Abstract
Purpose
Frequent ventricular premature depolarizations (VPDs) may cause cardiomyopathy (VPDCM), which often improves after VPD suppression. This study aimed to evaluate whether ablation of outflow tract ventricular arrhythmias (OT VAs) in patients with VPDCM improves renal in addition to left ventricular (LV) function.
Methods
We retrospectively evaluated 153 patients with OT VAs and examined VPD burden and LV ejection fraction (LVEF), as well as estimated glomerular filtration rate (eGFR) pre- and post-ablation. LV dysfunction was defined as LVEF <50 % and impaired renal function was defined as eGFR of <60 mL/min/1.73m2.
Results
Fifty-five patients had VPDCM. During mean follow-up of 14 months, 140 (92 %) were free from arrhythmia. In patients with VPDCM, patients with baseline LVEF 40–50 % had greater improvement in the post-ablation LVEF compared to patients with baseline LVEF <40 % (p < 0.01). At baseline, 36 (72 %) patients had renal dysfunction, 29 (81 %) of whom had improvement in eGFR from baseline after successful ablation from eGFR 51 to 57 mL/min/1.73m2. There was a significant association between cardiac (ΔLVEF ≥10 %) and renal (ΔeGFR ≥10 %) improvement (r = 0.54, p = 0.04). Using logistic regression analysis, procedural success was an independent predictor of improvement of cardiac (odds ratio [OR] = 13.7, p = 0.03) and renal function (OR = 21.0, p = 0.047).
Conclusions
Successful catheter ablation of OT VA reduces VPD burden and is associated with improved cardiac and renal function in patients with VPDCM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Ventricular premature depolarizations (VPDs) and non-sustained ventricular tachycardia may lead to development of left ventricular (LV) dilatation [1, 2] and reversible cardiomyopathy [3, 4]. Baman et al. indicated that a VPD burden of >24 % was independently associated with the development of cardiomyopathy [5]. Recently, it has been shown that long VPD QRS duration is a marker of risk for development of VPD-mediated cardiomyopathy (VPDCM) [6]. Longstanding rapid atrial fibrillation (AF) is also associated with the development of cardiomyopathy [7, 8], and in patients with AF and chronic kidney disease (CKD), renal function has been shown to improve after successful radiofrequency catheter ablation (RFCA) for AF [9]. For example, Navaravong et al. reported an 8 % improvement in estimated glomerular filtration rate (eGFR) for patients with CKD stage 2 following successful RFCA of AF [10]. Whether reduction of VPD burden in patients with VPDCM has a similar beneficial effect on renal function as AF suppression remains unknown. Therefore, this study aimed to evaluate whether renal function improves in addition to LV function after ablation of outflow tract (OT) ventricular arrhythmias (VAs) in patients with VPDCM.
2 Methods
2.1 Patient population
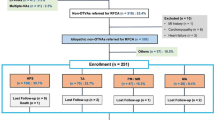
There were 474 patients referred for RFCA of OT VAs from 2006 to 2013. In these patients, all data on VPD burden, LV ejection fraction (LVEF), and eGFR pre- and post-ablation were obtained in 153 patients after excluding patients with potential alternative causes of their cardiomyopathy, including history of infarction, active ischemia, severe valvular disease, or inherited cardiomyopathy. The presence of an ischemic etiology was evaluated based upon history, electrocardiogram, and either coronary angiography or stress testing. All data were collected in a registry database approved by the Institutional Review Board of the University of Pennsylvania. Waiver of consent was obtained through the institutional review board for retrospective review of the clinical data.
2.2 Measured parameters
All patients underwent echocardiography prior to ablation to assess baseline LV systolic function and LV dimensions. LVEF was calculated using the Simpson’s biplane method. LV dysfunction was defined as LVEF <50 % by echocardiography. The modified diet in renal disease equation was used to determine the eGFR, expressed as mL/min/1.73m2. Renal dysfunction was defined as an eGFR <60 mL/min/1.73m2 as classified by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative guidelines [11]. Twenty-four hour Holter monitoring was performed at baseline and after RFCA to quantify the VPD burden. Patient demographic data including history of hypertension, AF, congestive heart failure (CHF), and diabetes mellitus were recorded. The most recent available biochemistry results assessing renal function obtained from the HUP laboratories were retrieved for analysis. In the absence of such data, the most recent data available from outside laboratories obtained prior to the index ablation procedure was constituted as baseline data.
