Abstract
The number of patients with atrial fibrillation undergoing percutaneous coronary intervention (PCI) is increasing. Since these patients have a CHA2DS2-VASc score of 1 or higher, they should be treated with oral anticoagulation to prevent stroke. However, combination therapy with oral anticoagulation for prevention of embolic stroke and dual platelet inhibition for prevention of coronary thrombosis significantly increases bleeding complications. The optimal combination, intensity and duration of antithrombotic combination therapy is still not known. In the rather small randomized WOEST trial, the combination of a vitamin K antagonist (VKA) and clopidogrel decreased bleeding compared to the conventional triple therapy with VKA, clopidogrel and aspirin. In the PIONEER AF-PCI trial, two rivaroxaban-based treatment regimens significantly reduced bleeding complications compared to conventional triple therapy without increasing embolic or ischemic complications following PCI. Dual therapy with rivaroxaban and clopidogrel appeared to provide an optimal risk–benefit ratio. In the RE-DUAL PCI trial, dual therapy with dabigatran also reduced bleeding complications compared to conventional triple therapy. With respect to the composite efficacy end point of thromboembolic events (myocardial infarction, stroke, or systemic embolism), death, or unplanned revascularization dabigatran-based dual therapy was non-inferior to VKA-based triple therapy. The upcoming trials AUGUSTUS with apixaban and ENTRUST-PCI with edoxaban will further examine the use of NOACs in this setting. While recent guidelines recommend NOAC-based dual therapy in only a subset of patients (those who are at increased risk of bleeding), the available data now suggest that this should be the preferred choice for the majority of patients. Adding aspirin to this primary choice for up to 4 weeks in patients at especially high ischemic risk would likely prevent atherothrombotic events, but this needs further investigation. Taken together, it is time to adjust our practice and move to dual therapy consisting of a NOAC plus clopidogrel in most patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The optimal antithrombotic management strategy for patients with non-valvular atrial fibrillation (AF) presenting with an acute coronary syndrome or undergoing percutaneous coronary intervention (PCI) for stable coronary artery disease continues to represent a clinical challenge. As with the use of any antithrombotic drug, clinicians need to balance the risks of ischemic stroke and thromboembolism, recurrent cardiac ischemia and/or stent thrombosis, and bleeding and hemorrhagic stroke. This document provides an update of the evidence after publication of the PIONEER AF-PCI and RE-DUAL PCI studies and presents statements on a best practice antithrombotic therapy guideline for the management of antithrombotic therapy in AF patients requiring PCI [1, 2].
Background
Randomized trials comparing placebo with antithrombotic therapies and different antithrombotic therapies have provided evidence that oral anticoagulation (OAC) is the most effective therapy to protect patients with AF from embolic stroke and systemic embolism. Therefore, current guidelines recommend considering OAC in patients with a CHA2DS2-VASc score of ≥ 1 class I recommendation with a CHA2DS2-VASc score ≥ 2 (male) or ≥ 3 (female patients) and class IIa recommendation with a score of 1 (male) and 2 (female patients) [3]. In the early days of stent implantation, dual antiplatelet therapy (DAPT) with aspirin and ticlopidine or clopidogrel was superior to aspirin and intense anticoagulation with high dose unfractionated heparin followed by oral anticoagulation with vitamin K antagonists (VKA) [4]. Since then DAPT has been recommended after stent implantation to protect the patient from stent thrombosis [5,6,7]. In consequence, conventional triple antithrombotic therapy with OAC, aspirin and clopidogrel has been considered as standard of care by expert consensus for a long time, even without direct evidence from randomized trials and despite the knowledge of its limitation of an increased bleeding risk [3, 5, 8,9,10,11,12,13,14,15,16,17,18,19].
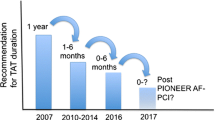
This paradigm has been challenged by the randomized WOEST study, in which the combination of a VKA and clopidogrel reduced bleeding complications compared to the conventional triple therapy, without increasing thrombotic or embolic events [20] (Fig. 1a). However, this trial was not powered for efficacy (with approximately 280 patients in each arm) and many interventionalists continued to use triple therapy, because of the fear of stent thrombosis. In the ISAR-TRIPLE study, a shortened course of triple therapy over 6 weeks was non-inferior compared to a 6-month therapy [21]. In a recent position statement of the ESC, triple therapy was still considered mandatory in most patients, with the option to shorten the duration in patients at increased risk for bleeding [22]. This scheme was also recommended in the current ESC revascularization and NSTE-ACS guidelines [6, 23]. More recently, the ESC issued guidelines for dual antiplatelet therapy (DAPT) following PCI that also contain recommendations for patients with an indication for oral anticoagulation undergoing PCI [24]. The DAPT guidelines recommend dual therapy with a NOAC for patients at high risk for bleeding based on the data from the PIONEER AF-PCI trial as described below.
