Abstract
Introduction
Ventricular arrhythmias (VAs) in patients with chronic heart failure (CHF) are sometimes refractory to antiarrhythmic drugs and cardiac ablation. This study aimed to investigate catheter-based renal sympathetic denervation (RDN) as antiarrhythmic strategy in refractory VA.
Methods
These are the first data from a pooled analysis of 13 cases from five large international centers (age 59.2 ± 14.4 years, all male) with CHF (ejection fraction 25.8 ± 10.1 %, NYHA class 2.6 ± 1) presented with refractory VA who underwent RDN. Ventricular arrhythmias, ICD therapies, clinical status, and blood pressure (BP) were evaluated before and 1–12 months after RDN.
Results
Within 4 weeks prior RDN, a median of 21 (interquartile range 10–30) ventricular tachycardia (VT) or fibrillation (VF) episodes occurred despite antiarrhythmic drugs and prior cardiac ablation. RDN was performed bilaterally with a total number of 12.5 ± 3.5 ablations and without peri-procedural complications. One and 3 months after RDN, VT/VF episodes were reduced to 2 (0–7) (p = 0.004) and 0 (p = 0.006), respectively. Four (31 %) and 11 (85 %) patients of these 13 patients were free from VA at 1 and 3 months. Although BP was low at baseline (116 ± 18/73 ± 13 mmHg), no significant changes of BP or NYHA class were observed after RDN. During follow-up, three patients died from non-rhythm-related causes.
Conclusions
In patients with CHF and refractory VA, RDN appears to be safe concerning peri-procedural complications and blood pressure changes, and is associated with a reduced arrhythmic burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implantable cardioverter-defibrillators (ICDs) are well established for the primary and secondary prevention of sudden cardiac death in patients with cardiomyopathy and chronic heart failure (CHF) [1–3]. However, recurrent ventricular arrhythmias (VAs) with subsequent ICD shocks are associated with increased mortality and deterioration of ventricular function [4], as well as psychological trauma. In addition to beta-blockers, the use of antiarrhythmic agents such as amiodarone may reduce VA further, but its use is often limited by serious side effects [5]. Cardiac catheter ablation of VA has been shown to be an effective treatment option for VA with a reasonable safety profile, in particular in scar-related heart disease [1]. However, even in experienced centres, a significant proportion of patients with CHF experience recurrent VA episodes despite catheter ablation and treatment with antiarrhythmic agents. In particular in non-ischemic dilated cardiomyopathy (NIDCM), up to 50 % of patients will suffer from recurrent VA [6, 7]. Given the involvement of the sympathetic nervous system (SNS) in the initiation and maintenance of VA, sympathomodulatory treatments, such as left cardiac sympathetic denervation, have been proposed as adjunctive or rescue treatment options [8, 9].
Catheter-based RDN has been shown to reduce sympathetic activity and blood pressure (BP) in certain hypertensive patients in open, non-controlled studies [10–14], in a real-life registry on 1000 patients [15] and in sham-controlled studies [16, 17]. Also, potentially beneficial electrophysiological effects, such as reduction in heart rate [18], alterations in ventricular refractoriness [19], reduction of ventricular premature beats [20], occurrence of VA during acute myocardial ischemia [21], and atrial arrhythmias [22], have been described following RDN in both experimental and clinical settings. Case reports and some smaller series have reported the first experiences of RDN as treatment of VA [18, 23–28]. The present multicenter international registry was designed to evaluate the safety and efficacy of RDN for treatment of refractory VA in patients with CHF using the largest published experience to date.
Methods
Study design and patient selection
The present analysis represents first data from a pooled analysis of 13 cases from five large international centers undergoing RDN for treatment of VA. Patients with CHF were included if recurrent VA occurred when medical antiarrhythmic treatment and cardiac ablation were ineffective or not technically feasible. Patients were excluded if VA occurred in the setting of acute myocardial infarction, sepsis, or any reversible cause, such as electrolyte disturbances. Also, patients with hemodynamically relevant renal artery stenosis or patients in whom RDN could not be performed due to other reasons were excluded. Patients were informed of the experimental approach and provided written consent. Review of the clinical data accords with the guidelines of the local ethic committees.
Renal denervation procedure
The RDN procedure was performed as previously described [18]. An ICD interrogation was performed to assess the number of VT/VF-episodes in the 4 weeks prior to the procedure. The choice of the RDN catheter was left to the discretion of the treating physicians. Renal angiograms were performed via femoral access to confirm anatomic eligibility. Peri-procedural complications and the number of ablations on each side were documented. Heparin was given to achieve an activated clotting time during the procedure of more than 250 s.
