Abstract
Purpose
We aimed to explore whether sarcopenia diagnosed with the third lumbar vertebra skeletal muscle index (L3 SMI) can be a predictor of prognosis for colorectal cancer (CRC) patients.
Methods
A systematic review and meta-analysis was conducted using PubMed, Embase, and the Web of Science databases. All original comparative studies published in English that were related to sarcopenia versus non-sarcopenia in non-metastatic CRC patients based on postoperative and survival outcomes were included. Data synthesis and statistical analysis were carried out using Stata software.
Results
A total of 12 studies including 5337 patients were included in our meta-analysis. In our overall analyses of postoperative outcomes, we indicated that CRC patients with sarcopenia would have longer hospital stays, higher incidence of total postoperative morbidity (OR = 1.70, 95% CI = 1.07–2.70, P < 0.01), mortality (OR = 3.45, 95% CI = 1.69–7.02, P < 0.01), and infection (OR = 2.21, 95% CI = 1.50–3.25, P < 0.01) but not anastomosis leakage or intestinal obstruction when compared to non-sarcopenia patients. Regarding survival outcomes, our results showed that sarcopenia predicted a decreased overall survival (HR = 1.63, 95% CI = 1.24–2.14, P < 0.01), disease-free survival, and cancer-specific survival for non-metastatic CRC patients. Moreover, our subgroup analyses showed similar tendency with our overall analyzed results.
Conclusions
Sarcopenia diagnosed with L3 SMI can be a negative predictor of postoperative and survival outcomes for non-metastatic CRC patients. Prospective studies with a uniform definition of sarcopenia are needed to update our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer worldwide with high morbidity and mortality [1]. Although surgical resection remains as the main treatment for non-metastatic CRC patients, approximately 20–30% patients may still suffer from postoperative complications such as surgical site infection, anastomosis leakage, or intestinal obstruction [2,3,4,5]. Meanwhile, prognostic stratification of patients is usually guided by tumor pathology after potentially curative surgery; however, long-term survival outcomes can also be negatively impacted by postoperative complications and baseline host-related factors [4, 6,7,8,9]. Therefore, exploring host-related factors, which can predict the prognosis of postoperative and survival outcomes, are very important to identify the subgroup population that can benefit more from colorectal resection.
Body composition is a common host-related factor, and there is mounting evidence that indicates that patients with cancer undergo a variety of changes in body composition that alters their portion of muscle, fat, and bone [10]. Notably, sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass (SMI) [11]. To date, according to the consensuses of the European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AWGS), muscle strength and physical performance are also important components of sarcopenia [12, 13]. However, there is still no uniform standard to measure and define sarcopenia to date [14]. The most common method in measuring SMI is using preoperative computerized tomography (CT) scan at the third lumbar vertebra (L3) [15], which is commonly used in retrospective studies to measure SMI and to define sarcopenia. Growing evidence indicates that patients with sarcopenia have a negative prognosis of postoperative or survival outcomes in various types of tumors, such as gastric, pancreatic, or lung cancer [16,17,18,19]. However, the impact of sarcopenia in patients with non-metastatic CRC remains controversial. Hence, it is urgent to provide comprehensive evidence to evaluate the impact of sarcopenia in CRC patients.
Based on the aforementioned, we aimed to explore whether sarcopenia diagnosed with L3 SMI can be a negative prognostic predictor in terms of postoperative and survival outcomes for patients with non-metastatic CRC.
Methods
Search strategy
Based on the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (http://www.prisma-statement.org/), we conducted our systematic review and meta-analysis. Our search was restricted to the English language based on the following MeSH/main keywords: “colorectal,” “rectal,” “colonic,” “colon,” “rectum,” “sarcopenia,” “myopenia,” and “muscle mass” using datasets from PubMed, Embase, and Web of Science (up to April 4, 2018). To avoid redundant studies, we checked all authors and organizations and evaluated the recruitment period and population of patients enrolled in each study. In addition, the lists of references in the relevant studies were also screened for additional studies.
