Abstract
Based on a biodegradable cross-linker, acryloyloxyethylaminopolysuccinimide (AEA-PSI), a series of looser cross-linked poly(N-isopropylacrylamide-co-acrylic acid) [P(NIPAAm-co-AAc)] hydrogels were prepared, and their water content, swelling/deswelling kinetics, and the morphology of the gels were investigated. The swelling behaviors of AEA-PSI-cross-linked P(NIPAAm/AAc) hydrogels were investigated in Dulbecco’s phosphate-buffered saline (pH = 7.4), in the distilled water, and in the simulated gastric fluids (pH = 1.2), respectively. The water contents of the hydrogels were controlled by the monomer molar ratio of NIPAAm/AAc, swelling media, and the temperature. In the swelling kinetics, all the dried hydrogels exhibited fast swelling behavior, and the swelling ratios were influenced significantly by the amounts of AEA-PSI and AAc content. The deswelling kinetics of the hydrogel were independent of the content of AAc and cross-linker. Lastly, the morphology of the hydrogels was estimated by the field scan electron microscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(N-isopropylacrylamide; PNIPAAm) hydrogel has been extensively studied as an intelligent polymeric matrix. The reversible phase transition of PNIPAAm hydrogel can be induced by a small external temperature change about its volume phase transition temperature (VPTT; ~32 °C) in aqueous media [1–3]. When the external temperature is below the VPTT, the hydrogel hydrates and absorbs plenty of water, but it dehydrates quickly at the temperature above its VPTT. Because of this unique property, significant attention has been focused on its application in the biotechnology and bioengineering fields [4–8]. However, the PNIPAAm homopolymeric hydrogel is not a favored matrix for biomedical applications because of its transition temperature and rigid network structure. A desirable phase transition temperature of the three dimensional matrix should be at or near the physiologic temperature (37 °C). In addition, the gel matrix should possess high water content but still exhibit temperature sensitive properties at 37 °C [9]. Thus, incorporating a hydrophilic monomer, acrylic acid (AAc), into the PNIPAAm backbone is a good approach to modulate the properties of PNIPAAm hydrogel.
However, an important limitation of PNIPAAm hydrogel for biomedical application is their lack of bioactivity and biodegradability. By incorporating degradable linkages into hydrogel, the material can accomplish a number of interesting biomedical applications such as temporary implants [10]. Such degradable hydrogel comprises of cross-linking molecules with degradable segments. As degradation occurs, degradable linkages in each “arm” of the cross-linking molecules are cleaved systematically, lowering the average number of cross-links per kinetic chain with time and causing eventual mass loss [11]. One of our work’s objectives was to develop a new biodegradable cross-linker. In previous studies [12], we synthesized the biodegradable cross-linker, acryloyloxyethylaminopolysuccinimide (AEA-PSI) and prepared a series of looser cross-linked P(NIPAAm-co-AAc) hydrogels using AEA-PSI as cross-linker. Their phase transition behavior, lower critical solution temperature or volume phase transition temperature, was investigated. By alternating the NIPAAm/AAc molar ratio, hydrogels were synthesized to have VPTT in the vicinity of 37 °C. The VPTT of the hydrogels was significantly influenced by monomer ratio of the NIPAAm/AAc but not by the cross-linking density within the polymer network.
In this paper, the swelling behaviors of AEA-PSI-cross-linked P(NIPAAm/AAc) hydrogels in Dulbecco’s phosphate-buffered saline (PBS; pH = 7.4), in the distilled water (DW), and in simulated gastric fluids (SGF; pH = 1.2) were investigated, respectively. The swelling/deswelling kinetics of the hydrogels were measured gravimetrically and the morphology of the hydrogels was estimated by the field scan electron microscopy (SEM).
