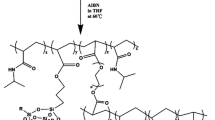
Abstract
Biodegradable cross-linkers acryloyloxyethylaminopolysuccinimide (AEA-PSI) were obtained by microwave irradiation using maleic anhydride as materials. With AEA-PSI cross-linker, cross-linked poly(N-isopropylacrylamide-co-acrylic acid) [P(NIPAAm-co-AAc)] hydrogels were prepared, and their phase transition behavior, lower critical solution temperature (LCST), water content, thermodynamics stability, and enzymatic degradation properties were investigated. By alternating the NIPAAm/AAc molar ratio, hydrogels were synthesized to have LCST in the vicinity of 37 °C. The LCST of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels was significantly influenced by monomer ratio of the NIPAAm/AAc but not by the cross-linking density within the polymer network. The water content of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels was more than 90% even at 37 °C, which was controlled by the monomer molar ratio of NIPAAm/AAc, swelling media, and the cross-linking density. The thermodynamics stability was also characterized by thermogravimetry. In enzymatic degradation studies, breakdown of the AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels was dependent on the cross-linking density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many hydrogels have recently been developed as biomaterials for applications in the medical and pharmaceutical fields. Research studies have showed that the swelling behavior of hydrogels depends on the external environment [1, 2]. They can exhibit abrupt changes in the swelling behavior of the network structure, permeability, or mechanical strength in response to changes in pH, ionic strength, temperature, and electromagnetic radiation. The most commonly studied hydrogels having environmental sensitivity are responsive to either pH or temperature [3, 4]. Poly(N-isopropylacryamide) (PNIPAAm) is a thermosensitive hydrogel that has received much attention for biomedical use because of its lower critical solution temperature (LCST) behavior at around 32 °C in an aqueous solution [5, 6]. PNIPAAm chains hydrate to form expanded structures in water when the solution temperature is below its LCST but become a compact gel structure by dehydration when heated to a temperature above its LCST. Below its LCST, PNIPAAm is extremely soluble in water and appears transparent. However, as its temperature is increased above the LCST, it becomes hydrophobic from the increased interactions between the isopropyl groups, and PNIPAAm precipitates out from the aqueous solution, appearing opaque. PNIPAAm hydrogels possess a three-dimensional network structure which is insoluble but has characteristics of reversible swelling [2]. The polymer chains undergo a coil (soluble)–globule (insoluble) transition when the external temperature cycles across its LCST at about 33 °C [1, 7]. Thus, at a temperature below the LCST, PNIPAAm hydrogels absorb water and exist in a swollen state but shrink and display an abrupt volume decrease when the environmental temperature is higher than the LCST. With this quick response to phase transition at body temperature, PNIPAAm may be an excellent candidate for drug delivery or injectable soft-tissue replacement.
However, an important limitation of PNIPAAm hydrogel for biomedical application is their lack of bioactivity and biodegradability. By incorporating degradable linkages into hydrogel, the material can accomplish a number of interesting biomedical applications such as temporary implants [8]. Such degradable hydrogel comprises cross-linking molecules with degradable segments. As degradation occurs, degradable linkages in each “arm” of the cross-linking molecules are cleaved systematically, lowering the average number of cross-links per kinetic chain with time and causing eventual mass loss [9]. Kim and Healy [10] synthesized Poly(N-isopropylacrylamide-co-acrylic acid) [P(NIPAAm-co-AAc)] hydrogels with degradable peptide cross-linkers. One of our work’s objectives was to develop a new biodegradable cross-linker.
Polyaspartic acid (PASP) is considered to be an environmentally friendly chemical. Not only is PASP well compatible with the global environment owing to its excellent biodegradability, but it also has excellent biocompatibility with human beings. It has been found that, even when swallowed by human beings, it is digested and absorbed by enzymatic action, and, moreover, it does not exhibit antigenecity in human bodies, and their metabolites are free of toxicity [11]. So we would use polyaspartic acid as material to develop a new biodegradable cross-linker.