Inclusion and exclusion criteria
According to the PICOS criteria (population, intervention, comparison, outcomes, and study design), studies were selected in our present meta-analysis according to the following eligibility criteria: (1) population: patients with primary colorectal cancer without metastases; (2) intervention: sarcopenia diagnosed by preoperative CT scan with L3SMI and underwent colorectal resection; (3) comparison: sarcopenia versus non-sarcopenia colorectal cancer patients; (4) outcomes: postoperative (hospital stay, total postoperative complications, postoperative mortality, anastomosis leak, infection, intestinal obstruction), and survival outcomes (OS, DFS and CSS) compared between the two groups; and (5) study design: comparative studies (retrospective and prospective studies). In addition, the exclusion criteria were (1) patients with other cancer types or metastases; (2) sarcopenia diagnosed by other methods; and (3) studies with insufficient data or absence of the outcomes of interest.
Data extraction and quality assessment of included studies
Two reviewers (GuangweiSun and Yalun Li) reviewed and assessed each of the included studies independently, and the following information was collected: first author, year of publication, country, study type, number of patients enrolled, age, body mass index (BMI), male/female percentage, and cutoff value of L3SMI. In addition, data extraction of postoperative and survival outcomes (OS was defined as event of death due to any cause; DFS was defined as event of disease recurrence or death; and CSS was defined as event of death due to cancer) was also performed by the two reviewers independently. Moreover, the Newcastle–Ottawa Scale (NOS) criterion was used to evaluate the quality of the studies included [20]. All disagreements in terms of the aforementioned studies were resolved by discussion between the two reviewers (GuangweiSun and Yalun Li).
Statistical analysis
In our systematic review and meta-analysis, continuous variables were analyzed by the weighted mean difference (WMD), and dichotomous variables were analyzed by the odds ratios (ORs). If the study did not provide values for the mean and standard deviation (SD), we used the method of Hozo et al. to calculate the mean and SD for analyses [21]. Meanwhile, the most appropriate statistic to use for evaluating survival outcomes (time-to-event outcomes) was the hazard ratio (HR). If studies did not provide the HR directly, we obtained an estimated HR by methods designed by Tierney [22]. All analyses were performed using Stata software, version 12.0 (2011; Stata Corp., College Station, TX, USA). All the analyses in this study used a random-effects model because it provided more conservative estimates and was tailored to multicenter studies in which heterogeneity was usually present [23]. All statistical values were reported with the 95% confidence interval (CI) and a two-tailed P value less than 0.05 was defined as statistical significance. Subgroup analyses were conducted in terms of study type, total patients number, NOS scores, country, and cutoff values of sarcopenia based on OS and total postoperative complications. Finally, publication bias was assessed using Begg’s and Egger’s tests [24, 25].
Results
Selected studies
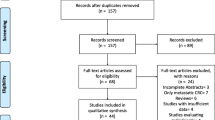
Based on our search strategy, a total of 376 published studies were identified. After removing the duplicates, screening the title and abstract, and further evaluating, finally, 12 comparative studies were included in the systematic review and meta-analysis [11, 26,27,28,29,30,31,32,33,34,35,36]. A flow chart of the search strategies, which includes the reasons for exclusion of studies, is illustrated in Fig. 1. Among the included studies, five studies were prospective comparative studies [26, 29, 30, 33, 36], and the other seven were designed retrospectively [11, 27, 28, 31, 32, 34, 35]. In addition, six studies were conducted in Asian countries [26, 27, 29, 31, 32, 35], the other six studies were from non-Asian countries [11, 28, 30, 33, 34, 36]. Meanwhile, six studies included a total number patients larger than 200 [11, 28, 30,31,32, 34] and eight studies had the NOS score larger than 5 [26, 29,30,31,32,33,34,35]. The detailed characteristics of the included studies are summarized in Table 1. Moreover, the definition and cutoff values of sarcopenia from the included studies are summarized in Table 2.