Eighty-five patients (56 %) received follow-up at the HUP Ambulatory Arrhythmia Center Clinic who underwent pre- and post-ablation laboratory testing at the HUP laboratories. For the sixty-eight patients (44 %) who received follow-up by physicians from outside of the University of Pennsylvania Health System, the laboratory data were obtained from the same laboratory pre- and post-ablation for consistency.
Routine ECGs were performed at 6 weeks post-ablation and follow-up echocardiograms, Holter monitors, and serum creatinine levels 3 to 6 months after ablation. For patients not followed at the University of Pennsylvania Health System, individual referring cardiologists were contacted and medical records reviewed. Procedural success was defined as ≥80 % documented reduction in VPD burden on follow-up Holter monitor [12]. Accordingly, cardiac and renal improvements were arbitrarily defined as improvements of LVEF ≥10 % and eGFR ≥10 %, respectively.
2.3 Catheter ablation
Standard surface 12-lead ECGs were recorded. A 6Fr quadripolar catheter was positioned in the right ventricular apex for pacing. The integration of 3D mapping and phased array intracardiac echocardiography (CARTOSOUND®, Biosense Webster Inc., Diamond Bar, CA, USA) was used to delineate the complex OT relationships. For patients with frequent spontaneous VPDs, activation mapping was used to identify the site with the earliest presystolic activity. Mapping was started in the right ventricular OT (RVOT), unless the VPD morphology on ECG was inconsistent with RVOT origin or the information obtained from coronary sinus recordings suggested LV origin.
Intravenous heparin was administered to maintain an activated clotting time >250 s during aortic cusp and/or LV endocardial mapping. The ablation catheter was inserted via the right femoral artery and advanced to the aortic cusp region in a retrograde fashion. If activation times in the aortic cusp region were not sufficiently early with respect to the QRS onset, additional detailed mapping was performed in the great cardiac vein and anterior interventricular vein.
Radiofrequency energy was delivered with a conventional irrigated 3.5-mm-tip ablation catheter (ThermoCool®, Biosense Webster Inc., Diamond Bar, CA, USA) in a power control mode with initial settings of 20 W and titration to 40 W for 60–120 s. Catheter tip was irrigated by a continuous flow of saline at a rate of 30 mL/min during ablation. Burst atrial and ventricular pacing before and during isoproterenol infusion (up to a rate of 20 μg/min) was repeated following ablation to assess for inducible VPD [12, 13].
2.4 Statistical analysis
Statistical analyses were performed using SPSS version 21.0 software (SPSS Inc, Chicago, IL, USA). A p value <0.05 was considered statistically significant. Continuous data are presented as mean and standard deviation or median and interquartile range in variables that were not normally distributed, while categorical data are presented as percentages. Comparisons between groups were made using two-sample T test, one-way ANOVA, or the non-parametric equivalent for continuous variables. Chi-square or Fisher’s exact tests were used for categorical data. Pearson and Spearman correlation coefficients (r) were used to quantify correlations between variables. A simple and multivariable linear regression analysis was performed between the changes of eGFR pre- and post-VPD ablation. We present the results from univariate and multivariate models that controlled for sex, past history of hypertension, taking β-blockers, and procedural success for the predictors of improvement of cardiac function, and controlled for sex, taking angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB), ΔLVEF >10 % post-ablation, and procedural success for the predictors of improvement of renal function. Multivariable analysis was performed on items that showed a p value smaller than 0.1 by univariable analysis.
3 Results
3.1 Patient characteristics
There were 98 patients with normal LVEF (control group), and 55 patients with VPDCM in this study (mean age 53 ± 14 years; 66 [43 %] female). Of the patients with OT VAs, 55 (36 %) patients were identified as having a VPD site of origin from the RVOT, and 98 (64 %) were successfully ablated from the LV outflow tract respectively. In patients with VPDCM, 25 (45 %) patients underwent cardiac magnetic resonance imaging before ablation. Of these, 9 (36 %) patients had delayed enhancement in a small region (5: basal septum, 3: infero-lateral, 1: infero-septum). However, all patients had no evidence of extensive structural heart disease, and most importantly, the sites (if any) where delayed enhancement was detected were not associated with the origin of VPD. Furthermore, in patients without imaging, the electrogram at the site of origin of the VPD in all cases had normal voltage and other characteristics. During median follow-up period of 14 months (IQR 7–26), 140 patients (92 %) were free from arrhythmia (Table 1). Of these patients, 12 (9 %) were maintained on antiarrhythmic drugs (AADs) (control group: 5 sotalol and 2 flecainide; VPDCM group: 2 amiodarone, 2 dofetilide, and 1 mexiletine), while 128 (91 %) remained arrhythmia-free off AAD. Thirteen patients recurred due to the following factors: (1) the VPD origin was too close to major epicardial coronary arteries (four patients); (2) VPD origin was in close proximity to the conduction system (three patients); (3) multifocal VPDs that were not completely amenable to suppression despite extensive ablations (four patients); and (4) clinical VPD was too infrequent at the time of the procedure despite pacing and pharmacological stimulation for mapping and ablation to be performed (two patients). In patients who underwent successful ablation, VPD burden was significantly reduced (baseline vs. post-RFCA = 24 % [IQR 14–34] vs. 0.4 % [IQR 0–3.3], p < 0.01), whereas VPD burden remained at 13 % [IQR 11–23] following failed ablation procedures (Fig. 1). Systolic blood pressure increased significantly following successful ablation in patients with VPDCM (baseline vs. post-RFCA = 118 mmHg [IQR 107–130] vs. 125 mmHg [IQR 117–137], p = 0.01; Table 2).
