure grade), PBS (pH = 7.4 ± 0.1), MAH (analytic ultrapure grade), and acetone (analytic ultrapure grade), except gelatin, papain, and NIPAAm, were purchased from Shanghai Fine Chemical Co. Ltd., China, without further purification.
Synthesis of N-MAGEL
As shown in Scheme 1, N-MAGEL was prepared by acylation reaction between free amino groups in the branch chain of gelatin and maleic anhydride. Briefly, acetone solution containing maleic anhydride was added in gelatin solution (20 %, w/v) with stirring, then the pH of mix solution was adjusted to 9 with NaOH (30 %, w/v) and the solution was heated to 40 °C for 7 h. The product was collected after dialysis and lyophilized using a freeze dryer (LGJ-10, Beijing Song Yuan Hua Xing Technology, China) connected to a vacuum pump (2XZ-2, Zhejiang Qiujing Vacuum, China). The structure of N-maleyl gelatin was characterized by 1H NMR with a 500-MHz spectrometer (Bruker, AV-500) with D2O as solvent, and TNBS [8] was used to measure the content of residual free amino groups. The absorbance of samples in experiment was measured by a microplate spectrophotometer (BioTek, Power Wave XS-2). The degree of substitution (DS) was calculated as follows:
where m n is the weight of free amino groups in N-maleyl gelatin, and m g is the weight of free amino groups in gelatin.
Synthesis P(NIPAAm-co-N-MAGEL) hydrogel
As shown in Fig. 2, the hydrogel samples with various weight ratios of N-MAGEL/NIPAAm were prepared by free radical polymerization. For instance, NIPAAm (0.9 g), N-MAGEL (0.1 g), and 2 wt% of Bis were dissolved in deionized water by stirring, and nitrogen gas was bubbled into the solution for 10 min. APS (4 %) and TEMED were added in as an initiator and accelerator, respectively. The solution continued to react for 24 h at room temperature after thoroughly stirring at 30 s. The hydrogels were washed with deionized water to extract unreacted compounds, and freeze drying was used to remove the water of hydrogels.
The BSA was entrapped in hydrogels by physical method. In all the steps described above, 10 mL of deionized water containing BSA (0.5 %, w/v) was used as replacement. The washing water used to extract unreacted compounds was collected to measure encapsulation efficiency of BSA by bovine BSA ELISA kit (Mlbio). The encapsulation efficiency (EE) of BSA was calculated as follows [9]:
where m 1 is the total weight of BSA in deionized water, and m 0 is the total weight of BSA in washing water.
Temperature-sensitive measurement
The LCST of hydrogels was measured by spectrophotometry method. Exactly, the transmittance of hydrogel samples from 26 to 36 °C was measured by ultraviolet/visible (UV–VIS) spectrophotometer (723 P, Shanghai Spectrum Instruments, China) attached to high-constant temperature bath (CH-1015, The DC Instrument of Shanghai Precision Scientific Instrument, China) at visible light (λ = 569 nm, path length = 3 cm). The temperature of high-constant temperature bath increased 0.5–1 °C every 15 min, and all transmittance of hydrogel samples was calibrated by deionized water.
Water content studies
The swelling ratios of hydrogels were measured by gravimetric method. All hydrogels were immersed in deionized water at 25 °C, and the weight of hydrogels at intervals was determined. After full swelling of hydrogels at 25 °C, the deionized water was heated to 40 °C, and the weight of hydrogels at intervals again was determined. The swelling ratios (SR) of hydrogels were calculated as follows:
where W t is the weight of hydrogel at t time, and W 0 is the initial weight of dry hydrogel.
Drug delivery behaviors in vitro
The dried hydrogels containing BSA were placed in PBS with pH of 7.4 and incubated at 25 and 37 °C in a vapor-bathing constant temperature vibrator (rpm = 100, Taicang Instruments, China), respectively. One milliliter of PBS was removed and replaced by the same volume fresh PBS at intervals. The concentration of BSA in PBS also was measured by ELISA, and the cumulative release ratio (CR) of BSA was calculated as follows:
where m t is the sum weight of BSA in PBS at t time and previous removed solution, and m h is the weight of BSA in dried hydrogel.
Biodegradation behaviors
Papain was selected to evaluate the biodegradation behaviors of hydrogels. Hydrogel samples with different content of N-MAGEL were immersed in PBS solution containing papain (0.6 %, w/v) [10]. The hydrogel samples were washed with deionized water, and freeze drying at intervals after incubated at 37 °C in the vapor-bathing constant temperature vibrator was performed. The degradation ratio (DR) of hydrogel was calculated as follows:
where W t ′ is the weight of dried hydrogel at t time, and W 0 is the weight of dried hydrogel at initial time.