Overall analyses of postoperative outcomes
Our pooled analyses indicated that patients with sarcopenia showed a significant longer hospital stay when compared to patients without sarcopenia after colorectal resection (WMD = 1.29, 95% CI = 0.50–2.08, P < 0.01). In terms of postoperative complications, the sarcopenia group showed a significantly higher total morbidity and mortality in comparison with the non-sarcopenia group (morbidity: OR = 1.70, 95% CI = 1.07–2.70, P < 0.01; mortality: OR = 3.45, 95% CI = 1.69–7.02, P < 0.01). Infection, anastomosis leakage and intestinal obstruction were the most common complications for patients after colorectal surgery and we observed that patients with sarcopenia showed a significant higher rate of incidence of postoperative infection but not anastomosis leakage and intestinal obstruction when compared with non-sarcopenia patients (infection: OR = 2.21, 95% CI = 1.50–3.25, P < 0.01; anastomosis leakage: OR = 0.73, 95% CI = 0.51–1.05, P = 0.09; OR = 1.13, 95% CI = 0.58–2.19, P = 0.73). The detailed results in terms of postoperative outcomes are shown in Fig. 2.
Overall analyses of survival outcomes
Six studies had provided survival outcome data [27, 28, 30,31,32, 35] and our pooled analyses demonstrated that the sarcopenia group showed a significant decreased OS in comparison with the non-sarcopenia group (HR = 1.63, 95% CI = 1.24–2.14, P < 0.01). In addition, we also observed that patients with sarcopenia had a significant decreased DFS and CSS in comparison with patients with non-sarcopenia after colorectal resection (DFS: HR = 1.70, 95% CI = 1.24–2.31, P < 0.01; CSS: HR = 1.62, 95% CI = 1.16–2.27, P < 0.01). The detailed results of survival outcomes are shown in Fig. 3. Moreover, five included studies had provided the results of multivariate analyses in OS [27, 28, 30, 31, 35], three studies in DFS [30, 31, 35], and three studies in CSS [28, 31, 35]; therefore, we pooled the results of multivariate analyses to further verify our overall results. The pooled results based on multivariate analyses data showed a similar tendency with our overall results significantly (OS: HR = 1.73, 95% CI = 1.28–2.35, P < 0.01; DFS: HR = 1.95, 95% CI = 1.36–2.80, P < 0.01; CSS: HR = 1.62, 95% CI = 1.16–2.27, P < 0.01).
Subgroup analyses and publication bias
Based on study type, total number of patients, NOS scores, country, and criterion of sarcopenia, we conducted subgroup analyses in terms of total postoperative complications and OS. Although we observed a similar tendency that the sarcopenia group had a higher incidence of postoperative complications and a decreased OS among different subgroups, some subgroups were without statistical significance. Regarding the total postoperative complications, the sarcopenia group showed a significantly higher incidence of postoperative complications in subgroups of retrospective studies (OR = 1.57, 95% CI = 1.08–2.29, P = 0.02), total patients smaller than 200 (OR = 2.24, 95% CI = 1.10–4.55, P = 0.03), NOS scores larger than 5 (OR = 1.77, 95% CI = 1.05–2.99, P = 0.03), studies conducted in Asia (OR = 2.69, 95% CI = 1.29–5.59, P < 0.01), and sarcopenia defined by SMI plus muscle strength and function (OR = 4.01, 95% CI = 2.16–7.44, P < 0.01). In addition, in terms of OS, except the subgroup of NOS scores larger than 5, all the other subgroups showed a significant improved OS in patients in the non-sarcopenia group. The detailed results of subgroup analyses are shown in Table 3. Meanwhile, we did not observe publication bias in terms of total postoperative complications (Begg’s test: P = 1.00, Egger’s test: P = 0.32) and OS (Begg’s test: P = 0.26, Egger’s test: P = 0.10) using Begg’s and Egger’s tests.