Follow-up
Follow-up examinations were performed at 1, 3, 6, and 12 months after RDN, which included an assessment of the clinical status, review of current medication, office BP measurements, 12-lead electrocardiogram, and an ICD interrogation. Programming of the ICD, in particular detection criteria and treatment of ventricular arrhythmias, was not changed during follow-up. Short-term follow-up of 3 of the 13 patients has been reported previously as case reports [9, 24, 25].
Statistical analysis
Data are presented as mean ± standard deviation (SD) or number (percentage) unless otherwise specified. Comparisons within groups were performed using the Pearson Chi-square test for categorical variables and the paired t test or the Wilcoxon rang sum test for continuous variables where appropriate. A two-tailed p value of <0.05 was regarded as statistically significant. All statistical analyses were performed with SPSS statistical software (version 23.0, SPSS Inc., Chicago, IL).
Results
Baseline characteristics of the 13 patients are shown in Table 1. All patients were male with a mean age of 59.2 ± 14.4 years. Cardiovascular comorbidities were highly prevalent with 85 % of the patients having a history of hypertension, 54 % diabetes, and 46 % chronic kidney disease with an estimated glomerular filtration rate ≤60 ml/min. Etiology of heart failure was ischemic in 7 (54 %) and IDCM in 6 (46 %) patients. The ejection fraction was 25.8 ± 10.1 % (range 15–44 %), and NYHA functional class was 2.6 ± 1.0. Beta-blockers and sotalol were prescribed in 11 (85 %) and 2 (15 %) patients, respectively. Amiodarone was used in 8 (62 %) patients with a daily dose of 394 ± 343 mg at the time of RDN. Another 2 (15 %) patients were treated with class-I antiarrhythmics. In total, the mean number of current antiarrhythmic agents was 1.8 ± 0.7.
Arrhythmic burden and procedural details
Data on the arrhythmic burden, previous cardiac ablations, as well as details on the RDN procedure are summarized in Table 2. A median number of 21 (10–30) VA occurred with the need for 14 (9–30) ICD shocks and 5 (0–12) anti-tachycardia pacing (ATP). Monomorphic VTs were prevalent in 7 (54 %) patients, polymorphic VTs in 6 (46 %), whereas VF episodes occurred in 8 (62 %) patients. Previous cardiac ablations were performed in 9 (85 %) patients; in 2 (15 %) patients with ICM and 2 (15 %) patients with NIDCM, cardiac ablation was not performed due to polymorphic arrhythmias or according to patient’s request. In 5 (38 %) patients, RDN was performed as an acute treatment of electrical storm.
RDN was performed without any peri-procedural complications. Unilateral RDN was performed in one patient with a single-sided kidney. In 8 (62 %) patients, the procedure was done with a single electrode (Medtronic, Symplicity Flex catheter) or multielectrode RDN catheter (St. Jude EnligHTN), whereas in the remaining 5 (38 %), an irrigated cardiac ablation catheter was used. A total of 12.5 ± 3.5 ablations points were generated, which were equally distributed between both sides. An accessory renal artery was present in one patient, which was also treated.
Follow-up
At 1, 3, 6, and 12 months after RDN, 13, 10, 9, 8 patients presented for follow-up, respectively. Three patients died within the first year after the procedure. Causes of death were progressive heart failure in two patients (6 and 7 months after RDN) and septic shock with respiratory failure in one patient (2 months after RDN).
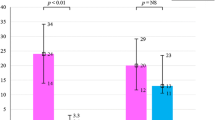
The number of sustained VA and ICD shocks 1 month before RDN and during follow-up is shown in Fig. 1. The median number of VT/VF episodes decreased from 21 (10–30) to 2 (0–7) at 1 month (p = 0.004) and 0 at 3 months (p = 0.006). Likewise, the numbers of ICD interventions were reduced: ATP from 5 (0–12) to 0 (p = 0.157) and ICD shocks from 14 (9–30) to 1 (0–7) at 1 month (p = 0.004). In the first month after RDN, 4 (31 %) patients were completely free from any VA. After the first month, 11 (85 %) patients were completely free from any further VT/VF episodes. Changes of antiarrhythmic drugs were documented in 3 (23 %, two patients with recurrent VA) and 1 (8 %) patients at 1- and 3-month follow-up and, thereafter, in none of the remaining patients.