3.2 Cardiac function
Post-ablation LVEF improved significantly compared to baseline in patients with VPDCM (50 % [IQR 45–55] vs. 35 % [IQR 30–40], p < 0.01; Table 2), and 84 % of patients post successful ablation showed an improvement in their LVEF ≥10 %. Furthermore, patients with baseline LVEF 40–50 % had a greater improvement in the post-ablation LVEF as compared to patients with baseline LVEF <40 % (p < 0.01; Fig. 2). In the control group, LVEF did not differ following successful ablation. Using a logistic regression analysis, procedural success yielded an odds ratio (OR) of 13.7 (95 % confidence interval [CI] 1.31–143.4, p = 0.03) which was a significant covariate for the improvement of cardiac function in patients with VPDCM (Table 3).
Comparison of LVEF pre- and post- ablation for patients with a baseline LVEF <40 % versus baseline LVEF 40–50 % in patients with VPD-mediated cardiomyopathy versus baseline LVEF ≥50 % in patients with structurally normal heart. Patients with baseline LVEF 40–50 % had greater improvement in the post-ablation LVEF compared to patients with baseline LVEF <40 % and baseline LVEF ≥50 %. LVEF left ventricular ejection fraction
3.3 Renal function
At baseline, 36 (72 %) patients with VPDCM had renal dysfunction. Of these, 29 patients (81 %) had an improvement in the eGFR and 24 patients (67 %) had a significant improvement in eGFR ≥10 % from baseline following successful ablation (Fig. 3). For the entire cohort, there was a significant improvement in serum creatinine and eGFR from baseline following successful ablation (creatinine, 0.8 mg/dL [IQR 0.7–1.1] vs. 1.0 mg/dL [IQR 0.9–1.2], p < 0.05; eGFR, 57 mL/min/1.73m2 [IQR 46–66] vs. 51 mL/min/1.73m2 [IQR 44–63], p < 0.05; Table 2). In control group, 50 (51 %) patients had impaired renal function at baseline. Of these, 28 (56 %) patients improved renal function after successful PVC ablation. However, serum creatinine and eGFR did not differ following successful ablation. In addition, there is no difference in the responses of eGFR to RVOT versus LVOT ectopy-induced cardiomyopathy (RVOT vs. LVOT = 8.6 mL/min/1.73m2 [IQR 3.2–11.9] vs. 6.2 mL/min/1.73m2 [IQR 1–10.7], p = 0.20). Finally, five (10 %) patients had non-sustained ventricular tachycardia (NSVT); however, existence of NSVT did not affect both baseline LVEF and eGFR.
Flow chart of the impact of successful VPD ablation on patients with baseline renal dysfunction in patients with VPD-mediated cardiomyopathy. Seventy-two percent of patients had baseline renal dysfunction. Of these, 81 % had an improvement in the eGFR and 67 % had a significant improvement in eGFR ≥10 % from baseline following successful ablation. eGFR estimated glomerular filtration rate
3.4 Relationship between cardiac and renal function in patients with VPDCM
In this study, eight patients experienced improvement of LVEF <10 % following successful ablation. In this group, the pre- and post-ablation eGFR did not differ significantly (53 mL/min/1.73m2 [IQR 46–58] vs. 54 mL/min/1.73m2 [IQR 47–61], p = NS). The degree of improvement in eGFR following successful ablation was independent of baseline LVEF. Importantly, there was a significant association between cardiac (ΔLVEF ≥10 %) and renal (ΔeGFR ≥10 %) improvement (r = 0.54, p = 0.04; Fig. 4).