Discussion
Sarcopenia is a geriatric syndrome affecting older adults, which was first described by Rosenbery [37] as the loss of muscle mass in seniors. Previous evidence indicated that sarcopenia can be a predictor of all-cause mortality among community-dwelling older people [38], and to date, the association between sarcopenia and cancer had drawn the attention of clinicians throughout the world. Recently, a systematic review indicated that low SMI at cancer diagnosis is associated with worse survival in patients with solid tumors [39]. However, to the best of our knowledge, whether sarcopenia can be a predictor of prognosis of CRC patients remains controversial; therefore, we conducted this systematic review and meta-analysis to resolve this issue.
Based on our results, we indicated that CRC patients with sarcopenia diagnosed with L3SMI would have longer hospital stays, higher incidence of total postoperative morbidity, mortality, and infections when compared to non-sarcopenia patients. Our results of the meta-analysis are in accordance with previous single-center investigation findings. Lieffers et al. indicated that sarcopenia is independently predictive of postoperative infections [11], and multivariate logistic regression analysis also showed that sarcopenia is significantly associated with total postoperative morbidity by studies of Huang et al. [29] and Nakanishi et al. [32]. Hence, sarcopenia not only negatively impacted the recovery of patients undergoing colorectal resection but also brought significant higher costs for relations of patients [40]. Moreover, sarcopenia is also a negative long-term prognostic factor for CRC patients based on our results. Evidence also demonstrated that sarcopenia in cancer survivors is associated with increased cardiovascular disease risk [41]. In addition, Nipp et al. also indicated that sarcopenia is associated with quality of life and depression in patients with advanced cancer [42]. Hence, the negative impacts of sarcopenia have highlighted the importance of prevention and curing sarcopenia among clinicians and patients.
What are the factors associated with the prevalence of sarcopenia in CRC patients? A recent investigation indicated that BMI, serum albumin, phase angle, muscle attenuation, and scored patients-generated subjective global assessment were independent predictors of sarcopenia in CRC patients by multivariable analyses [43]. Based on the baseline information of our included studies (Table 1), we also observed that patients in the sarcopenia group had a relatively lower BMI in comparison with non-sarcopenia group patients. Hence, changes in BMI may be a clinical signal for earlier detection of sarcopenia for CRC patients. Exercise and diet are the two main common ways for prevention of sarcopenia for cancer patients. Physical activity of cancer patients is associated with maintenance or significant improvements in aerobic capacity and muscle strength [44, 45]. Evidence also demonstrated that resistance exercise is more effective for improving muscle strength than aerobic exercise [44, 45]. In addition, there is indication that resistance training induces increase in muscle mass and strength can be enhanced by a high-protein diet and certain nutrients [46, 47].
A clear and uniformed definition of sarcopenia is important, since the number of publications on this syndrome is increasing. However, to date, no study has comprehensively evaluated the definitions and tools used in the literature to define and determine the presence of sarcopenia [14]. A systematic review on how to define and measure sarcopenia is being conducted and we expect the publication of this systematic review to standardize the criteria of sarcopenia [14]. To decrease the heterogeneity among studies caused by the diagnosis method of sarcopenia, we only included studies of sarcopenia diagnosed by preoperative CT scan with L3 SMI. Among the included studies, five studies defined the cutoff values only based on sex-specific L3 SMI, two studies also contained muscle strength and function assessments in addition to sex-specific L3 SMI, and five studies were based on both sex- and BMI-specific L3 SMI. Based on our subgroup analyses, we could still not determine the best criterion and cutoff values of sarcopenia for CRC patients because of the limited included studies and the lack of individual data. However, based on the current evidence, we proposed that gender, BMI and race should be taken into consideration for the cutoff values of the criteria of sarcopenia. In addition, muscle mass, strength, and function should be comprehensively evaluated to define sarcopenia.