Experimental
Materials
The AEA-PSI cross-linker was synthesized according to the literature [12]. The materials were N-isopropylacrylamide (Tokyo Kaset Kogyo), acrylic acid (analytic ultrapure grade), ammonium persulfate (APS; analytic ultrapure grade), N,N,N′,N′-tetramethylethylenediamine (TEMED; analytic ultrapure grade), Dulbecco’s phosphate-buffered saline (pH = 7.4±0.1), and simulated gastric fluids (pH = 1.2±0.1). All materials excepted AEA-PSI and NIPAAm were purchased from Shanghai Fine Chemical, China, used as received without further purification.
Synthesis of P(NIPAAm-co-AAc) hydrogels
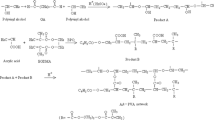
The P(NIPAAm-co-AAc) hydrogels were prepared with AEA-PSI as cross-linker by redox polymerization in the presence of APS as initiator and TEMED as accelerator in aqueous media. The method used to synthesize the AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels was similar to that published previously (Scheme 1) [12]. A series of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels were prepared by varying the molar ratios of NIPAAm/AAc and the amounts of the cross-linker. The polymerization formulations of the hydrogel samples were described in Table 1. The total monomer amount of NIPAAm and AAc in the feed was always 5% w/v. Briefly, NIPAAm, AAc, and AEA-PSI were dissolved in PBS and nitrogen gas was bubbled into the solution for 15 min to eliminate dissolved oxygen. Following the nitrogen gas purge, 0.8 wt.% (based on total monomer) of APS and 8% w/v (based on total monomer) of TEMED were added as the initiator and accelerator, respectively. The mixture was stirred vigorously for 30 s and the polymerization was allowed to proceed at room temperature for 24 h. Following the polymerization, the AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels were washed three times for 15 min each in excess distilled water to extract unreacted compounds.
Phase transition determination
The phase transition of the hydrogel samples was measured by ultraviolet–visible (UV–VIS) spectrophotometer (723 P, Shanghai Spectrum Instruments, China) attached to high constant temperature bath (CH-1015, The DC Instrument of Shanghai Precision Scientific Instrument, China). The transmittance of visible light (λ = 546 nm, path length = 3 cm) through the hydrogel was recorded as a function of temperature. Distilled water was used to calibrate the spectrophotometer. The heating rate was 0.5 ~1 °C every 10 min. The VPTT of the hydrogel samples was determined as the abscissa of the inflection point of the transmittance vs. temperature curves.
Water content studies
The freeze-dried hydrogel samples were weighed upon removal from the freeze dryer and immersed in excess PBS, SGF, or distilled water for 24 h at 25 °C and 37 °C, respectively. The water content was calculated on the basis of the weight difference of the hydrogel samples before and after swelling.
where W s is the weight of the swollen hydrogel and W d is the weight of the dry hydrogel.
Measurement of swelling kinetics
The swollen hydrogel samples were first freeze dried for at least 24 h, and then the dried hydrogel samples were immersed in DW or PBS to swell at 25 °C and 37 °C, respectively. The swelling kinetics of the hydrogels was measured gravimetrically. The samples were removed from water at regular time intervals. After the sample surfaces had been wiped with moistened filter paper to remove water, the weights of the hydrogels were recorded. Swelling ratio is defined as follows:
where W t is the weight of the wet hydrogel at regular time intervals and W d is the weight of the dry hydrogel.
Measurement of deswelling kinetics
The deswelling kinetics of the hydrogels were measured gravimetrically at 45 °C after the sample surfaces had been wiped with moistened filter paper to remove the water. Before the measurements, the hydrogel samples were immersed in distilled water at room temperature until they reached swelling equilibrium. The weight changes of the hydrogels were recorded at regular time intervals during the course of deswelling. Water retention was defined as follows:
where the symbols are the same as defined above.
Scanning electron microscopy analysis
For the morphological studies, the hydrogel samples were first immersed in PBS or DW for 0.5 h. Then, the swollen hydrogel samples were freeze dried for 24 h. Finally, the freeze-dried specimens were fractured in liquid nitrogen and coated with gold for 30 s. The morphology of the freeze-dried hydrogels was investigated by using a scanning electron microscope (6700F, JEOL).
