Abstract
Objectives
To investigate, whether renal denervation (RDN) has a direct effect on cardiac sympathetic activity and innervation density.
Background
RDN demonstrated its efficacy not only in reducing blood pressure (BP) in certain patients, but also in decreasing cardiac hypertrophy and arrhythmias. These pleiotropic effects occur partly independent from the observed BP reduction.
Methods
Eleven patients with resistant hypertension (mean office systolic BP 180 ± 18 mmHg, mean antihypertensive medications 6.0 ± 1.5) underwent I-123-mIBG scintigraphy to exclude pheochromocytoma. We measured cardiac sympathetic innervation and activity before and 9 months after RDN. Cardiac sympathetic innervation was assessed by heart to mediastinum ratio (H/M) and sympathetic activity by wash out ratio (WOR). Effects on office BP, 24 h ambulatory BP monitoring, were documented.
Results
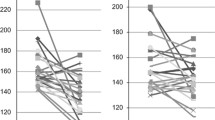
Office systolic BP and mean ambulatory systolic BP were significantly reduced from 180 to 141 mmHg (p = 0.006) and from 149 to 129 mmHg (p = 0.014), respectively. Cardiac innervation remained unchanged before and after RDN (H/M 2.5 ± 0.5 versus 2.6 ± 0.4, p = 0.285). Cardiac sympathetic activity was significantly reduced by 67 % (WOR decreased from 24.1 ± 12.7 to 7.9 ± 25.3 %, p = 0.047). Both, responders and non-responders experienced a reduction of cardiac sympathetic activity.
Conclusion
RDN significantly reduced cardiac sympathetic activity thereby demonstrating a direct effect on the heart. These changes occurred independently from BP effects and provide a pathophysiological basis for studies, investigating the potential effect of RDN on arrhythmias and heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Efferent fibers from the brain to the kidneys increase renin release, sodium tubular reabsorption, water retention, and renal vascular resistance in response to chemical or mechanical stimuli, thereby contributing to the development and maintenance of hypertension [1]. Renal afferent fibers regulate the kidney-brain feedback creating a self-maintaining sympathetic activation loop, which contributes to the development and maintenance of resistant hypertension and end organ damage [2]. Recent studies and registries demonstrated that the interruption of this pathway by renal denervation (RDN) is effective to treat resistant hypertension in some, but not all patients [3–5]. Further, RDN appears to have not only BP lowering effects, but also effects on other organs. In fact, RDN showed positive cardiac remodeling effects in terms of LV mass reduction and diastolic function improvement in severe hypertension [6, 7] and exhibited beneficial effects on atrial remodeling [8]. Interestingly, these changes were independent from BP lowering effects. Moreover, it has been reported that RDN prevents arrhythmic events in electric storm [9] and ventricular arrhythmias [10]. To date, evidence suggests that RDN reduces peripheral sympathetic activity measured by muscle sympathetic nerve activity (MSNA) [11] and renal norepinephrine (NE) spillover [12]. However, clinical evidence investigating changes in cardiac sympathetic activity following RDN are lacking. We used I-123-metaiodobenzylguanidine (MIBG), a radiopharmaceutical sharing the uptake into sympathetic nerve with NE, to investigate if RDN has effects on cardiac sympathetic innervation and activity. Like NE, MIBG is stored in vesicles in the presynaptic ends but it does not interact with the post-synaptic alpha- and beta receptors permitting the measurement of cardiac sympathetic activity without affecting sympathetic function and activation [13]. Analysis of cardiac MIBG images consists of quantitative analysis of global uptake (heart-to-mediastinum ratio, H/M) and the difference in tracer uptake/retention between early and late images (washout ratio, WOR). Recently, low H/M and/or high WOR have been associated with increased risk of worsening NYHA class, life-threatening arrhythmias and cardiac death in patients with heart failure (HF) [13].