PASP is obtained by base hydrolysis of polysuccinimide (PSI) synthesized by some raw materials. According to the difference of the raw materials, synthesis methods of PSI can be categorized into two kinds [12–14]. Firstly, aspartic acid is directly polymerized into PSI with or without solvent and catalyst. Secondly, dicarboxylic acid or anhydride having four carbon atoms, such as maleic anhydride and fumaric acid, reacts with ammonia or amine salt to produce d,l-aspartic acid, then PSI can be obtained by polymerizing d,l-aspartic acid. Methods for the preparation of PSI from maleic acid or anhydride and ammonia are known for having inexpensive starting material. The reaction steps and material state directly affect the molecular weight and product yield. The product from maleic anhydride and ammonia has high molecular weight by two reaction steps, but the yield is low. Increasing the length of the reaction and the amount of ammonia relative to the amount of maleic anhydride can increase yield. Thus, the reaction time becomes long.
It is well known that microwave-assisted organic synthesis has become an available technique for the generation of polymer, which usually leads to shorter reaction times and higher yields and purity. Many solvent-free reactions using microwaves have been developed as this reduces the risks of hazard due to pressure build-up in the reaction vessel, and the scale-up is easier.
Therefore, the objective of this study was to develop P(NIPAAm-co-AAc) hydrogels with polyaspartic acid derivatives [acryloyloxyethylaminopolysuccinimide (AEA-PSI)] cross-linkers. PSI was obtained by the microwave irradiation using maleic anhydride and ammonia as materials. The PSI was converted into hydroxyethylaminopolysuccinimide (HEA-PSI) by the partial ring-opening reaction with ethanolamine. HEA-PSI was then acrylated by acryloyl chloride to obtain the biodegradable AEA-PSI cross-linker. A series of thermoresponsive AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels were obtained by varying the molar ratio of NIPAAm/AAc. In addition, some hydrogels of different aggregation density were synthesized by altering the amount of cross-linker. To evaluate the thermoresponsive property, the LCST and water content of hydrogels were characterized. The thermodynamic stability was also characterized by thermogravimetry. The enzymatic degradation of hydrogels was determined by mass loss.
Experimental sections
Materials
Maleic anhydride, ammonium hydroxide solution, ethanolamine, triethylamine, N,N,N′,N′-tetramethylethylenediamine (TEMED; Shanghai Qianjin Chemical Agent, China, BR), N-isopropylacrylamide (NIPAAm; Tokyo Kaset Kogyo), acryloyl chloride, absolute ethanol, N,N′-methylenebisacrylamide (BIS), N,N′-dimethylformamide (DMF), ammonium peroxydisulfate (AP), Dulbecco’s phosphate-buffered saline (PBS, pH = 7.4), dimethyl sulfoxide (DMSO). All other agents were analytical grade purchased from Shanghai Fine Chemical, China, without purification.
Synthesis of polysuccinimide
The polysuccinimide (PSI) was prepared with maleic anhydride and ammonium hydroxide solution in a domestic microwave (MW) oven. Maleic anhydride (9.8 g, 0.1 mol) was dissolved in 20 ml distilled water, and ammonium hydroxide solution (28%, 17 ml, 0.12 mol) was added dropwise to the solution with stirring at 83 °C. After all of the ammonium hydroxide solution was added, the reaction continued with stirring for another 2 h at 83 °C. The mixture was subsequently irradiated in a domestic MW oven at 350 W for 0.5 min periodically up to total irradiation time of 5 min, gaining white solids. The white solids were irradiated continuously at 700 W for 5 min, and dry nitrogen was bubbled into the system to prevent oxidation. The product was washed three times with distilled water and dried at 40 °C using a dryer attached to a vacuum (YB-1A, Tianjin Tuopu Instruments, China). The reaction is described in Scheme 1.