Results and discussion
Water content
Table 1 showed the water contents of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels in different swelling media at 25 °C and 37 °C. At 25 °C, all of the hydrogel samples exhibited water contents of >15 g/g in DW, PBS, and SGF. The water contents of the hydrogels were lower in PBS and SGF than in DW. This tendency was greater above the VPTT as delineated in Table 1. The effect of the media on the swelling behavior could be attributed to the shielding of COO– repulsion, which prevented collapse of the hydrogels, by the interactions between COO– groups in AAc and the ions present in the PBS. As discussed previously, the hydrophilic –COO– groups hinder the dehydration of the polymer chains, expanding the collapsed structure. But in PBS, ions were introduced into P(NIPAAm-co-AAc)-based systems, ionic shielding of the –COO– groups occurred. This ionic shielding disrupted the solubility and repulsion of the –COO– groups, and the –COO– groups were unable to effectively counteract the hydrophobic NIPAAm interactions. As a result, the water contents decreased [13]. The swelling behavior in SGF was low due to the ionization/deionization of the carboxylic acid groups. At low pH, such as pH value about 1.2, the –COOH groups were not ionized and less hydrophility than when –COOH groups were ionized. The hydrogel was less swollen. At high pH values, the –COOH groups were ionized, and the charged –COO– groups generated electrostatic repulsive forces between the polymer chains, which leaded to swelling of the hydrogel network. Also, the ionized groups created an osmotic pressure in the network and therefore prompted the swelling process.
In addition, the water contents of AEA-PSI-cross-linked hydrogels (feed of AEA-PSI = 2.5 wt.%) decreased with increasing the AAc contents in the hydrogels in SGF, probably due to the presence of additional –COOH hydrogen bonds, whereas the water contents of the hydrogels in the DW and PBS gradually increased with the AAc content in the hydrogels (see sample 3, 6, 7, 8, and 9 in Table 1) at room temperature, below the VPTT. When heated to 37 °C, the water content of the hydrogels dropped significantly regardless of the swelling media. At 37 °C, the water contents of the hydrogels in SGF did not change significantly with the monomer molar ratio, whereas the water contents of the hydrogels in the DW and PBS gradually increased with the AAc content in the hydrogels. The results proved that the hydrogels with higher AAc contents were not in the collapsed state in the DW and PBS at 37 °C (which corresponded with the results of the VPTT measurement in Table 1); however, the hydrogels with higher AAc contents were also in the collapsed state in SGF at 37 °C because the –COOH groups were not ionized in SGF.
The effect of the amounts of the AEA-PSI cross-linker on the water contents of the hydrogels with the same NIPAAm/AAc molar ratio of 97.5/2.5 was also investigated (see sample 1, 2, 3, 4, and 5 in Table 1). As the amounts of the AEA-PSI cross-linker within the hydrogels increased, the water content difference was not statistically significant regardless of swelling media.
Swelling kinetics
Figure 1 showed the swelling kinetics of the dried hydrogel samples (NIPAAm/AAc monomer molar ratios 97.5/2.5) with different amounts of cross-linker 1.5, 2.5 and 3.5 wt.% in DW and PBS at 25 °C. As seen in Fig. 1, all the dried hydrogels exhibited fast swelling kinetics. They reached swelling equilibrium within 960 min. Compared to the hydrogels with higher amount of cross-linker (see line L3 and L6 in Fig. 1), the hydrogels with lower amount of cross-linker exhibited much faster swelling kinetics. The observed difference might be attributed to the hydrogels with higher amount of cross-linker, which could make the hydrogel material denser, reinforcing the hydrogel and leading to a more physical restriction. So, water molecules could not easily diffuse into hydrogel network which cause a lower swelling kinetics. As discussed above, the swelling rate of the dried hydrogel samples in PBS was lower than in DW due to the shielding of the –COO− groups by ions present in PBS.