Methods
Patients
Eleven patients with resistant hypertension underwent MIBG imaging to exclude pheochromocytoma and NE depletion after RDN. Eligible patients for RDN had an office systolic BP of ≥160 mmHg (≥150 mmHg for type 2 diabetics) and a 24 h systolic BP measured by ambulatory BP monitoring (ABPM) ≥130 mmHg despite antihypertensive treatment with ≥3 antihypertensive drugs at optimal doses (including a diuretic) with no changes in medication for at least 2 weeks before enrolment. Patients and physicians were instructed not to change antihypertensive medication during the study period, except when medically required. Inclusion criteria were age ≥18 years, glomerular filtration rate of ≥45 mL/min/1.73 m2. Eligible renal artery anatomy was length >20 mm and diameter >4 mm without stenosis or prior intervention (including balloon angioplasty or stenting). Exclusion criteria were secondary forms of hypertension or pseudo-hypertension, pregnancy, type 1 diabetes mellitus, myocardial infarction, unstable angina pectoris, cerebrovascular event within the last 6 months or hemodynamically significant valvular heart disease. Follow-up examinations were performed at 6 months. The analyses were approved by the local ethic committee in accordance with the Declaration of Helsinki.
Renal artery denervation procedure
Bilateral RDN using the Symplicity Flex catheter (Medtronic/Ardian USA) were performed as described elsewhere [3, 14]. All RDN procedures were performed by experienced operators (i.e., >15 RDN procedures/interventionist).
MIBG scintigraphy
All patients underwent MIBG scintigraphy at baseline and after a mean follow-up of 9 months. MIBG was performed to exclude pheochromocytoma at baseline and as a safety measure at follow-up. Following thyroid gland blocking with potassium perchlorate, intravenous injection of I-123-MIBG (GE Healthcare Buchler, Braunschweig, Germany) with a specific activity of 259 to 370 MBq/mg was given. The injected activity of MIBG ranged from 180 to 250 MBq. Planar whole body images in anterior and posterior views were obtained 15 min (early) and 4 h (delayed) after injection using a dual-head large-field camera (Multispect 2, Siemens, or Millenium Hawkeye, GE Healthcare) equipped with low energy high-resolution parallel-hole collimators. MIBG uptake of myocardium and mediastinum was quantified after drawing of a rectangular region of interest over the upper mediastinum and an irregularly configured region outlining the LV myocardium. Then, the H/M was calculated, which was defined as the average counts per pixel in the myocardium divided by that of the upper mediastinum. After correcting for the physical decay of 123I-MIBG, early and delayed H/M values were then used to compute the myocardial washout rate of MIBG as previously reported [15]. Myocardial rest perfusion imaging was performed with 99mTc-Sestamibi (MIBI, Cardiolite®, Bristol Myers Squibb, 500 MBq) or 99mTc-Tetrofosmin (Myoview®, GE Healthcare Buchler, 500 MBq) and SPECT. This perfusion imaging was used to exclude a myocardial hypoperfusion that may influence MIBG imaging adversely. Two operators blinded from patients’ baseline characteristics and from the effects of RDN during follow-up performed image analyses. Patients underwent imaging acquisition after taking their usual medication as recommended by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Acquisitions were all performed in the morning hours.
Office and 24 h ambulatory BP monitoring
Office BP measurements were performed in supine position after at least 5 min of rest with an automatic oscillometric monitor (Omron HEM-705 monitor; Omron Healthcare, Vernon Hills, Illinois), as indicated in recent guidelines. Baseline measurements are available for the whole population. All patients underwent baseline 24 h ambulatory blood pressure monitoring (ABPM) with an automatic portable device (Mobil-O-Graph, Medispec Deutschland GmbH, Krefeld, Germany) with readings taken every 15 min in daytime (7 am–10 pm) and every 30 min at nighttime (10 pm–7 am). Patients should perform their usual diurnal activities and sleep routine.
Statistical analysis
Data are presented as mean ± standard deviation (SD) or numbers (percentage) unless otherwise specified. Variables were checked for normal distribution. Correlation analyses were performed using the Spearman´s rank correlation. Pre-post comparisons were performed with the Student’s T test or the Wilcoxon rank sum test as appropriated. A p value <0.05 was considered significant. Statistical analyses were calculated using the SPSS statistical software (version 20.0, SPSS, Inc, Chicago, IL).