Synthesis of AEA-PSI cross-linker
As shown in Scheme 2, the AEA-PSI cross-linker was prepared with PSI, ethanolamine, and acryloyl chloride. Specifically, PSI (5.7 g) was dissolved in DMF (15 ml), and ethanolamine (1.1 ml) was added dropwise to the solution with stirring. The reaction was allowed to proceed for 3 h at 45 °C. Subsequently, the mixture was precipitated with absolute ethanol, filtered, and dried in a vacuum dryer to obtain the dry HEA-PSI. In succession, the dry HEA-PSI (4.7 g) was dissolved in DMF (25 ml) with triethylamine, and acryloyl chloride (5.4 ml) was added dropwise to the solution with stirring. The reaction temperature was kept at 0 ∼ 5 °C. After all of the acryloyl chloride was added, the reaction continued with stirring for 4 h at 0 ∼ 5 °C and 24 h at room temperature. Then the triethylamine hydrochloride salts were removed by filtration. The filtrate was dialyzed against distilled water for 2 days with periodic bath changes (every 8 h) to eliminate unreacted compounds. The final dialysis product was lyophilized for 24 h using a freeze dryer (LGJ-10, Beijing Song Yuan Hua Xing Technology, China) connected to a vacuum pump (2XZ-2, Zhejiang Qiujing Vacuum, China).
Synthesis of P(NIPAAm-co-AAc) hydrogels
The P(NIPAAm-co-AAc) hydrogels were prepared by redox polymerization in aqueous media. The AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels were prepared by varying the molar ratio of NIPAAm/AAc and the amount of the cross-linker. The total monomer amount of NIPAAm and AAc in the feed was always 5% w/v, and the NIPAAm/AAc molar ratio of 97.5:2.5, 96.5:3.5, and 95.5:4.5 were taken. In addition, the P(NIPAAm-co-AAc) hydrogels cross-linked with BIS were synthesized by a similar method to compare with the AEA-PSI-cross-linked hydrogels. The polymerization formulations of the hydrogel samples are described in Table 1. NIPAAm, AAc and AEA-PSI cross-linker (or BIS) were dissolved in PBS, and dry nitrogen gas was bubbled into the solution for 15 min to eliminate dissolved oxygen. After the nitrogen gas purged, 0.8 wt% (based on total monomer) of AP and 8% v/w (based on total monomer) of TEMED were added as the initiator and accelerator, respectively. The mixture was stirred vigorously for 30 s, and the polymerization was allowed to proceed at room temperature for 24 h. After the polymerization, the BIS- and AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels were washed three times for 15 min each in excess distilled water to extract unreacted compounds. The process is described in Scheme 3.
Characterization of cross-linker
1H NMR and 13C NMR spectroscopy were used to identify the synthesis of the cross-linker. The chemical shifts of PSI, HEA-PSI, and AEA-PSI were measured using nuclear magnetic resonance (NMR) spectroscopy with a 500 MHz spectrometer (Bruker, AV-500); the spectrum was recorded in deuterated DMSO at room temperature.
In addition, gel permeation chromatography (GPC; Shimadzu, 10 VP) was carried out to estimate the molecular weight of PSI. The mixture solution of tetrahydrofuran and DMF were used as the solvent, and standard poly (ethylene glycol) was used for calibrating the molecular weight.
Phase transition determination
The phase transition of the hydrogel samples was measured by ultraviolet/visible (UV-VIS) spectrophotometer (723 P, Shanghai Spectrum Instruments, China) attached to high constant-temperature bath (CH-1015, The DC Instrument of Shanghai Precision Scientific Instrument, China). The transmittance of visible light (λ = 546 nm, path length = 3 cm) through the hydrogel was recorded as a function of temperature. Distilled water was used to calibrate the spectrophotometer. The heating rate was 0.5 ∼ 1 °C every 10 min. The LCST of the hydrogel samples was determined as the abscissa of the inflection point of the transmittance vs temperature curves.