Safety issues and complications
No acute peri-procedural complications occurred. BP and heart rate were not significantly altered after RDN (Fig. 2), and more importantly, no symptomatic hypotension was reported during follow-up. Furthermore, NYHA functional class remained unchanged with a mean of 2.5 ± 0.8 (p = 0.501), while 3 (23 %) patients had a worsening by one class and 2 (15 %) patients had an improvement by one class (Fig. 3). At 3-month follow-up, ejection fraction assessed by echocardiography was available in 8 (62 %) patients, and remained unchanged compared with baseline measurements (24.9 ± 12.1 %; p = 0.8).
Discussion
Herein, we report the largest cohort of patients with severe CHF undergoing RDN for treatment of recurrent VA. RDN appeared to be safe with respect to the procedure and functional parameters, such as NYHA class and BP, during follow-up. Moreover, in patients resistant to cardiac ablation and antiarrhythmic drugs, arrhythmic burden was significantly reduced by RDN in the setting of VT storm or unstable and frequent VA with frequent ICD shocks and interventions.
Most patients suffering from VA have underlying structural heart disease. Cardiac ablation and antiarrhythmic drug therapy may not sufficiently suppress VA in certain patients. The involvement of the SNS in the development and progression of CHF as well as its detrimental role in the onset and maintenance of VA has been well described [29, 30]. The electrophysiological effects of SNS activity in the ventricle include a reduction of the effective refractory period, increased automaticity, and a higher susceptibility to VA [30]. Surgical cardiac denervation or cervical sympathectomy has been considered in patients with refractory VA, but this approach is limited by its invasive nature and adverse effects [8, 31].
Renal sympathetic afferent and efferent nerves are important mediators of renal and whole-body sympathetic activity and contribute to the development and progression of hypertension [32]. Catheter-based RDN has been shown to reduce BP in several observational studies and in three randomized, controlled trials (Symplicity HTN-2, Prague 15 and DENERHTN) [16, 33, 34]. In the Symplicity HTN-3 trial, RDN was safe but not superior to drug therapy in reducing office or ambulatory BP [17]. Inappropriate patient selection and performance of an ineffective procedure have been suggested by some authors as possible causes for this negative result [35–38]. Sham-controlled trials in patients with hypertension are ongoing (NCT02439775, NCT02439749, NCT02392351).
In patients with CHF, cardiac but also renal sympathetic activity is elevated [39], which, in turn, can be modulated by RDN [10, 12]. Based on these considerations, the rationale for treating VA with a sympathomodulatory approach such as RDN is sound [40, 41]. Since the first-in-man report in 20 [9, 12], several other case reports and case series documented the ability of RDN to significantly improve arrhythmic burden in patients with different entities of chronic heart failure [25–28]. A recent study included ten patients undergoing RDN for treatment of refractory VA [26]. Six of these patients had Chagas disease, two had NIDCM, and two ICM. In these patients with previous failed or contraindicated cardiac ablation on amiodarone, RDN was safely performed with an irrigated-tip cardiac ablation catheter without deleterious effects on BP or HR during follow-up. After RDN, the median number of VA was reduced from 28.5 to 1 and 0 at 1- and 6-month follow-up [26].
In this report, patients with mainly ischemic cardiomyopathy (54 %) or non-ischemic dilated cardiomyopathy were included. Medical treatment of CHF was maximally tolerated doses and guideline-recommended with patients receiving renin–angiotensin system blocking agents, beta-blockers, and aldosterone antagonists. Despite antiarrhythmic agents and previous cardiac ablation, patients in the present registry had recurrent VA, mainly polymorphic VT or VF. In line with a recent study [26], the number of VA was significantly reduced after RDN without any deterioration of BP or functional status during follow-up. Thereby, our data provide further evidence on probable antiarrhythmic effects of RDN in patients with CHF.
Three prospective, randomized multicenter studies investigating RDN as treatment for VA were initiated. The ARDEVAT trial (NCT02071511) and the RESET-VT trial (NCT01858194) investigate the value of RDN as adjunctive treatment to VT ablation alone. While the RESET-VT trial enrolls all patients with structural heart disease planned for cardiac ablation, the ARDEVAT trial was focused on patients with ischemic cardiomyopathy and an ejection fraction ≥30 %. The ARDEVAT trial has been terminated earlier following the results of Symplicity HTN-3. The RESCUE-VT trial (NCT01747837), which is currently enrolling patients, aims to investigate the effects of RDN as adjunctive treatment in patients receiving an ICD for either secondary prevention or primary prevention with inducible VT. More studies are needed to further investigate the safety and efficacy of this therapy.