Results
Baseline characteristics
Eleven patients (five women and six men) with a mean age of 66.3 ± 5.7 years were investigated. Mean BMI was 32.2 ± 5.8 kg/m2, 45 % of the patients were diabetics. Despite a mean antihypertensive medication of 6.0 ± 1.5 drugs, mean office systolic BP was 179.6 ± 17.6 mmHg and mean systolic ABP was 149.3 ± 17.1 mmHg. All patients were treated with diuretics, 91 % received ACE inhibitors or angiotensin receptor blockers, 91 % beta blockers, 82 % calcium channel blockers (CCBs) and 64 % sympatholytics. Only one patient was on permanent AF. Table 1 depicts the baseline characteristics of the study population.
None of the patients experienced any acute or follow-up procedure related complication. Procedural characteristics are reported in Table 2. An average of 10.5 ± 3.1 complete 120 s ablation treatments was delivered in each patient. In detail, 4.7 ± 1.7 complete radiofrequency applications in the right and 5.8 ± 2.1 in the left renal artery were performed. One patient was not treated as mandated and underwent single sided left RDN due to impossible engagement of the dedicated ablation catheter into the right renal artery. Serum creatinine and glomerular filtration rate (calculated with the MDRD formula) remained unchanged after RDN (1.1 ± 0.2 to 1.1 ± 0.3 mg/dl, p = 0.482; 62.7 ± 12.3 to 59.2 ± 14.5 ml/min/1.73 m2, p = 0.489, respectively).
Medication adherence was not chemically assessed. According to visit questionnaire, five patients modified their anti-hypertensive treatment during the follow-up. In particular, three patients needed medication reduction; one patient underwent therapy changes and one patient increased drug regimen.
MIBG scintigraphy
Cardiac innervation measured by delayed H/M remained unchanged during follow-up (2.52 ± 0.47 versus 2.61 ± 0.44, p = 0.285). However, RDN significantly reduced cardiac sympathetic activity by 67 % (WOR from 24.1 ± 12.7 % to 7.9 ± 25.3 %, mean reduction −16.2 ± 23.7 %, p = 0.047). Results are depicted in Fig. 1 and Table 3. There was no correlation between the alterations in I-123-metaiodobenzylguanidine-uptake (WOR), as a measure of cardiac sympathetic activity, and baseline characteristics (office systolic BP r = −0.191, p = 0.574; systolic ABP r = −0.256, p = 0.448). There were also no correlations between WOR changes and BP changes (office systolic BP r = −0.364, p = 0.272, systolic ABPM r = −0.346, p = 0.297). Both, responders (i.e., office systolic BP reduction ≥10 mmHg at 6 months) and non-responders experienced cardiac sympathetic activity reduction after RDN (−16.7 ± 26.4 versus −13.9 ± 3.4 %, for responders and non-responders, respectively).
Office BP and ABPM
Nine patients (82 %) were responders to RDN, defined as a reduction in office BP ≥10 mmHg at 6 month follow-up. Mean office systolic BP was reduced by 38.9 ± 30.0 mmHg (from 179.7 ± 17.6 to 140.7 ± 28.7 mmHg, p = 0.006), office diastolic BP was reduced from 96.7 ± 13.7 to 77.9 ± 12.4 mmHg (−18.8 ± 10.8 mmHg, p = 0.001), respectively. Systolic ABP decreased after RDN by 20.5 ± 22.8 mmHg (from 149.3 ± 17.1 to 128.7 ± 15.4 mmHg, p = 0.014), and also diastolic ABP significantly reduced from 85.0 ± 12.8 to 71.4 ± 8.2 mmHg (−13.6 ± 13.9 mmHg, p = 0.005), respectively.