Univariate and multivariate predictors of improvement of renal function were assessed using logistic regression analysis. Improvement in LVEF (OR = 11.4, 95 % CI 1.02–127.1, p = 0.04) and procedural success (OR = 23.4, 95 % CI 1.04–525.0, p = 0.04) were associated with improvement of renal function in univariate analysis (Table 4). In multivariate analysis, only procedural success (OR = 21.0, 95 % CI 1.04–421.4, p = 0.047) was associated with improvement of renal function.
3.5 Medication during follow-up in patients with VPDCM
Following ablation, 37 (67 %) patients were discharged on beta-blockers, 22 (40 %) on ACE inhibitors, 14 (25 %) on ARBs, 20 (36 %) on statins, and 18 (33 %) on diuretics. In patients with AADs at baseline, 6 (50 %) patients were discharged off AADs following successful ablation.
During the subsequent 14-month follow-up period, β-blockers were discontinued in eight patients (22 %), ACE inhibitors in three (14 %), ARBs in one (7 %), and diuretics were discontinued in six patients (33 %). Importantly, assessment of renal function was performed before discontinuation of medications that may have adversely affected renal function (including ACE inhibitors, angiotensin receptor blockers, and diuretics).
4 Discussion
4.1 Main findings
We investigated whether renal function recovers in addition to LV function after ablation of OT VAs. The present study demonstrated that 92 % of patients were free from arrhythmia during a follow-up period of 14 months. Post-ablation LVEF improved significantly compared to baseline, and 84 % of patients with VPDCM demonstrated an improvement in LVEF ≥10 % after successful ablation. Furthermore, patients with baseline LVEF 40–50 % had greater improvement of post-ablation LVEF compared to patients with baseline LVEF <40 %. Cardiac improvement was associated with procedure success.
At baseline, 36 (72 %) patients had renal dysfunction, of whom 81 % had improved eGFR after successful ablation in patients with VPDCM. Interestingly, renal improvement was associated with both procedure success and cardiac improvement. There were no significant differences in cardiac and renal function recovery following successful ablation in the control group.
4.2 Cardiac function
The mechanism of VPDCM may be related to the higher average heart rates and a short coupling interval in patients with frequent VPDs, LV dyssynchrony during VPDs, and the chronic effects of extra-systolic potentiation that increase intracellular calcium and myocardial oxygen consumption [14]. LV dyssynchrony also causes global reduction of cardiac mechanical efficiency, induces changes in regional hypertrophy, and alters myocardial blood flow [15]. However, it is well known that VPDCM is often reversible after successful ablation. Consistent with prior studies, LVEF improved dynamically following successful ablation in the present study [5, 12]. Mountantonakis et al. described that a reduction of 80 % or a burden of <5000 residual VPDs per day predicted improvement in LVEF and normalization of LV size following ablation [12]. Interestingly, in this study, 84 % of patients post successful ablation improved ΔLVEF ≥10 %. Furthermore, patients with baseline LVEF 40–50 % had greater improvement in their post-ablation LVEF compared to patients with baseline LVEF <40 %. This may indicate the presence of pre-existing structural abnormalities such as intramyocardial scar which might limit the degree of LV function recovery.
4.3 Renal function
Elevated levels of angiotensin II may play an integral role in renal dysfunction for patients with chronic renal hypoperfusion due to congestive cardiac failure [16]. Among various vasoactive mediators, local activation of the renin-angiotensin system is especially important because it can lead to constriction of efferent arterioles, hypoperfusion of post-glomerular peritubular capillaries, and subsequent hypoxia of the tubulointerstitium in the downstream compartment. In addition, angiotensin II directly damages endothelial cells. Accumulation of angiotensin II can lead to the loss of peritubular capillaries, an effect that is alleviated by angiotensin receptor blockade. A second important mechanism of angiotensin II-induced ischemia is inefficient cellular respiration and hypoxia via oxidative stress. Thus, angiotensin II induces tubulointerstitial hypoxia via both hemodynamic and non-hemodynamic mechanisms [16]. Moreover, Damman et al. described that an increased central venous pressure is associated with impaired renal function and is independently related to all-cause mortality in a broad spectrum of patients with cardiovascular disease [17]. From our study, recovery of cardiac function (ΔLVEF ≥10 %) is likely to be an underlying etiological factor for the increase in systolic blood pressure independent of changes in antihypertensive medication post-ablation. One possible mechanistic explanation for the clinical lag observed in renal function recovery is that increased systolic blood pressure may have generated more effective peripheral arterial perfusion of vital organs. In addition, it is reported that endothelial dysfunction is associated with renal dysfunction [18], and endothelial dysfunction is improved due to successful catheter ablation of AF [19]. Maintenance of sinus rhythm may result into improvement of renal dysfunction.