Swelling studies
The swelling characteristics of the hydrogel samples were investigated in triplicate. Hydrogel samples were weighed after freeze-drying overnight and immersed in excess distilled water, PBS for 3 days at 25 °C and 37 °C, respectively. The water content of hydrogel samples was defined as
Where W s is the weight of the swollen hydrogel sample and W d is the weight of the dry hydrogel sample.
Thermodynamic stability behavior
Thermogravimetry and differential thermogravimetry curves of hydrogel samples were performed by a NETZSCH TG 209 (Germany) from 20 to 600 °C at a heating rate of 1 °C/min. The Al 203 was used as the crucible, and nitrogen was the protective gas with flowing rate 20 ml/min.
Enzymatic degradation properties
The enzymatic degradation behavior of the hydrogel should be dependent on both the concentration of enzyme solution and the cross-linking density within hydrogel. In this paper, we only focused on the degradation behavior depending on the cross-linking density. Dry hydrogel samples were immersed in PBS solution having 0.005 mg/ml trypsin at 37 °C. At predetermined time points, samples were removed from the solvent, freeze-dried over night, and weighed to determine weight loss. After weighing, the samples were placed back in fresh trypsin PBS solution for continuous degradation. The percent mass loss of hydrogel was calculated using the formula
where W ti is the initial weight of hydrogel and W t is the weight of hydrogel at different time intervals in degradation medium.
Results and discussion
Synthesis of PSI and AEA-PSI cross-linker
As shown in Scheme 1, ammonium maleate was obtained at 83 °C by the reaction of maleic anhydride and ammonium hydroxide solution. The PSI was prepared with the ammonium maleate solution under microwave irradiation. This method used the low-cost maleic anhydride to substitute the expensive aspartic acid and shortened the reaction time of the polycondensation comparing with the traditional polycondensation [15]. Scheme 2 shows the process of AEA-PSI cross-linker synthesis; the PSI was converted into HEA-PSI by partial ring-opening reaction with ethanolamine. HEA-PSI was then acrylated by acryloyl chloride to obtain the AEA-PSI cross-linker with acryl groups.
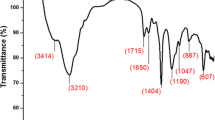
The weight-average molecular weight (Mw) of synthesized PSI was 1,009, number-average molecular weight (Mn) was 817, and the Mw/Mn was 1.24. The 1H NMR spectrum of PSI showed signals at 2.7, 3.2 ppm assigned to methylene protons of the succinimide repeat unit and 5.3 ppm belonged to methine protons of the succinimide repeat unit. The 1H NMR spectrum of HEA-PSI showed new signals at 3.6 ppm of methylene which belonged to –CH2–OH. The degree of reaction was about 20% which is calculated by comparing the integral of the peak related to methylene protons at 3.6 ppm, with that related to methylene protons (belonging to succinimide) at 3.2 ppm. The 1H NMR of AEA-PSI showed new signals at 5.9–6.3 ppm, which contributed to the acryl group. Figure 1 shows the 13C NMR spectra of the PSI, HEA-PSI, and AEA-PSI. As seen in Fig. 1a, two main carbonyl peaks were observed at 173 and 174 ppm, representing the two inequivalent carbonyls of the PSI repeat unit. The repeat unit methylene and methine were observed at 33 and 47 ppm, respectively. The 13C NMR spectrum of the HEA-PSI showed new signals at 61 ppm (belonging to –CH2–OH, Fig. 1b). The chemical shift peaks at 130 ppm in 13C NMR of AEA-PSI (Fig. 1c) also indicated that PSI was indeed acrylated by acryloyl chloride.