a Results of the randomized WOEST trial comparing triple therapy with a vitamin K antagonist plus clopidogrel plus aspirin versus dual therapy with a vitamin K antagonist and clopidogrel in patients undergoing PCI with stent implantation and an indication for oral anticoagulation [20]. MI myocardial infarction. b Cumulative incidence rates of the primary safety endpoint of clinically significant bleeding and secondary efficacy endpoints in the PIONEER AF-PCI trial [1, 25]. CS clinically significant, CV cardiovascular. c Cumulative incidence rates of the denoted endpoints in the RE-DUAL PCI trial [2]
PIONEER AF-PCI trial
The PIONEER AF-PCI trial was the first large-scale trial to compare the conventional, VKA-based combination therapy with a NOAC-based therapy in patients with AF undergoing PCI [1]. In this trial, 2,124 participants with AF undergoing PCI with stenting were randomly assigned to receive, in a 1:1:1 ratio, low-dose rivaroxaban (15 mg once daily with further reduction to 10 mg in patients with creatinine clearance of 30–50 ml/min) plus a P2Y12 inhibitor for 12 months (“Riva 15 regimen”), very low-dose rivaroxaban (2.5 mg twice daily) plus DAPT for 1, 6, or 12 months (“Rivaroxaban 2 x 2.5 mg regimen”), or standard therapy with a dose-adjusted VKA plus DAPT for 1, 6, or 12 months (“VKA regimen”). Duration of DAPT and type of P2Y12 inhibitor were specified by the investigator before randomization. Triple therapy was maintained for 12 months in about 50% of the patients and the indication for PCI was acute coronary syndrome (ACS) in about 50% of the patients. The primary safety outcome was clinically significant bleeding [a composite of major bleeding or minor bleeding according to thrombolysis in myocardial infarction (TIMI) criteria or bleeding requiring medical attention]. While there was a significantly lower incidence of bleedings with both rivaroxaban regimens compared to VKA plus DAPT, ischemic complications, death or stroke rates were similar (but the trial was not powered for establishing non-inferiority of efficacy endpoints). Hospitalization rates were significantly lower in the rivaroxaban groups than in the VKA group in a post hoc analysis [25]. The results of the primary endpoint and secondary bleeding and ischemic endpoints are summarized in Fig. 1b.
RE-DUAL PCI trial
The RE-DUAL PCI trial was the second large-scale trial in this setting [2]. In this trial, 2725 participants with AF undergoing PCI were randomly assigned to receive conventional triple therapy with a VKA or one of the two doses of dabigatran approved for stroke prevention in AF (2× 110 mg or 2× 150 mg) in combination with a P2Y12 inhibitor (predominantly clopidogrel). Triple therapy was maintained for 1 month in patients who received a bare-metal stent and for 3 months after implantation of a drug-eluting stent. Indication for PCI was ACS in about 50% of the patients. The primary safety outcome was major or clinically relevant non-major bleeding according to the International Society of Thrombosis and Haemostasis (ISTH) criteria. The risk for bleeding was significantly reduced with the dabigatran 2× 110 mg regimen as compared to triple therapy and non-inferior with the dabigatran 2× 150 mg regimen (Fig. 1c). RE-DUAL PCI also demonstrated non-inferiority of dual therapy with dabigatran (both doses combined) to triple therapy with warfarin in a composite efficacy end point of thromboembolic events (myocardial infarction, stroke, or systemic embolism), death, or unplanned revascularization.
Clinical implications
The results of the WOEST, PIONEER and RE-DUAL trials challenge the role of triple therapy with VKA as standard of care in patients with atrial fibrillation undergoing PCI. While in the periprocedural phase aspirin in combination with a P2Y12 inhibitor should be given to all patients, in contrast to earlier assumptions, longer term dual antiplatelet therapy does not seem mandatory after PCI in patients treated with OAC. From a pathophysiological view, this seems plausible, because thrombin is the most potent stimulus for platelet activation and aggregation and direct factor Xa inhibition with rivaroxaban has been associated with a significant reduction in stent thrombosis in the ATLAS ACS2-TIMI 51 trial [26]. In addition, improved stent designs and better implantation techniques have reduced the rate of stent thrombosis, which is now < 1% in most trials. However, in certain situations, longer DAPT might still be desirable. It is therefore tempting to speculate about implications for subgroups of patients, such as those with STEMI or those undergoing complex PCI (e.g., bifurcation or left main PCI or PCI with multiple stents), who are considered at highest risk for stent thrombosis and recurrent ACS.