Discussion
Cardiac sympathetic activity reduction after RDN
To the best of our knowledge, we report for the first time direct effects of catheter-based RDN on cardiac sympathetic activity measured by MIBG scintigraphy. Herein, RDN significantly reduced WOR by 67 % (p = 0.047) in 11 resistant hypertensive patients without affecting cardiac innervation (delayed H/M remained unchanged, p = 0.285). The patient who underwent single sided RDN experienced little reduction of WOR (from 20 to 18 %). The effect of RDN on renal sympathetic efferent activity was previously described by Krum et al. [12]. Authors reported a 47 % reduction of renal NE spillover after RDN. Afferent sympathetic nerves from the kidneys to the central nervous system perpetuate the feedback, ultimately leading to sympathetic over-activation. Central sympathetic outflow modulation measured by MSNA has been shown to significantly decrease 3, 6 and 12 months after RDN [11]. However, it has been shown that sympathetic activity obtained from MSNA is inconsistently related with cardiac sympathetic activity [16]. We found a normal cardiac MIBG uptake in our patient population, with a higher degree of sympathetic activity at baseline (normal values for MIBG are 2.2 ± 0.3 for delayed H/M and 10 ± 9 % for WOR [17] ). This is in contrast with previous published studies in patients with essential hypertension, in whom H/M was reduced and WOR was increased [18, 19]. It has to be highlighted that, unlike essential hypertensives, our patients were treated with 6.0 ± 1.5 antihypertensive drugs on average, including beta blockers, ACE inhibitors, and mineralocorticoid receptor antagonists, which could have influenced H/M and WOR [18]. WOR has to be considered more closely associated with sympathetic activity than MIBG uptake, because it is independent of the number of neurons available, in contrast to H/M [20]. Importantly, MIBG was performed as a safety measure, since reduced H/M is associated with higher mortality in comorbidities such as HF [21] and the effect of RDN on cardiac sympathetic innervation were unknown. Herein, there was no deficit of sympathetic innervation following RDN, which adds important information to the safety profile of the approach.
Cardiac sympathetic activity and HF
HF patients are characterized by increased activation of the sympathetic nervous system. This adversely impacts cardiac structure and function and increases mortality risk [22]. In the recent ADMIRE-HF trial, delayed H/M was a predictor of cardiac and all cause death independent of other clinical and imaging parameters, including age, NYHA functional class, LVEF, and brain natriuretic peptide [21]. In particular, patients with delayed H/M <1.6 were at higher risk. In line, HF patients with a WOR ≥27 % showed a significantly higher mortality, morbidity and risk of sudden cardiac death than those with WOR <27 % [23, 24]. There is clinical evidence that reduction of cardiac sympathetic activity with beta-blockers positively affects HF progression [25]. Recently, the safety and efficacy of RDN has been tested in a very small group of HF patients. In 7 normotensive patients with reduced LV systolic function Davies et al. observed no hospital readmissions for HF symptoms during the 6-month follow-up and improvements in 6-min walking distance [26]. Taborsky et al. reported the effects of RDN in 51 patients with severe HF (mean LV-EF 25 ± 12 % and most NYHA class III/IV). Patients undergoing RDN experienced a significant improvement in systolic function and in NT-proBNP compared to the medical treated group [27]. In the current analyses, we report a significant WOR reduction in hypertensive patients after RDN. If the results can be reproduced in HF patients, this could provide the pathophysiological rationale for sympatho-modulation by RDN in patients with HF.
Cardiac sympathetic activity and arrhythmic recurrences
The significant WOR reduction after RDN could also help to explain previous positive effects of RDN in reducing arrhythmic recurrences. In fact, there is evidence that inappropriate and excessive activation of the sympathetic nervous system has further deleterious effects on the heart, as it may trigger ventricular and atrial arrhythmias [28, 29]. Pokushalov et al. tested in 27 patients the hypothesis that BP lowering, together with sympathetic modulation by RDN, could prevent AF recurrences after pulmonary vein ablation (PVI) [30]. They documented a consistent reduction in AF recurrence in patients treated with PVI combined with RDN when compared to patients treated with PVI alone [30]. The exact mechanisms are unknown but several factors may account for the beneficial effects of RDN: (i) the combination of local cardiac and whole body sympathetic activity reduction, which may inhibit the systemic and local cardiac renin angiotensin aldosterone system activity, reduces negative atrial anatomic and electric remodeling, (ii) the effective reduction in sympathetic activity inhibits the triggers in certain AF patients, and (iii) a direct effect on atrial remodeling [8]. Our data support for the first time two out of three effects and could explain, at least in part, the pathophysiological action of RDN on atrial arrhythmias. Only one patient in our population was on permanent AF. RDN reduced his WOR from 26 to 16 %. In a study on 88 patients Arimoto et al. [31] demonstrated that WOR is a multivariate predictor of AF recurrence (hazard ratio: 1.6, 95 % confidence interval: 1.004–1.125, p = 0.037) after AF ablation. This makes attractive the possibility to prevent AF recurrences reducing WOR by RDN [32]. The results from larger ongoing studies are needed to confirm the positive results of RDN in AF patients.