LCST characterization
The effect of temperature on the transmittance of visible light (λ = 546 nm) through the BIS-cross-linked PNIPAAm hydrogel sample and AEA-PSI- and BIS-cross-linked P(NIPAAm-co-AAc) hydrogel samples is shown in Fig. 2. Each line represents the average of three experiments with one hydrogel sample. The PNIPAAm hydrogel appeared a sharp phase transition around 31 °C, while the P(NIPAAm-co-AAc) hydrogels showed an increase in LCST and a much broader phase transition. The extremely sharp transition of PNIPAAm hydrogel had been attributed to the hydrophobic/hydrophilic balance of the side groups in polymers, which led to rapid dehydration of the polymer as the temperature increased above LCST. The increased LCST of the P(NIPAAm-co-AAc) hydrogels was attributed to hydrophilic monomers. It was the hydrophilic monomer AAc that strongly affected the balance of the hydrophilic/hydrophobic nature in copolymers [16, 17]. The more hydrophilic monomers in PNIPAAm copolymer, the higher LCST is because hydrophilic monomer hindered the dehydration of the polymer chains, especially the ionized –COO– groups that were sufficiently soluble to counteract aggregation affection of the hydrophobic temperature-sensitive groups. The P(NIPAAm-co-AAc) hydrogels exhibited a broader phase transition temperature than PNIPAAm hydrogels besides a higher LCST. This indicated that the hydrophilic monomer decreased swelling thermosensitivity of the NIPAAm copolymer because the hydrophilic monomer (AAc) prevented the formation of a compact shrunken structure. It was important to note that the LCST of the P(NIPAAm-co-AAc) hydrogels remolded by altering the molar ratio of AAc in the polymerization process. In addition, the dosage of the AEA-PSI cross-linker did not affect LCST apparently, as seen in Fig. 3.
Transmittance as a function of temperature for (filled triangles) BIS-cross-linked PNIPAAm hydrogel; (filled circles) BIS-cross-linked P(NIPAAm-co-AAc) hydrogel with NIPAAm/AAc molar ratio of 97.5/2.5; AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels with NIPAAm/AAc molar ratio of (open circles) 97.5/2.5, (filled inverted triangles) 96.5/3.5, and (open squares) 95.5/4.5; all the hydrogels with cross-linker density 0.226% take PBS as solvent
Transmittance as a function of temperature for AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels, (filled circles) cross-linker density 0.174% and (filled squares) cross-linker density 0.226%, and (filled traingles) cross-linker density 0.276%; all the hydrogels with NIPAAm/AAc molar ratio of 97.5/2.5 take PBS as solvent
Water content
Water content of BIS-cross-linked PNIPAAm hydrogel sample and BIS-cross- and AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogel samples in distilled water and PBS at 25 and 37 °C were shown in Table 2. At 25 °C, all the hydrogel samples presented high water contents of >90% in distilled water and PBS. The water content of BIS-cross-linked PNIPAAm hydrogel showed a significant drop at 37 °C regardless of swelling medium. All the P(NIPAAm-co-AAc) hydrogels presented high water contents of >90% even when the temperature was above LCST.
The water content of BIS-cross-linked PNIPAAm hydrogel was temperature dependent, while the P(NIPAAm-co-AAc) hydrogels cross-linked with BIS and AEA-PSI presented medium dependence at 25 and 37 °C. The water contents of both AEA-PSI- and BIS-cross-linked hydrogels were higher in distilled water than in PBS; this phenomenon was greater at 37 °C. This behavior was the interaction between COO– groups in AAc and the ions presented in PBS.
In addition, the water contents of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels with different NIPAAm/AAc monomer molar ratios did not change extensively at 25 °C. The hydrophilic monomer AAc did not significantly affect the water contents of P(NIPAAm-co-AAc) hydrogels. At 37 °C, the water content of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels did not change distinctly with the monomer molar ratio in distilled water, whereas the water content of the hydrogels gradually increased with the AAc monomer molar added in PBS. This result suggested that the unshielded COO– groups increased with increasing of the AAc monomer in PBS [17].