The role of the present international multicenter experience is to provide data of VA reduction by RDN, when the procedure is performed under real-life conditions in patients with a few or no therapeutic options. The registry in being expanded and will continue to enroll international patients with VA who have undergone RDN.
Limitations
Based on the design and size, the study has some potential limitations. Although it represents the largest cohort of patients published so far, the overall number of patients is relatively small, and the follow-up period is rather short. Given the uncontrolled nature of the collected data, a possible placebo or Hawthorne effect or a change of adherence to medication cannot be ruled out. Moreover, the patient cohort is heterogeneous (different etiologies of heart failure), and the RDN procedure was not strictly standardized. Eight patients were treated with a dedicated RDN catheter and five with conventional cardiac ablation catheters. Although these catheters are not intended to be used in renal arteries, data on safety and effectiveness in patients with hypertension or atrial fibrillation have been published [42, 43]. The use of cardiac ablation catheter in the present registry reflects the real-life condition of this analysis. Furthermore, no measurements of sympathetic tone were conducted. In hypertensive patients, reduction of blood pressure is regarded as parameter of successful treatment. This criterion cannot be applied to CHF, as these patients typically have normal or low blood pressure, which remains unchanged after the procedure. Identification of parameters of successful RDN treatment is of utmost importance, in particular in patient with VA. Considering these limitations, the presented registry offers only limited conclusions on the efficacy and safety of RDN as antiarrhythmic treatment, and further controlled trials are needed.
Conclusions
RDN for treatment of recurrent VA was not associated with adverse events in patients with CHF. Furthermore, the number of VA was significantly reduced following RDN possibly mediated by a decreased sympathetic activity after the procedure. Further randomized, prospective clinical trials studies are urgently needed to scrutinize the efficacy of RDN as adjunct to or as a replacement of standard therapy of VA. Until then, RDN remains a rescue therapy in patients with recurrent VA despite conventional therapies, such as antiarrhythmic drugs and cardiac ablation.
References
Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al (2015) ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 36:2793–2867
McMurray JJ, Adamopoulos S, Anker SD et al (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33:1787–1847
Yancy CW, Jessup M, Bozkurt B et al (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62:e147–e239
Poole JE, Johnson GW, Hellkamp AS et al (2008) Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 359:1009–1017
Connolly SJ, Dorian P, Roberts RS et al (2006) Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA 295:165–171
Dinov B, Fiedler L, Schonbauer R et al (2014) Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 129:728–736
Della Bella P, Baratto F, Tsiachris D et al (2013) Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation 127:1359–1368
Vaseghi M, Gima J, Kanaan C et al (2014) Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm 11:360–366
Ukena C, Bauer A, Mahfoud F et al (2012) Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol 101:63–67
Krum H, Schlaich M, Whitbourn R et al (2009) Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373:1275–1281
Böhm M, Linz D, Ukena C et al (2014) Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond? Circ Res 115:400–409
Hering D, Marusic P, Walton AS et al (2014) Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 64:118–124
Donazzan L, Mahfoud F, Ewen S et al (2016) Effects of catheter-based renal denervation on cardiac sympathetic activity and innervation in patients with resistant hypertension. Clin Res Cardiol 105:364–371
Sharp AS, Davies JE, Lobo MD et al (2016) Renal artery sympathetic denervation: observations from the UK experience. Clin Res Cardiol 105:544–552
Böhm M, Mahfoud F, Ukena C et al (2015) First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension 65:766–774
Rosa J, Widimsky P, Tousek P et al (2015) Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 65:407–413
Bhatt DL, Kandzari DE, O’Neill WW et al (2014) A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370:1393–1401
Ukena C, Mahfoud F, Spies A et al (2013) Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol 167:2846–2851
Lubanda JC, Kudlicka J, Mlcek M et al (2015) Renal denervation decreases effective refractory period but not inducibility of ventricular fibrillation in a healthy porcine biomodel: a case control study. J Transl Med 13:4