In summary, our systematic review and meta-analysis has provided valid evidence in evaluating the significance of sarcopenia in patients after colorectal resection. However, there are some limitations to our study. First, the definitions among studies were different and retrospective studies had no restriction with muscle strength and function. Although we conducted subgroup analyses based on definitions of sarcopenia and study type, prospective studies with uniform standards and definition of sarcopenia are needed to update our results. Second, neoadjuvant chemoradiotherapy and adjuvant chemotherapy were also important factors that affect the prognosis of CRC patients; however, included studies did not provide the detailed information and data based on neoadjuvant or adjuvant therapies. Hence, these factors might affect our pooled results and future studies based on individual data are needed to verify our findings. Finally, we could not conduct subgroup analyses based on the primary location of tumors, such as rectal, right, or left colon cancer. The prognosis was different based on the primary location of CRC patients [48]; therefore, the mixed baseline information might restrict our further exploration of the significance of sarcopenia in rectal and colon cancer, respectively.
Conclusion
Sarcopenia diagnosed by L3SMI can be a predictor of postoperative and survival outcomes for patients with CRC. However, prospective studies with uniform standards and definition of sarcopenia are needed to update our results.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Dornfeld M, Lovely JK, Huebner M, Larson DW (2017) Surgical site infection in colorectal surgery: a study in antibiotic duration. Dis Colon Rectum 60(9):971–978. https://doi.org/10.1097/DCR.0000000000000807
Eto K, Urashima M, Kosuge M, Ohkuma M, Noaki R, Neki K, Ito D, Takeda Y, Sugano H, Yanaga K (2018) Standardization of surgical procedures to reduce risk of anastomotic leakage, reoperation, and surgical site infection in colorectal cancer surgery: a retrospective cohort study of 1189 patients. Int J Color Dis 33:755–762. https://doi.org/10.1007/s00384-018-3037-3
Takahashi H, Haraguchi N, Nishimura J, Hata T, Yamamoto H, Matsuda C, Mizushima T, Doki Y, Mori M (2018) The severity of anastomotic leakage may negatively impact the long-term prognosis of colorectal cancer. Anticancer Res 38(1):533–539. https://doi.org/10.21873/anticanres.12255
Chapman SJ, Pericleous A, Downey C, Jayne DG (2018) Postoperative ileus following major colorectal surgery. Br J Surg 105:797–810. https://doi.org/10.1002/bjs.10781
Aoyama T, Oba K, Honda M, Sadahiro S, Hamada C, Mayanagi S, Kanda M, Maeda H, Kashiwabara K, Sakamoto J, Saji S, Yoshikawa T (2017) Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients’ data from three large phase III randomized trials. Cancer Med 6(7):1573–1580. https://doi.org/10.1002/cam4.1126
Slankamenac K, Slankamenac M, Schlegel A, Nocito A, Rickenbacher A, Clavien PA, Turina M (2017) Impact of postoperative complications on readmission and long-term survival in patients following surgery for colorectal cancer. Int J Color Dis 32(6):805–811. https://doi.org/10.1007/s00384-017-2811-y
Toiyama Y, Hiro J, Shimura T, Fujikawa H, Ohi M, Tanaka K, Inoue Y, Mohri Y, Kusunoki M (2016) The impact of body mass index on oncological outcomes in colorectal cancer patients with curative intent. Int J Clin Oncol 21(6):1102–1110. https://doi.org/10.1007/s10147-016-1016-7
Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, Feliciano EC, Castillo AL, Quesenberry CP, Kwan ML, Prado CM (2017) Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomark Prev 26(7):1008–1015. https://doi.org/10.1158/1055-9965.EPI-17-0200