The effect of cross-linker density on the water content for AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels with the same NIPAAm/AAc monomer molar ratio (97.5/2.5) was shown in Table 2. The hydrogels with higher cross-linker density contained high water content than hydrogels with lower cross-linker density in PBS at 37 °C. This result indicated that the effect of interactions between COO– group in AAc in the hydrogels and the ions in PBS was reduced by increase in the cross-linking density [17].
Thermal stability behavior
Thermogravimetric apparatus was used to record the thermal degradation behavior of the hydrogel. The thermogravimetry (TG) and the corresponding derivative thermogravimetry (DTG) curves of the AEA-PSI-cross-linked hydrogel and BIS-cross-linked hydrogel were shown in Figs. 4 and 5, respectively. The first thermal event occurred at 61 °C, where all samples presented a mass loss ranging from 5.57 ∼ 5.84%. This was attributed to the evaporation of water, which was a function of the morphology and crystallinity of the polymers. The TG and the DTG curves of the two samples differed at the second stage. The second stage was attributed to the thermal degradation of AEA-PSI cross-linker reaching a maximum at 130.7 °C, with weight loss of 12.69%. However, the BIS-cross-linker reached a maximum at 149.9 °C, with weight loss of 8.24%. The third stage was the degradation of the backbone of the AEA-PSI- and BIS-cross-linked hydrogels reaching a maximum about 389 °C, with weight loss of 66.05 and 74.03%, respectively. This result indicated that the thermodynamic stability of AEA-PSI-cross-linked hydrogel was lower than BIS-cross-linked hydrogel; it was because the AEA-PSI-cross-linked hydrogel contained ester and more amido bond than BIS-cross-linked hydrogel.
Enzymatic degradation behavior
Figure 6 showed the enzymatic degradation behavior of AEA-PSI-cross-linked hydrogel samples prepared by using different feed molar ratios of the AEA-PSI cross-linker (detailed compositions of hydrogels were given in Table 1) in PBS solution having 0.005 mg/ml trypsin. The weight of all the samples decreased more than 40% within the first day and reached a relatively stable stage on the second day. The driving force for the weight loss was the hydrolysis of the amido and ester bands in the AEA-PSI cross-linker of the hydrogel. As the cross-links broke down, P(NIPAAm-co-AAc) chains linked with the degraded cross-links were freed up and diffused into the solutions. The degradation rate gradually decreased with time mostly due to decrease in the concentration of the cross-links. Besides, the ester hydrolytic action reached the equilibrium as degradation carried on. As a result, the weight loss of hydrogel samples became negligible after 1 day [18].
Degradation behavior as a function of time for AEA-PSI cross-linked P(NIPAAm-co-AAc) hydrogel with different cross-linking density in PBS solution having 0.005 mg/mltrypsin. (Filled squares) Cross-linker density 0.174%; (filled circles) cross-linker density 0.226%; (filled triangles) cross-linker density 0.276%
In general, the degradation of hydrogel in solution (mass loss from the networks) was linked to several network parameters such as number of cross-links per backbone chain, molecular weight of backbone, and proportion of degradable groups in the main and side chain [17]. The degradation rate gradually decreased with increasing cross-linking density. As the cross-linking density increased, additional degradable units must be broken to degrade the hydrogel. This effect resulted in longer inhibition time for the mass loss with increasing cross-linking density [17].
Conclusions
A novel biodegradable cross-linker acryloyloxyethylaminopolysuccinimide (AEA-PSI) was obtained by microwave irradiation using maleic anhydride as materials. With AEA-PSI cross-linker, cross-linked poly(N-isopropylacrylamide-co-acrylic acid) [P(NIPAAm-co-AAc)] hydrogels were prepared in phosphate-buffered saline (PBS). The LCST of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels was significantly influenced by NIPAAm/AAc monomer molar ratio, while the cross-linking density did not affect the LCST. The water content of AEA-PSI-cross-linked P(NIPAAm-co-AAc) hydrogels was more than 90% at 37 °C, which was controlled by the monomer molar ratio of NIPAAm/AAc, swelling media and the cross-linking density. The thermodynamic stability of AEA-PSI-cross-linked hydrogel was lower than BIS-cross-linked hydrogel. The AEA-PSI-cross-linked hydrogels were potentially biodegradable. The enzymatic degradation rate depended on the cross-linking density of hydrogel.