Importantly, PIONEER AF-PCI was not powered for assessing ischemic endpoints, especially not in subgroups (only a total of 257 patients had STEMI and data about complex PCI were not reported). Reassuringly, the rate of stent thrombosis was low in all rivaroxaban groups (< 1%, without significant differences). The rate of MACE for patients with STEMI was numerically higher in the 2× 2.5 mg rivaroxaban group, but this did not reach statistical significance (5.1% with the Riva 15 regimen, 10.8% with the Rivaroxaban 2 x 2.5 mg regimen, and 9.5% with the VKA regimen, n.s.). However, the relative risk reduction in the primary (bleeding) endpoint was especially large in the STEMI subgroup (14.6% with the Riva 15 regimen, 19.2% with the Rivaroxaban 2 x 2.5 mg regimen, and 35.9% with the VKA regimen, p < 0.05). Taken together, it is unclear whether patients with STEMI or complex PCI would benefit from DAPT in combination with 2× 2.5 mg rivaroxaban or if a single P2Y12 inhibitor in combination with 15 mg rivaroxaban would be a “one fits all” solution. Extrapolating from the available data and the guideline-derived assumption that DAPT is beneficial, it seems reasonable to consider adding aspirin for up to 4 weeks to the 15 mg rivaroxaban regimen in patients at especially high risk for atherothrombotic events, although this has not been tested in PIONEER AF-PCI [16]. Figure 2 provides a scheme favoring the “one fits all” solution, allowing this consideration.
With the dabigatran 2× 110 mg regimen, the risk for myocardial infarction was numerically higher than with 2× 150 mg in the RE-DUAL PCI trial. For high-risk patients undergoing PCI, most clinicians may therefore prefer the 2× 150 mg dabigatran regimen if dabigatran is chosen over rivaroxaban.
It is also not entirely clear, which P2Y12 inhibitor should be preferred [27]. Only 3–5% of study participants had been assigned to ticagrelor and < 2% to prasugrel in PIONEER AF-PCI and 12% to ticagrelor in RE-DUAL PCI by their treating physician, while most patients received clopidogrel in both trials. According to subgroup analyses from PIONEER AF-PCI as well as from the GEMINI-ACS-1 trial (with 56% treated with ticagrelor) [28], clopidogrel may be the ideal combination partner for rivaroxaban.
Whether protection from stroke is as effective with lower doses of a NOAC when combined with clopidogrel, as the full dose tested in the AF trials is a matter of debate. PIONEER AF-PCI tested a 25% lower dose than the recommended dose for stroke prevention in lone AF of 20 mg. Although the study was not powered to test efficacy, there was no important signal suggesting that stroke risk was elevated, especially with the 15 mg rivaroxaban regimen (1.3 versus 1.2% with triple therapy, HR 1.07, 95% CI 0.39–2.96). In RE-DUAL PCI, only AF-approved doses were tested. Numerically, the stroke risk with the dabigatran 2× 110 mg regimen appeared slightly higher than with VKA-based triple therapy, but this was not significant and possibly due to chance (HR 1.3, 95% CI 0.63–2.67). The extended efficacy endpoint defined in RE-DUAL PCI allowed testing for non-inferiority for both dabigatran arms compared to triple therapy, which was achieved. Taken together, the efficacy data in both trials suggest that there was no sign for increased stroke rates in patients assigned to dual therapy.
Outlook
Two additional large randomized trials are still ongoing (AUGUSTUS and ENTRUST-PCI) examining different combination therapies in patients with non-valvular AF undergoing PCI. After the publication of these trials, at least a year from now, the role of all four NOACs and also the dosing (full or reduced) in the setting after PCI will be clearer, and broader recommendations for the optimal antithrombotic combination therapy might be possible. However, at present, patients should be treated according to the available evidence and the conclusions presented in this report.