Cardiac sympathetic activity reduction has also been demonstrated to be effective in the treatment of life-threatening ventricular arrhythmic episodes. Arrhythmia recurrences in long-QT syndrome and in patients with catecholaminergic polymorphic ventricular tachycardia were reduced after surgical left cardiac sympathetic denervation [33]. Moreover, cardiac sympathetic nerve firing reduction during high thoracic epidural anesthesia was associated with a subsequent decrease in arrhythmia burden in patients with refractory electrical storm [34]. Interestingly, Ukena et al. [9] reported the first-in-man experience with RDN in two patients in therapy-resistant electrical storm. RDN resulted in an arrhythmia-free period of >6 months. These observations were also confirmed by others [10, 35, 36]. Hitherto, only indirect evidence of cardiac sympathetic activity after RDN has been reported. We report a significant WOR reduction (without cardiac de-innervation) after RDN, which could be the possible pathophysiological explanation of previous RDN results and is of interest for the potential application of RDN as additional treatment for certain ventricular arrhythmias [37, 38].
Interestingly, we found no correlation between BP fall and cardiac sympathetic activity reduction. Likewise, previous RDN studies in hypertensive patients showed that LV remodeling assessed with CMR [7] and echocardiography [6], as well as HR reduction [39] were independent from BP reduction. Mechanistically, cardiovascular phenotype of high sympathetic innervation is not always BP dependent; therefore RDN effects on heart are independent from BP effects. This is evident for ventricular arrhythmias, severe HF and certain AF subtypes, which are all conditions characterized by high sympathetic cardiac activity, but only rarely by high BP. Two studies were recently performed in normotensive animals to investigate the effects of RDN on arrhythmias [40, 41]. In one study [40], RDN reduced atrial sympathetic nerve sprouting, structural alterations and AF complexity in goats with persistent AF independently from BP changes. Transcardiac and renal NE were significantly lower in RDN goats as compared to the sham group at follow-up. In the other study [41], RDN reduced ventricular rate during AF episodes, which were also shorter after RDN compared with sham. Once more, BP was not modified. As further evidence, seven normotensive patients, who underwent RDN for severe HF improved their functional status without experiencing BP reduction [26]. Further studies are needed to better investigate these findings.
Limitations
Our analyses may have some limitations. First, the number of patients who underwent MIBG before and after RDN is small, thereby limiting statistical power. However, the observed BP reductions in our population (both in office and ABPM) is in line and superimposable to that of previous larger studies. Second, the use of beta blockers, CCBs and RAAS blockers could have influenced the results. However, medical therapy was not significantly changed during the follow-up period so that the same drug interference was present before and after RDN. Third, we did not provide a control group and the present analysis was a retrospective evaluation of clinically driven examinations. The results and implications are therefore hypothesis generating. Data from a control group (both BP and MIBG data) as well as data from a sham group in a controlled study are important to clarify the effectiveness of RDN in reducing WOR.
Conclusions
This study is the first to report a reduction of cardiac sympathetic activity in the presence of unchanged sympathetic innervation. In our population of 11 resistant hypertensive patients, RDN significantly reduced cardiac sympathetic activity by 67 % partly independent from BP lowering effects, while it did not reduce delayed H/M as a measure of sympathetic innervation. These results may help to explain previous findings of positive cardiac remodeling and beneficial effects on arrhythmia recurrence after RDN. Moreover, cardiac sympathetic reduction could be helpful in HF patients. Future studies are urgently needed to assess the potential of RDN in treating arrhythmias and HF.