Results and discussion
Synthesis of N-MAGEL and P(NIPAAm-co-N-MAGEL) hydrogel
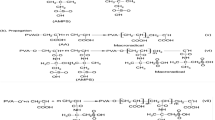
As was shown in Scheme 1, the free amino groups in the branch chain of gelatin reacted with anhydride bond on maleic anhydride by acylation reaction. Compared to the chemical shift changes of N-MAGEL and gelatin in 1H NMR spectra, new peak appeared at 5.90 and 6.2 ppm in N-MAGEL, which was assigned to –CH=CH–. It indicated that maleic anhydride was grafted onto gelatin by forming new amido bond.
As was shown in Fig. 1, the DS was determined by the weighting ratio of MAH/gelatin in reaction system. The DS of amino groups reached maximum (96.7 %) rapidly and then remained unchanged as the weighting ratio of MAH/gelatin increased. In consideration of few free amino groups in gelatin [11], the weighting ratios of MAH/gelatin from 0.05:1 to 0.06:1 were chosen as feeding ratios in preparation of N-MAGEL to offer more cross-links.
As was shown in Fig. 2, P(NIPAAm-co-N-MAGEL) hydrogel was composed of P(NIPAAm) chains and N-MAGEL chains that cross-link with Bis to form a three-dimensional network. The N-MAGEL was fixed in hydrogel by forming a network. The biodegradability and biocompatibility of N-MAGEL also were introduced in hydrogel by this way.
Temperature sensitivity
Figure 3 showed the light transmittance of hydrogel samples (the N-MAGEL/NIPAAm weight ratios in samples were 0:100, 10:90, 20:80, and 30:70, respectively) ranged from 27 to 35 °C at 569 nm. All samples which have undergone a sudden decrease of light transmittance about 32 °C indicate that the addition of N-MAGEL has no obvious effect on the LCST of hydrogel. The LCST of hydrogel samples was decided by the ratio of hydrophilicity and hydrophobicity on P(NIPAAm) chains [4]; the addition of N-MAGEL and Bis had no influence on the structure of P(NIPAAm) chains, and hydrophilic/hydrophobic phase transition of hydrogels always occurred at about 32 °C.
Water content studies
Figure 4 showed the equilibrium SR of hydrogel samples (the N-MAGEL/NIPAAm weight ratios in samples were 0:100, 5:95, 10:90, 15:85, 20:80, 25:75, and 30:70, respectively) in deionized water at 25 and 37 °C. As was shown in Fig. 4, at the temperature of 25 °C, the SR of hydrogel gradually increased with the additional dosage of N-MAGEL in hydrogel. The SR of hydrogel with 30:70 weight ratios of N-MAGEL/NIPAAm had doubled compared with hydrogel without N-MAGEL. These results indicated that increasing content of N-MAGEL in hydrogel had a promotion to SR. N-MAGEL chains absorbed more water molecules than P(NIPAAm) chains because the content of hydrophilic groups on gelatin was higher. In addition, hydrophilic groups abounded on backbone and branch chain of N-MAGEL were beneficial to enhancing cooperative effect in the intermolecular and that helped to absorb extra water molecules for hydrogels.
As the weight ratios of N-MAGEL/NIPAAm in hydrogel increased from 25:75 to 30:70, the SR also increased from 20 to 30, rapidly. Gelatin transformed into gel state by self-aggregation rapidly when meeting with water. Compared to the structure of gelatin [12] with NIPAAm, the gel had better water absorption capacity because hydrogen bond formed by hydrophilic groups on N-MAGEL was stronger. The hydrogel with higher weight ratios of N-MAGEL/NIPAAm was more likely to form gel by self-aggregation of N-MAGEL in hydrogel.
The equilibrium SR of hydrogel samples at 37 °C decreased several times compared to its SR at 25 °C. The hydrophilic/hydrophobic phase transition of P(NIPAAm) backbone when the temperature was higher than LCST led to the initial intermolecular hydrogen bond formed by hydrophilic groups with surrounding water broken, the three-dimensional porous structural in the hydrogels also shrinking as hydrophobic chains gathering together. These results made the water to be released from hydrogels. The SR of hydrogel samples broadly stable as the weighting ratios of N-MAGEL/NIPAAm were increasing indicated that N-MAGEL had a limited influence on the SR of hydrogel when temperature was higher than LCST. Because P(NIPAAm) was accounted for the major proportion in hydrogel, hydrophobic forces of isopropyl on P(NIPAAm) exceed the total hydrophilic forces of hydrogel, and the hydrophilic groups in N-MAGEL were embedded by P(NIPAAm) chains as the shrinking of three-dimensional porous structure. As the weight ratios of N-MAGEL/NIPAAm were higher than 20:80 in hydrogel, the SR increased slowly. Increasing the proportion of hydrophilic groups in hydrogel had an inhibiting effect for the shrinking of three-dimensional porous structure. More hydrophilic groups were introduced in hydrogels as the weight ratios of N-MAGEL/NIPAAm were increasing, and hydrophobic chains of P(NIPAAm) were not enough to embedding hydrophilic groups led to the increase of SR.