Summary
The results of the PIONEER AF-PCI and the RE-DUAL PCI trials suggest that antithrombotic combination therapy with a reduced dose of rivaroxaban of 15 mg or a regular dose of dabigatran in combination with clopidogrel seems to provide a better risk–benefit ratio than conventional triple therapy. Based on these considerations, triple therapy with VKA after PCI should no longer be standard of care.
References
Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA (2016) Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 375(25):2423–2434. https://doi.org/10.1056/NEJMoa1611594
Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH, Investigators R-DPSCa (2017) Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 377(16):1513–1524. https://doi.org/10.1056/NEJMoa1708454
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K (2016) 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37(38):2893–2962. https://doi.org/10.1093/eurheartj/ehw210
Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO, Pocock SJ, Kuntz RE (1998) A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 339(23):1665–1671. https://doi.org/10.1056/NEJM199812033392303
Authors/Task Force M, Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S (2015) 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. https://doi.org/10.1093/eurheartj/ehv320
Authors/Task F, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A (2014) 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35(37):2541–2619. https://doi.org/10.1093/eurheartj/ehu278
Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van’t Hof A, Widimsky P, Zahger D (2012) ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33(20):2569–2619. https://doi.org/10.1093/eurheartj/ehs215 pii].
Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P, Advisors (2016) Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. https://doi.org/10.1093/eurheartj/ehw058
Moser M, Olivier CB, Bode C (2013) Triple antithrombotic therapy in cardiac patients: more questions than answers. Eur Heart J. https://doi.org/10.1093/eurheartj/eht461
Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, Kober L, Torp-Pedersen C, Gislason GH, Hansen ML (2012) Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 126(10):1185–1193. https://doi.org/10.1161/CIRCULATIONAHA.112.114967
Proietti M, Airaksinen KEJ, Rubboli A, Schlitt A, Kiviniemi T, Karjalainen PP, Lip GYH, Group AS (2017) Synergic impact of oral anticoagulation control and renal function in determining major adverse events in atrial fibrillation patients undergoing percutaneous coronary intervention: insights from the AFCAS registry. Clin Res Cardiol 106(6):420–427. https://doi.org/10.1007/s00392-016-1071-0
Ruile P, Jander N, Blanke P, Schoechlin S, Reinohl J, Gick M, Rothe J, Langer M, Leipsic J, Buettner HJ, Neumann FJ, Pache G (2017) Course of early subclinical leaflet thrombosis after transcatheter aortic valve implantation with or without oral anticoagulation. Clin Res Cardiol 106(2):85–95. https://doi.org/10.1007/s00392-016-1052-3
Proietti M, Nobili A, Raparelli V, Napoleone L, Mannucci PM, Lip GY, investigators R (2016) Adherence to antithrombotic therapy guidelines improves mortality among elderly patients with atrial fibrillation: insights from the REPOSI study. Clin Res Cardiol 105(11):912–920. https://doi.org/10.1007/s00392-016-0999-4
Fauchier L, Clementy N, Bisson A, Stamboul K, Ivanes F, Angoulvant D, Babuty D, Lip GY (2017) Prognosis in patients with atrial fibrillation and a presumed “temporary cause” in a community-based cohort study. Clin Res Cardiol 106(3):202–210. https://doi.org/10.1007/s00392-016-1040-7
Gunawardene M, Willems S, Schaffer B, Moser J, Akbulak RO, Jularic M, Eickholt C, Nuhrich J, Meyer C, Kuklik P, Sehner S, Czerner V, Hoffmann BA (2017) Influence of periprocedural anticoagulation strategies on complication rate and hospital stay in patients undergoing catheter ablation for persistent atrial fibrillation. Clin Res Cardiol 106(1):38–48. https://doi.org/10.1007/s00392-016-1021-x
Seeger J, Bothner C, Dahme T, Gonska B, Scharnbeck D, Markovic S, Rottbauer W, Wohrle J (2016) Efficacy and safety of percutaneous left atrial appendage closure to prevent thromboembolic events in atrial fibrillation patients with high stroke and bleeding risk. Clin Res Cardiol 105(3):225–229. https://doi.org/10.1007/s00392-015-0910-8
Stahli BE, Gebhard C, Gick M, Ferenc M, Mashayekhi K, Buettner HJ, Neumann FJ, Toma A (2017) Outcomes after percutaneous coronary intervention for chronic total occlusion according to baseline renal function. Clin Res Cardiol. https://doi.org/10.1007/s00392-017-1179-x