Bianchini F, Kaaks R, Vainio H (2002) Overweight, obesity, and cancer risk. Lancet Oncol 3(9):565–574
Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE (2012) Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 107(6):931–936. https://doi.org/10.1038/bjc.2012.350
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older P (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39(4):412–423. https://doi.org/10.1093/ageing/afq034
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15(2):95–101. https://doi.org/10.1016/j.jamda.2013.11.025
Carvalho do Nascimento PR, Poitras S, Bilodeau M (2018) How do we define and measure sarcopenia? Protocol for a systematic review. Syst Rev 7(1):51. https://doi.org/10.1186/s13643-018-0712-y
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006. https://doi.org/10.1139/H08-075
Jin WH, Mellon EA, Frakes JM, Murimwa GZ, Hodul PJ, Pimiento JM, Malafa MP, Hoffe SE (2018) Impact of sarcopenia in borderline resectable and locally advanced pancreatic cancer patients receiving stereotactic body radiation therapy. J Gastrointest Oncol 9(1):24–34. https://doi.org/10.21037/jgo.2017.09.13
Shen Y, Hao Q, Zhou J, Dong B (2017) The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: a systematic review and meta-analysis. BMC Geriatr 17(1):188. https://doi.org/10.1186/s12877-017-0569-2
Miller JA, Harris K, Roche C, Dhillon S, Battoo A, Demmy T, Nwogu CE, Dexter EU, Hennon M, Picone A, Attwood K, Yendamuri S (2018) Sarcopenia is a predictor of outcomes after lobectomy. J Thorac Dis 10(1):432–440. https://doi.org/10.21037/jtd.2017.12.39
Rossi S, Di Noia V, Tonetti L, Strippoli A, Basso M, Schinzari G, Cassano A, Leone A, Barone C, D'Argento E (2018) Does sarcopenia affect outcome in patients with non-small-cell lung cancer harboring EGFR mutations? Future Oncol 14:919–926. https://doi.org/10.2217/fon-2017-0499
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16. https://doi.org/10.1186/1745-6215-8-16
Schmidt FL, Oh IS, Hayes TL (2009) Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol 62(Pt 1):97–128. https://doi.org/10.1348/000711007X255327
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Chen WZ, Chen XD, Ma LL, Zhang FM, Lin J, Zhuang CL, Yu Z, Chen XL, Chen XX (2018) Impact of visceral obesity and sarcopenia on short-term outcomes after colorectal cancer surgery. Dig Dis Sci 63:1620–1630. https://doi.org/10.1007/s10620-018-5019-2
Choi MH, Oh SN, Lee IK, Oh ST, Won DD (2018) Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle 9(1):53–59. https://doi.org/10.1002/jcsm.12234
Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, Xiao J, Alexeeff S, Corley D, Weltzien E, Castillo AL, Caan BJ (2017) Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS Study. JAMA Oncol 3(12):e172319. https://doi.org/10.1001/jamaoncol.2017.2319
Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, Zhou CJ, Shen X, Yu Z (2015) Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Color Dis 17(11):O256–O264. https://doi.org/10.1111/codi.13067
Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, Kennedy RH, Fearon KC, Jenkins JT (2016) Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg 103(5):572–580. https://doi.org/10.1002/bjs.10075
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Yoshida M, Watanabe M, Baba H (2015) Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol 22(8):2663–2668. https://doi.org/10.1245/s10434-014-4281-6
Nakanishi R, Oki E, Sasaki S, Hirose K, Jogo T, Edahiro K, Korehisa S, Taniguchi D, Kudo K, Kurashige J, Sugiyama M, Nakashima Y, Ohgaki K, Saeki H, Maehara Y (2018) Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today 48(2):151–157. https://doi.org/10.1007/s00595-017-1564-0