4.4 Clinical implications
The present study may improve the recognition and treatment of individuals with frequent OT VAs that are associated with the development of VPDCM. Due to the relatively high blood flow to the kidney, accounting for over 20 % of total cardiac output, chronic reduction in cardiac function can lead to progressive renal failure. Therefore, in patients with frequent VPDs and cardiomyopathy, successful suppression of VPDs may improve renal function in patients with new-onset kidney disease, and RFCA should therefore be considered.
5 Study limitations
The study has several limitations. Firstly, ours is a single-center retrospective observational study. Larger multicenter studies are warranted to confirm our findings. Secondly, we acknowledge that proteinuria is an important predictor of cardiovascular morbidity and mortality, and the role of proteinuria was not evaluated in this study. Thirdly, the results are not generalizable to patients with alternative causes for cardiomyopathy. Lastly, longer follow-up would be useful to determine the effects of ablation on renal function in the longer term.
6 Conclusions
This study suggests that successful catheter ablation of OT VA reduces VPD burden and is associated with improved LVEF and renal function for patients with VPDCM during medium term follow-up. Prospective studies with longer follow-up are warranted.
References
Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–65.
Sekiguchi Y, Aonuma K, Yamauchi Y, Obayashi T, Niwa A, Hachiya H, et al. Chronic hemodynamic effects after radiofrequency catheter ablation of frequent monomorphic ventricular premature beats. J Cardiovasc Electrophysiol. 2005;16:1057–63.
Yokokawa M, Good E, Crawford T, Chugh A, Pelosi Jr F, Latchamsetty R, et al. Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm. 2013;10:172–5.
Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–7.
Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–9.
Carballeira Pol L, Deyell MW, Frankel DS, Benhayon D, Squara F, Chik W, et al. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014;11:299–306.
Zipes DP. Atrial fibrillation. A tachycardia-induced atrial cardiomyopathy. Circulation. 1997;95:562–4.
Fujino T, Yamashita T, Suzuki S, Sugiyma H, Sagara K, Sawada H, et al. Characteristics of congestive heart failure accompanied by atrial fibrillation with special reference to tachycardia-induced cardiomyopathy. Circ J. 2007;71:936–40.
Takahashi Y, Takahashi A, Kuwahara T, Okubo K, Fujino T, Takagi K, et al. Renal function after catheter ablation of atrial fibrillation. Circulation. 2011;124:2380–7.
Navaravong L, Barakat M, Burgon N, Mahnkopf C, Koopmann M, Ranjan R, et al. Improvement in estimated glomerular filtration rate in patients with chronic kidney disease undergoing catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2015;26:21–7.
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266.
Mountantonakis SE, Frankel DS, Gerstenfeld EP, Dixit S, Lin D, Hutchinson MD, et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608–14.
Hutchinson MD, Garcia FC. An organized approach to the localization, mapping, and ablation of outflow tract ventricular arrhythmias. J Cardiovasc Electrophysiol. 2013;24:1189–97.
Huizar JF, Kaszala K, Potfay J, Minisi AJ, Lesnefsky EJ, Abbate A, et al. Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:543–9.
Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis. 2006;49:26–41.
Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25.
Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8.
Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. 2006;47:42–50.
Lim HS, Willoughby SR, Schultz C, Chakrabarty A, Alasady M, Lau DH, et al. Successful catheter ablation decreases platelet activation and improves endothelial function in patients with atrial fibrillation. Heart Rhythm. 2014;11:1912–8.
Acknowledgments
The authors thank M. Sato for support of statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All data were collected in a registry database approved by the Institutional Review Board of the University of Pennsylvania. Waiver of consent was obtained through the institutional review board for retrospective review of the clinical data.
Sources of funding
This study was funded in part by the Susan and Murray Bloom Research Fund.
Rights and permissions
About this article
Cite this article
Maeda, S., Chik, W.W., Liang, J.J. et al. Recovery of renal dysfunction after catheter ablation of outflow tract ventricular arrhythmias in patients with ventricular premature depolarization-mediated cardiomyopathy. J Interv Card Electrophysiol 48, 43–50 (2017). https://doi.org/10.1007/s10840-016-0190-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-016-0190-x