Swelling/deswelling kinetic studies
The swelling kinetics of the dried hydrogel samples (the N-MAGEL/NIPAAm weight ratios in samples were 0:100, 10:90, 20:80, and 30:70, respectively) in deionized water at 25 °C were shown in Fig. 5. The swelling rates of all dried hydrogel samples decreased with time, and the hydrogels with higher weighting ratios of N-MAGEL/NIPAAm exhibited much faster swelling rate. Water molecules absorbed in hydrogel rapidly at first might be attributed to all hydrophilic groups in hydrogel which were free as the dried hydrogels immersed in deionized water. The hydrogels with higher weighting ratios of N-MAGEL/NIPAAm had more hydrophilic groups, and the hydrogen bond formed by them was stronger which led to water molecules to be easily diffused into hydrogel network which caused a higher swelling kinetics.
The deswelling kinetics of equilibrium swelling hydrogel samples (the N-MAGEL/NIPAAm weight ratios in samples were 0:100, 10:90, 20:80, and 30:70, respectively) transferred to deionized water at 40 °C were shown in Fig. 6. All samples lost at least half of water in 50 min. When the temperature was higher than LCST, the hydrophilic/hydrophobic phase transition of P(NIPAAm) which leads to the hydrogen bond formed by hydrophilic was destroyed rapidly which caused the decrease of water retention. The deswelling kinetic curves also indicated that increasing the content of N-MAGEL in hydrogels helped to decrease deswelling rate and increase water retention. As discussed in the “Swelling/deswelling kinetic studies” section, the hydrophilic groups on N-MAGEL improved water absorbency of hydrogel. The hydrogen bond formed by N-MAGEL which stayed intact at 40 °C led to the increase of water molecules in hydrogels. As a result, hydrogels with higher weighting ratios of N-MAGEL/NIPAAm showed better water retention.
Drug delivery behaviors
The encapsulation efficiency of BSA in hydrogel samples was 92, 87, and 85 % (the N-MAGEL/NIPAAm weight ratios in samples were 5:95, 10:90, and 15:85, respectively). Due to a good volume phase transition character and high encapsulation efficiency (more than 80 %) for BSA, hydrogel samples with weight ratios of N-MAGEL/NIPAAm from 5:95 to 15:85 were selected as carriers of BSA to evaluate drug delivery behaviors of hydrogel. The BSA release characteristics of different hydrogel samples (the N-MAGEL/NIPAAm weight ratios in samples were 5:95, 10:90, and 15:75, respectively) at 25 °C are concluded in the percentage cumulative release profiles shown in Fig. 7. The release of BSA in all hydrogel samples began with an initial burst release, sometimes as high as 22–32 %. Then, the BSA was continuously released reaching 53–62 % in 8 h. When the samples immersed in PBS, the BSA absorbed on the surface of hydrogels rapidly release to PBS by diffusion led to the release of BSA rapidly. As the decrease of the concentration grads between network of hydrogel and PBS, BSA slowly diffused through tortuous channels of hydrogel for release from 1 to 8 h.
Figure 8 showed the CR of BSA in hydrogel samples (the N-MAGEL/NIPAAm weight ratios in samples were 5:95, 10:90, and 15:85, respectively) at 37 °C. The CR of BSA reached 44–69 % in 1 h and remained unchanged after 2 h with 50–79 %, faster than the release of BSA in hydrogel with same weight ratios of N-MAGEL/NIPAAm at 25 °C. The fast volume phase transition of P(NIPAAm) chains decreased the pore size of channels in hydrogel, which caused most of BSA in hydrogels to be extruded rapidly and the other part encapsulated in channels. What is more, P(NIPAAm) chains had a strong rejection to BSA at 37 °C and the intermolecular repulsion increased the release of BSA. So, compared to the CR of BSA at 25 °C, the hydrogel with same weight ratios of N-MAGEL/NIPAAm released more BSA with less time at 37 °C.
In addition, as the N-MAGEL/NIPAAm weight ratios in hydrogel increase, the BSA showed higher cumulative releasing ratios both at 25 and 37 °C. N-MAGEL in hydrogels contributed to enhancing water absorbency, and the tortuous channels of hydrogel were faster occupied by water molecules leading to the decrease of intermolecular attraction between BSA and hydrogel chains. What is more, N-MAGEL increased the pore size of channels in hydrogels by forming more hydrogen bond. These results led to BSA easier release to PBS.