Figure 2 showed the swelling kinetics of dried hydrogel samples (the amounts of cross-linker 2.5 wt.%) with different NIPAAm/AAc monomer molar ratios 97.5/2.5, 96.5/3.5, and 95.5/4.5 in DW and PBS. It was found that the samples had the similar swelling kinetics initially at 25 °C in PBS (see line L1, L2, and L3 in Fig. 2). In DW (see line L4, L5, and L6 in Fig. 2), however, their swelling behaviors were different. The swelling rate of the hydrogel with less AAc monomer was lower than others at the beginning of the swelling process. When reaching the swelling equilibrium, the water content increases with the increasing of hydrophilic monomer AAc which had discussed above.
Deswelling kinetics
Figures 3 and 4 present the deswelling kinetics of the hydrogels after transferring an equilibrated swollen sample at 25 °C (below its VPTT) to hot PBS at 45 °C (above its VPTT). As discussed above, due to the hydrogen bonds between the hydrophilic groups and water and the hydration shell around the hydrophobic groups, the whole gel network is well soluble when the external temperature is below the VPTT. When the temperature is raised, these hydrogen bonds are weakened and destroyed. As a consequence, the hydrophobic groups become naked and the interactions among the hydrophobic groups strengthen, which frees the entrapped water molecules. When the temperature is raised above the VPTT, the hydrophobic interactions turn to be dominant and the polymer chains contract and aggregate abruptly; this leaded to the shrinkage of the gel volume. Simultaneously, a lot of freed water molecules appear and have to diffuse out. The diffusion rate of the freed water through the network determines the deswelling rate of the hydrogel. The diffusion rate is controlled by the collective diffusion coefficient and hydrogel morphology. As seen in Figs. 3 and 4, all the hydrogel samples tended to shrink and lost a lot of water immediately once immersed into hot PBS at 45 °C. For instance, the water retention of hydrogels decreased less than 20% within 20 min. The deswelling kinetics of hydrogels was not significantly influenced by the increasing of hydrophilic monomer AAc and the cross-linking density. In the shrinking procedure, the entrapped water was rapidly squeezed out from hydrogel network due to the shielding effect caused by ions in PBS.
Figures 5 and 6 showed the deswelling kinetics of hydrogels in DW at 45 °C. It could be clearly seen that the shrinking rates of the hydrogels in DW were much slower than those in PBS (seen in Figs. 3 and 4). After being immersed in distilled water at 45 °C for 240 min, the hydrogels shrank and lost water less than 50%. The deswelling kinetics of hydrogels was not significantly influenced by the increasing of hydrophilic monomer AAc of the hydrogel in DW at 45 °C.
Morphological studies
When samples are freeze dried, movement of polymer chains is highly restricted since the entire sample is in the solid state (both polymer chains and water molecules). Thus, as water molecules are removed by sublimation, the polymer chains cannot move and remain in the same conformation [14].
The morphological characteristics of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels after exposure to solutions and subsequent freeze drying have been examined by SEM. Figure 7 were the surfaces (a, b) and fractures (c, d) SEM images of the hydrogels after exposure to the DW (a, c) and PBS (b, d) for 0.5 h. As seen in Fig. 7a, after exposure to the DW for 0.5 h and subsequent freeze-drying, the surface of the hydrogel was very dense and smooth with few pores, compared to the surface of the sample exposure to the PBS for 0.5 h (Fig. 7b) with numerous micropores, which was attributed to the PBS having an eroding effect on the hydrogel. In Fig. 7c, it could be seen that after exposure to the DW for 0.5 h, inner pores in scaffolds are arrayed tightly and regularly, whereas after exposure to PBS for 0.5 h (Fig. 7d), the inner pores of the hydrogels became larger and irregular, some parts of the scaffolds even vanished. It further proved that the hydrogel could be eroded by PBS.