Tsioufis C, Papademetriou V, Tsiachris D et al (2014) Drug-resistant hypertensive patients responding to multielectrode renal denervation exhibit improved heart rate dynamics and reduced arrhythmia burden. J Hum Hypertens 28:587–593
Linz D, Wirth K, Ukena C et al (2013) Renal denervation suppresses ventricular arrhythmias during acute ventricular ischemia in pigs. Heart Rhythm 10:1525–1530
Linz D, van Hunnik A, Ukena C et al (2014) Renal denervation: effects on atrial electrophysiology and arrhythmias. Clin Res Cardiol 103:765–774
Hilbert S, Rogge C, Papageorgiou P et al (2015) Successful single-sided renal denervation in drug-resistant hypertension and ventricular tachycardia. Clin Res Cardiol 104:279–281
Hoffmann BA, Steven D, Willems S et al (2013) Renal sympathetic denervation as an adjunct to catheter ablation for the treatment of ventricular electrical storm in the setting of acute myocardial infarction. J Cardiovasc Electrophysiol 24:1175–1178
Scholz EP, Raake P, Thomas D et al (2015) Rescue renal sympathetic denervation in a patient with ventricular electrical storm refractory to endo- and epicardial catheter ablation. Clin Res Cardiol 104:79–84
Armaganijan LV, Staico R, Moreira DA et al (2015) 6-Month outcomes in patients with implantable cardioverter-defibrillators undergoing renal sympathetic denervation for the treatment of refractory ventricular arrhythmias. JACC Cardiovasc Interv 8:984–990
Remo BF, Preminger M, Bradfield J et al (2014) Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm 11:541–546
Staico R, Armaganijan L, Moreira D et al (2014) Renal sympathetic denervation and ventricular arrhythmias: a case of electrical storm with multiple renal arteries. Eurointervention 10:166
Ferguson DW, Berg WJ, Sanders JS (1990) Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol 16:1125–1134
Zipes DP (2008) Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleve Clin J Med 75(Suppl 2):S94–S96
Hou Y, Zhou Q, Po SS (2015) Neuromodulation for cardiac arrhythmia. Heart Rhythm 13:584–592
DiBona GF (2005) Physiology in perspective: the wisdom of the body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol 289:R633–R641
Esler MD, Krum H, Sobotka PA et al (2010) Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376:1903–1909
Azizi M, Sapoval M, Gosse P et al (2015) Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 385:1957–1965
Mahfoud F, Böhm M, Azizi M et al (2015) Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 36:2219–2227
Lüscher TF, Mahfoud F (2014) Renal nerve ablation after SYMPLICITY HTN-3: confused at the higher level? Eur Heart J 35:1706–1711
Mahfoud F, Lüscher TF (2015) Renal denervation: symply trapped by complexity? Eur Heart J 36:199–202
Kandzari DE, Bhatt DL, Brar S et al (2015) Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 36:219–227
Rundqvist B, Elam M, Bergmann-Sverrisdottir Y et al (1997) Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation 95:169–175
Ukena C, Mahfoud F, Linz D et al (2013) Potential role of renal sympathetic denervation for the treatment of cardiac arrhythmias. Eurointervention 9 Suppl R:R110–R116
Huang B, Scherlag BJ, Yu L et al (2015) Renal sympathetic denervation for treatment of ventricular arrhythmias: a review on current experimental and clinical findings. Clin Res Cardiol 104:535–543
Kiuchi MG, Maia GL, de Queiroz Carreira MA et al (2013) Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur Heart J 34:2114–2121
Pokushalov E, Romanov A, Corbucci G et al (2012) A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 60:1163–1170
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CU, FM, and MB are supported by the Ministry of Science and Economy of the Saarland. FM and DL are supported by the Deutsche Hochdruckliga. CU and MB are supported by the Deutsche Forschungsgemeinschaft (KFO 196). FM, DL and MB are supported by Deutsche Gesellschaft für Kardiologie. CU, FM, and MB received scientific support and speaker honorarium from Medtronic Inc. and St. Jude Medical. DT was supported by the DZHK (Deutsches Zentrum für Herz-Kreislauf-Forschung—German Center for Cardiovascular Research) and the BMBF (German Ministry of Education and Research). JSS has served as consultant to Medtronic, Boston Scientific and Biosense Webster, and has received research support from Medtronic and Biosense Webster.
Rights and permissions
About this article
Cite this article
Ukena, C., Mahfoud, F., Ewen, S. et al. Renal denervation for treatment of ventricular arrhythmias: data from an International Multicenter Registry. Clin Res Cardiol 105, 873–879 (2016). https://doi.org/10.1007/s00392-016-1012-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1012-y