Pedziwiatr M, Pisarska M, Major P, Grochowska A, Matlok M, Przeczek K, Stefura T, Budzynski A, Klek S (2016) Laparoscopic colorectal cancer surgery combined with enhanced recovery after surgery protocol (ERAS) reduces the negative impact of sarcopenia on short-term outcomes. Eur J Surg Oncol 42(6):779–787. https://doi.org/10.1016/j.ejso.2016.03.037
Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewe KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, Poeze M (2015) Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 261(2):345–352. https://doi.org/10.1097/sla.0000000000000628
Sueda T, Takahasi H, Nishimura J, Hata T, Matsuda C, Mizushima T, Doki Y, Mori M (2018) Impact of low muscularity and myosteatosis on long-term outcome after curative colorectal cancer surgery: a propensity score-matched analysis. Dis Colon Rectum 61(3):364–374. https://doi.org/10.1097/DCR.0000000000000958
van der Kroft G, Bours D, Janssen-Heijnen DM, van Berlo D, Konsten D (2018) Value of sarcopenia assessed by computed tomography for the prediction of postoperative morbidity following oncological colorectal resection: a comparison with the malnutrition screening tool. Clin Nutr ESPEN 24:114–119. https://doi.org/10.1016/j.clnesp.2018.01.003
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127(5 Suppl):990S–991S
Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B (2017) Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas 103:16–22. https://doi.org/10.1016/j.maturitas.2017.04.007
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67. https://doi.org/10.1016/j.ejca.2015.12.030
Gani F, Buettner S, Margonis GA, Sasaki K, Wagner D, Kim Y, Hundt J, Kamel IR, Pawlik TM (2016) Sarcopenia predicts costs among patients undergoing major abdominal operations. Surgery 160(5):1162–1171. https://doi.org/10.1016/j.surg.2016.05.002
Lee SJ, Park YJ, Cartmell KB (2018) Sarcopenia in cancer survivors is associated with increased cardiovascular disease risk. Support Care Cancer 26:2313–2321. https://doi.org/10.1007/s00520-018-4083-7
Nipp RD, Fuchs G, El-Jawahri A, Mario J, Troschel FM, Greer JA, Gallagher ER, Jackson VA, Kambadakone A, Hong TS, Temel JS, Fintelmann FJ (2018) Sarcopenia is associated with quality of life and depression in patients with advanced cancer. Oncologist 23(1):97–104. https://doi.org/10.1634/theoncologist.2017-0255
Souza BU, Souza NCS, Martucci RB, Rodrigues VD, Pinho NB, Gonzalez MC, Avesani CM (2018) Factors associated with sarcopenia in patients with colorectal cancer. Nutr Cancer 70(2):176–183. https://doi.org/10.1080/01635581.2018.1412480
Stene GB, Helbostad JL, Balstad TR, Riphagen II, Kaasa S, Oldervoll LM (2013) Effect of physical exercise on muscle mass and strength in cancer patients during treatment—a systematic review. Crit Rev Oncol Hematol 88(3):573–593. https://doi.org/10.1016/j.critrevonc.2013.07.001
Strasser B, Steindorf K, Wiskemann J, Ulrich CM (2013) Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc 45(11):2080–2090. https://doi.org/10.1249/MSS.0b013e31829a3b63
Baracos VE (2015) Skeletal muscle anabolism in patients with advanced cancer. Lancet Oncol 16(1):13–14. https://doi.org/10.1016/S1470-2045(14)71185-4
Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, Mazurak VC (2011) Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 117(8):1775–1782. https://doi.org/10.1002/cncr.25709
Qiu MZ, Pan WT, Lin JZ, Wang ZX, Pan ZZ, Wang FH, Yang DJ, Xu RH (2018) Comparison of survival between right-sided and left-sided colon cancer in different situations. Cancer Med 7:1141–1150. https://doi.org/10.1002/cam4.1401
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent.
Rights and permissions
About this article
Cite this article
Sun, G., Li, Y., Peng, Y. et al. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis 33, 1419–1427 (2018). https://doi.org/10.1007/s00384-018-3128-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-018-3128-1