Conclusion
In summary, with a novel biodegradable AEA-PSI cross-linker, cross-linked P(NIPAAm-co-AAc) hydrogels were prepared in phosphate-buffered saline. The swelling behaviors of AEA-PSI-cross-linked P(NIPAAm/AAc) hydrogels were investigated in PBS (pH = 7.4), in DW, and in SGF (pH = 1.2), respectively. The water contents of the hydrogels were controlled by the monomer molar ratio of NIPAAm/AAc, swelling media, and the temperature. In the swelling kinetics, all the dried hydrogels exhibited fast swelling behavior. The swelling rate of the hydrogel in distilled water was higher than that in PBS, and it was also influenced by the amounts of the AEA-PSI and AAc content. The hydrogels deswelled fast in PBS at 45 °C. The deswelling rate of the hydrogel was independent of the content of AAc and AEA-PSI. The surface of the hydrogel was smooth but the hydrogel contained open and well-structure orientated porous network. The AEA-PSI-cross-linked hydrogels were potentially biodegradable in PBS.
References
Grinberg VY, Dubovik AS, Kuznetsov DV, Grinberg NV, Grosberg AY, Tanaka T (2000) Studies of the thermal volume transition of poly(N-isopropylacrylamide) hydrogels by high-sensitivity differential scanning microcalorimetry 2 thermodynamic functions. Macromolecules 33:8685–8692
Ju HK, Kim SY, Lee YM (2001) PH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly(N-isopropylacrylamide). Polymer 42(16):6851–6857
Kim JH, Lee SB, Kim SJ, Lee YM (2002) Rapid temperature/pH response of porous alginate-g-poly(N-isopropylacrylamide) hydrogels. Polymer 43(26):7549–7558
Stayton PS, Shimobji T, Long C, Chilkoti A, Chen GH, Harris JM, Hoffman AS (1995) Control of protein-ligand recognition using a stimuli-responsive polymer. Nature 378:472–474
Ramkissoon-Ganorkar C, Liu F, Baudys M, Kim SW (1999) Modulating insulin-release profile from pH/thermosensitive polymeric beads through polymer molecular weight. J Control Release 59:287–298
Gutowska A, Bae YH, Jacobs H, Mohammad F, Mix D, Feijen J, Kim SW (1995) Heparin release from thermosensitive polymer coatings: in vivo studies. J Biomed Mater Res 29:811–821
Vakkalanka SK, Brazel CS, Peppas NA (1996) Temperature and pH-sensitive terpolymers for modulated delivery of streptokinase. J Biomater Sci Polym Ed 8:119–129
Osada Y, Okuzaki H, Hori H (1992) A polymer gel with electrically driven motility. Nature 355:242–244
Vernon B, Kim SW, Bae YH (2000) Thermoreversible copolymer gels for extracellular matrix. J Biomed Mater Res 51:69–79
Metters A, Hubbell J (2005) Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules 6(1):290–301
Martens P, Metters AT, Anseth KS, Bowman CN (2001) A generalized bulk-degradation model for hydrogel networks formed from multivinyl cross-linking molecules. J Phys Chem B 105(22):5131–5138
Yu YQ, Li ZZ, Tian HJ (2007) Synthesis and characterization of thermoresponsive hydrogels cross-linked with acryloyloxyethylaminopolysuccinimide. Colloid Polym Sci 285:1553–1560
Stile RA, Burghardt WR, Healy KE (1999) Synthesis and characterization of injectable poly(N-isopropylacrylamide)-based hydrogels that support tissue formation in vitro. Macromolecules 32(22):7370–7379
Nakamatsu J, Torres FG, Troncoso OP, Min-Lin Y, Boccaccini AR (2006) Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromolecules 7(12):3345–3355
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No.20476049), the program for New Century Excellent Talents in the Universities (No. NCET-04-0649), and the Science Foundation of Shandong Province (No.Y2006B10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Yq., Xu, Y., Ning, H. et al. Swelling behaviors of thermoresponsive hydrogels cross-linked with acryloyloxyethylaminopolysuccinimide. Colloid Polym Sci 286, 1165–1171 (2008). https://doi.org/10.1007/s00396-008-1878-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-008-1878-y