References
Krum H, Sobotka P, Mahfoud F, Böhm M, Esler M, Schlaich M (2011) Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation 123:209–215
Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H (2011) Sympatho-renal axis in chronic disease. Clin Res Cardiol. 100:1049–1057
Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M (2010) Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376:1903–1909
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR (2014) Bakris GL; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370:1393–1401
Böhm M, Mahfoud F, Ukena C, Hoppe UC, Narkiewicz K, Negoita M, Ruilope L, Schlaich MP, Schmieder RE, Whitbourn R, Williams B, Zeymer U, Zirlik A (2015) Mancia G; on behalf of the GSR Investigators. First Report of the Global SYMPLICITY Registry on the Effect of Renal Artery Denervation in Patients With Uncontrolled Hypertension. Hypertension 65:766–774
Schirmer SH, Sayed MM, Reil JC, Ukena C, Linz D, Kindermann M, Laufs U, Mahfoud F, Böhm M (2014) Improvements in left ventricular hypertrophy and diastolic function following renal denervation: effects beyond blood pressure and heart rate reduction. J Am Coll Cardiol 63:1916–1923
Mahfoud F, Urban D, Teller D, Linz D, Stawowy P, Hassel JH, Fries P, Dreysse S, Wellnhofer E, Schneider G, Buecker A, Schneeweis C, Doltra A, Schlaich MP, Esler MD, Fleck E, Böhm M, Kelle S (2014) Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J 35:2224–2233
Schirmer SH, Sayed MMYA, Reil JC, Lavall D, Ukena C, Linz D, Mahfoud F, Böhm M (2015) Atrial remodeling following catheter-based renal denervation occurs in a blood pressure and heart rate—independent manner. JACC Cardiovasc Interv. 8:972–980
Ukena C, Bauer A, Mahfoud F, Schreieck J, Neuberger HR, Eick C, Sobotka PA, Gawaz M, Böhm M (2012) Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol. 101:63–67
Remo BF, Preminger M, Bradfield J, Mittal S, Boyle N, Gupta A, Shivkumar K, Steinberg JS, Dickfeld T (2014) Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm. 11:541–546
Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP (2014) Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 64:118–124
Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M (2009) Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373:1275–1281
Travin MI (2013) Cardiac autonomic imaging with SPECT tracers. J Nucl Cardiol. 20:128–143
Mahfoud F, Lüscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, Doevendans P, Fagard R, Fajadet J, Komajda M, Lefèvre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Böhm M (2013) European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 34:2149–2157
Carrió I, Cowie MR, Yamazaki J, Udelson J, Camici PG (2010) Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc Imaging. 3:92–100
Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G (1988) Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 11:3–20
Morozumi T, Kusuoka H, Fukuchi K, Tani A, Uehara T, Matsuda S, Tsujimura E, Ito Y, Hori M, Kamada T, Nishimura T (1997) Myocardial iodine-123-metaiodobenzylguanidine images and autonomic nerve activity in normal subjects. J Nucl Med 38:49–52
Sakata K, Shirotani M, Yoshida H, Kurata C (1998) Comparison of effects of enalapril and nitrendipine on cardiac sympathetic nervous system in essential hypertension. J Am Coll Cardiol 32:438–443
Sakata K, Shirotani M, Yoshida H, Nawada R, Obayashi K, Togi K, Miho N (1999) Effects of amlodipine and cilnidipine on cardiac sympathetic nervous system and neurohormonal status in essential hypertension. Hypertension 33:1447–1452
Imamura Y, Ando H, Mitsuoka W, Egashira S, Masaki H, Ashihara T, Fukuyama T (1995) Iodine-123 metaiodobenzylguanidine images reflect intense myocardial adrenergic nervous activity in congestive heart failure independent of underlying cause. J Am Coll Cardiol 26:1594–1599
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H (2010) Narula J; ADMIRE-HF Investigators. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 55:2212–2221
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J (2009) The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54:1747–1762