Biodegradation behaviors
Gelatin was degraded by a variety of proteases, such as neutral proteinase and trypsin [13]. Gelatin chains were degraded to low molecular weight peptide by papain in several hours, and enzyme activity of papain also reduced with increasing incubation time. As was shown in Fig. 9, the biodegradation behaviors of hydrogel samples (the N-MAGEL/NIPAAm weight ratios in samples were 5:95, 10:90, 15:85, 20:80, and 25:75, respectively) were explored. The DR increased from 12 to 24 % as the weight ratios of N-MAGEL/NIPAAm in hydrogels ranged from 5:95 to 25:75. The N-MAGEL in hydrogel degraded by papain led to the network being broken down, and chains in the broken network diffused into the solutions. Hydrogel with higher content of N-MAGEL developed more biodegradable components and cross-linkers. So, the DR of hydrogel increased with the N-MAGEL/NIPAAm weight ratios increasing.
The DR of all hydrogel samples increased rapidly from 0 to 7 h and almost no change from 7 to 10 h. Most of biodegradable components and cross-linkers were degraded within 7 h and led to the decrease of degradation rate later. Besides, enzyme activity of papain also reduced as degradation carried on. So, the weight loss of hydrogel samples became negligible after 7 h.
Conclusion
A novel, natural, biodegradable N-maleyl gelatin was prepared by acylation using gelatin and maleic anhydride as materials. It was added in NIPAAm as additives to synthesize thermoresponsive poly (N-isopropylacrylamide-co-N-maleyl gelatin) [P(NIPAAm-co-N-MAGEL)] hydrogel with Bis as cross-linker. The results indicated that increasing the content of N-MAGEL in hydrogel had no influence on LCST in spite of the weight ratios of N-MAGEL/NIPAAm reaching 30:70, but water content increased. What is more, hydrogels with higher N-MAGEL content exhibited faster swelling rate and slower deswelling rate. In addition, the release time and cumulative release ratios for BSA also raised with the increasing content of N-MAGEL in hydrogels. The enzymatic degradation rate depended on the N-MAGEL/NIPAAm weight ratios in hydrogel.
References
Buwalda SJ, Boere KWM, Dijkstra PJ (2014) Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release 190:254–273
Hoffman AS (2002) Hydrogels for biomedical applications. Adv Drug Deliv Rev 54(1):3–12
Yu Y, Li Y, Liu L (2011) Synthesis and characterization of pH-and thermoresponsive poly (N-isopropylacrylamide-co-itaconic acid) hydrogels crosslinked with N-maleyl chitosan. J Polym Res 18(2):283–291
Yu Y, Li Z, Tian H (2007) Synthesis and characterization of thermoresponsive hydrogels cross-linked with acryloyloxyethylaminopolysuccinimide. Colloid Polym Sci 285(14):1553–1560
Yu Y, Xu Y, Ning H (2008) Swelling behaviors of thermoresponsive hydrogels cross-linked with acryloyloxyethylaminopolysuccinimide. Colloid Polym Sci 286(10):1165–1171
Young S, Wong M, Tabata Y (2005) Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release 109(1):256–274
Cui L, Jia J, Guo Y (2014) Preparation and characterization of IPN hydrogels composed of chitosan and gelatin cross-linked by genipin. Carbohydr Polym 99:31–38
Loth T, Hötzel R, Kascholke C (2014) Gelatin-based biomaterial engineering with anhydride-containing oligomeric cross-linkers. Biomacromolecules 15(6):2104–2118
Wu ZX, Zou XY, Yang LL (2014) Thermosensitive hydrogel used in dual drug delivery system with paclitaxel- loaded micelles for in situ treatment of lung cancer. Colloids Surf B: Biointerfaces 122:90–98
West JL, Hubbell JA (1999) Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 32(1):241–244
Jamilah B, Harvinder KG (2002) Properties of gelatins from skins of fish—black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem 77(1):81–84
Duconseille A, Astruc T, Quintana N (2015) Gelatin structure and composition linked to hard capsule dissolution: a review. Food Hydrocoll 43:360–376
Nagai T, Tanoue Y, Kai N (2014) Collagen hydrolysates derived from Yezo sika deer (Cervus nippon yesoensis) tendon have highly health-promoting potentials. Int Food Res J 21(4):1395–1404
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 20876081) and the Science Foundation of Shandong Province (ZR2012BM015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, Y., Wang, B., Liu, M. et al. Synthesis and characterization of biodegradable thermoresponsive N-maleyl gelatin-co-P(N-isopropylacrylamide) hydrogel cross-linked with Bis-acrylamide for control release. Colloid Polym Sci 293, 1615–1621 (2015). https://doi.org/10.1007/s00396-015-3544-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3544-5