De Caterina R, Lip GYH (2017) The non-vitamin K antagonist oral anticoagulants (NOACs) and extremes of body weight-a systematic literature review. Clin Res Cardiol 106(8):565–572. https://doi.org/10.1007/s00392-017-1102-5
Hohnloser SH, Basic E, Nabauer M (2017) Comparative risk of major bleeding with new oral anticoagulants (NOACs) and phenprocoumon in patients with atrial fibrillation: a post-marketing surveillance study. Clin Res Cardiol 106(8):618–628. https://doi.org/10.1007/s00392-017-1098-x
Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van’t Hof AW, ten Berg JM, Investigators Ws (2013) Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 381(9872):1107–1115. https://doi.org/10.1016/S0140-6736(12)62177-1
Fiedler KA, Maeng M, Mehilli J, Schulz-Schupke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, Kristensen SD, Ibrahim T, Massberg S, Schunkert H, Laugwitz KL, Kastrati A, Sarafoff N (2015) Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE trial. J Am Coll Cardiol 65(16):1619–1629. https://doi.org/10.1016/j.jacc.2015.02.050
Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, Haeusler KG, Boriani G, Capodanno D, Gilard M, Zeymer U, Lane D, Document R, Storey RF, Bueno H, Collet JP, Fauchier L, Halvorsen S, Lettino M, Morais J, Mueller C, Potpara TS, Rasmussen LH, Rubboli A, Tamargo J, Valgimigli M, Zamorano JL (2014) Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J 35(45):3155–3179. https://doi.org/10.1093/eurheartj/ehu298
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J (2016) 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 37(3):267–315. https://doi.org/10.1093/eurheartj/ehv320
Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN, Group ESCSD., Guidelines ESCCfP, Societies ESCNC. (2017) 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. https://doi.org/10.1093/eurheartj/ehx419
Gibson CM, Pinto DS, Chi G, Arbetter D, Yee M, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Burton P, van Eickels M, Korjian S, Daaboul Y, Jain P, Lip GY, Cohen M, Peterson ED, Fox KA (2017) Recurrent hospitalization among patients with atrial fibrillation undergoing intracoronary stenting treated with 2 treatment strategies of rivaroxaban or a dose-adjusted oral vitamin K antagonist treatment strategy. Circulation 135(4):323–333. https://doi.org/10.1161/CIRCULATIONAHA.116.025783
Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM (2012) Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 366(1):9–19. https://doi.org/10.1056/NEJMoa1112277
Schoener L, Jellinghaus S, Richter B, Pfluecke C, Ende G, Christoph M, Quick S, Loehn T, Speiser U, Poitz DM, Mierke J, Strasser RH, Ibrahim K (2017) Reversal of the platelet inhibitory effect of the P2Y12 inhibitors clopidogrel, prasugrel, and ticagrelor in vitro: a new approach to an old issue. Clin Res Cardiol 106(11):868–874. https://doi.org/10.1007/s00392-017-1128-8
Ohman EM, Roe MT, Steg PG, James SK, Povsic TJ, White J, Rockhold F, Plotnikov A, Mundl H, Strony J, Sun X, Husted S, Tendera M, Montalescot G, Bahit MC, Ardissino D, Bueno H, Claeys MJ, Nicolau JC, Cornel JH, Goto S, Kiss RG, Guray U, Park DW, Bode C, Welsh RC, Gibson CM (2017) Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): a double-blind, multicentre, randomised trial. Lancet. https://doi.org/10.1016/S0140-6736(17)30751-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Duerschmied D: Speaker honoraria from Bayer, Daiichi-Sankyo, Pfizer, Brachmann J: none, Darius H: Speaker’s honoraria and consulting fees from Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, Boehringer Ingelheim, Frey N: none, Katus HA: none, Rottbauer W: none, Schäfer A: Speaker honoraria from Daiichi, Bristol-Myers Squibb/Pfizer; Consulting fees from Bayer, Boehringer Ingelheim, Thiele H: none, Bode C: Research grants from Bayer, GlaxoSmithKline, Merck; Speaker’s honoraria from Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, Boehringer Ingelheim; Consulting fees from Bayer, Zeymer U: Speaker’s honoraria and consulting fees from Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, Boehringer Ingelheim.
Rights and permissions
About this article
Cite this article
Duerschmied, D., Brachmann, J., Darius, H. et al. Antithrombotic therapy in patients with non-valvular atrial fibrillation undergoing percutaneous coronary intervention: should we change our practice after the PIONEER AF-PCI and RE-DUAL PCI trials?. Clin Res Cardiol 107, 533–538 (2018). https://doi.org/10.1007/s00392-018-1242-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1242-2