References
Andersson M, Axelsson A, Zacchi G (1998) Swelling kinetics of poly-(N-isopropylacrylamide) gel. J Control Release 50(1–3):273–281
Kost J, Langer R (1991) Responsive polymeric delivery systems. Adv Drug Deliv Rev 6(1):19–50
Hoffman AS (2002) Hydrogels for biomedical applications. Adv Drug Deliv Rev 54(1):3–12
Kopecek J (2003) Smart and genetically engineered biomaterials and drug delivery systems. Eur J Pharm Sci 20(1):1–16
Ju HK, Kim SY, Lee YM (2001) pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly(N-isopropylacrylamide). Polymer 42(16):6851–6857
Kim JH, Lee SB, Kim SJ, Lee YM (2002) Rapid temperature/pH response of porous alginate-g-poly(N-isopropylacrylamide) hydrogels. Polymer 43(26):7549–7558
Tanaka T (1986) Kinetics of phase transition in polymer gels. Physica A 140(1–2):261–268
Metters A, Hubbell J (2005) Network formation and degradation behavior of hydrogels formed by michael-type addition reactions. Biomacromolecules 6(1):290–301
Martens P, Metters AT, Anseth KS, Bowman CN (2001) A generalized bulk-degradation model for hydrogel networks formed from multivinyl cross-linking molecules. J Phys Chem B 105(22):5131–5138
Kim S, Healy KE (2003) Synthesis and characterization of injectable poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules 4(5):1214–1223
Li F, Ying Z, Tian WT (2006) Preparation and water absorbent behavior of superabsorbent polyaspartic acid resin. J Polym Res 13:145–152
Groth T, Joentgen W (1997) Process for preparing polysuccinimide and polyaspartic acid [P]. US Patent 5,610,255
Groth T, Joentgen W (1997) Process for preparing polyaspartic acid [P]. US Patent 5,714,558
Louis L (2000) Wood, Rockville, Md, salt of polyaspartic acid by high temperature reaction [P]. US Patent, 6,072,025
Wood LL, Calton GJ, Elkridge (1997) Process for preparing polysucinimide by high temperature reaction. US Patent 5,610,267
Ebara M, Aoyagi T, Sakai K, Okano T (2000) Introducing reactive carboxyl side chains retains phase transition temperature sensitivity in N-Isopropylacrylamide copolymer gels. Macromolecules 33(22):8312–8316
Zhang J, Peppas NA (2000) Synthesis and characterization of pH- and temperature-sensitive poly(methacrylic acid)/poly(N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules 33(1):102–107
Huang X, Lowe TL (2005) Biodegradable thermoresponsive hydrogels for aqueous encapsulation and controlled release of hydrophilic model drugs. Biomacromolecules 6(4):2131–2139
Acknowledgment
This research was supported by the National Natural Science Foundation of China (No. 20476049); the Program for New Century Excellent Talents in Universities (No. NCET-04-0649); and the Science Foundation of Shandong Province (Y2006B10).
Author information
Authors and Affiliations
Corresponding author
Additional information
Submitted to Colloid and Polymer Science, 2007-1-28.
Rights and permissions
About this article
Cite this article
Yu, Yq., Li, Zz., Tian, Hj. et al. Synthesis and characterization of thermoresponsive hydrogels cross-linked with acryloyloxyethylaminopolysuccinimide. Colloid Polym Sci 285, 1553–1560 (2007). https://doi.org/10.1007/s00396-007-1725-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-007-1725-6