Ogita H, Shimonagata T, Fukunami M, Kumagai K, Yamada T, Asano Y, Hirata A, Asai M, Kusuoka H, Hori M, Hoki N (2001) Prognostic significance of cardiac (123)I metaiodobenzylguanidine imaging for mortality and morbidity in patients with chronic heart failure: a prospective study. Heart 86:656–660
Kioka H, Yamada T, Mine T, Morita T, Tsukamoto Y, Tamaki S, Masuda M, Okuda K, Hori M, Fukunami M (2007) Prediction of sudden death in patients with mild-to-moderate chronic heart failure by using cardiac iodine-123 metaiodobenzylguanidine imaging. Heart 93:1213–1218
Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 106:2194–2199
Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP (2013) First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol 162:189–192
Taborsky M, Lazarova ML, Vaclavik J (2012) The effect of renal denervation in patients with advanced heart failure. Eur Heart J 33:517
Linz D, Ukena C, Mahfoud F, Neuberger HR, Böhm M (2014) Atrial autonomic innervation: a target for interventional antiarrhythmic therapy? J Am Coll Cardiol 63:215–224
Zipes DP (2008) Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleve Clin J Med. 75:S94–S96
Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS (2012) A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 60:1163–1170
Arimoto T, Tada H, Igarashi M, Sekiguchi Y, Sato A, Koyama T, Yamasaki H, Machino T, Kuroki K, Kuga K, Aonuma K (2011) High washout rate of iodine-123-metaiodobenzylguanidine imaging predicts the outcome of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 22:1297–1304
Linz D, van Hunnik A, Ukena C, Ewen S, Mahfoud F, Schirmer SH, Lenski M, Neuberger HR, Schotten U, Böhm M (2014) Renal denervation: effects on atrial electrophysiology and arrhythmias. Clin Res Cardiol. 103:765–774
Collura CA, Johnson JN, Moir C, Ackerman MJ (2009) Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 6:752–759
Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y, Shivkumar K (2010) Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 121:2255–2262
Hilbert S, Rogge C, Papageorgiou P, Hindricks G, Bollmann A (2015) Successful single-sided renal denervation in drug-resistant hypertension and ventricular tachycardia. Clin Res Cardiol. 104:279–281
Armaganijan LV, Staico R, Moreira DA, Lopes RD, Medeiros PT, Habib R, Melo Neto J, Katz M, Armaganijan D, Sousa AG, Mahfoud F, Abizaid A. 6-Month Outcomes in Patients With Implantable Cardioverter-Defibrillators Undergoing Renal Sympathetic Denervation for the Treatment of Refractory Ventricular Arrhythmias. JACC Cardiovasc Interv. 2015;8:984–90
Huang B, Scherlag BJ, Yu L, Lu Z, He B, Jiang H (2015) Renal sympathetic denervation for treatment of ventricular arrhythmias: a review on current experimental and clinical findings. Clin Res Cardiol. 104:535–543
Linz D, Böhm M (2015) Renal denervation for treatment of hypertension and beyond. Clin Res Cardiol. 104:87–88
Ukena C, Mahfoud F, Spies A, Kindermann I, Linz D, Cremers B, Laufs U, Neuberger HR, Böhm M (2013) Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol 167:2846–2851
Linz D, van Hunnik A, Hohl M, Mahfoud F, Wolf M, Neuberger HR, Casadei B, Reilly SN, Verheule S, Böhm M, Schotten U (2015) Catheter-based renal denervation reduces atrial nerve sprouting and complexity of atrial fibrillation in goats. Circ Arrhythm Electrophysiol 8:466–474
Linz D, Mahfoud F, Schotten U, Ukena C, Hohl M, Neuberger HR, Wirth K, Böhm M (2013) Renal sympathetic denervation provides ventricular rate control but does not prevent atrial electrical remodeling during atrial fibrillation. Hypertension 61:225–231
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Felix Mahfoud and Michael Böhm received speaker honorarium and consultancy fees from Medtronic/Ardian. Christian Ukena and Bodo Cremers received speaker honorarium from Medtronic. Murray Esler received honoraria for consultancy and educational activities from Medtronic; holds no shares in the company or patents for renal denervation. All other authors declare no conflicts.
Rights and permissions
About this article
Cite this article
Donazzan, L., Mahfoud, F., Ewen, S. et al. Effects of catheter-based renal denervation on cardiac sympathetic activity and innervation in patients with resistant hypertension. Clin Res Cardiol 105, 364–371 (2016). https://doi.org/10.1007/s00392-015-0930-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0930-4