Abstract
Purpose
The aim of this study was to assess the association between sarcopenia and cardiovascular disease (CVD) risk in cancer survivors.
Methods
We analyzed a consecutive series of 683 cancer survivors from the Korean National Health and Nutritional Exam Survey (2008–2011 years). Sarcopenia was defined as the appendicular skeletal muscle mass divided by weight (Kg) < 1 standard deviation below the sex-specific healthy population aged 20–39 years. CVD risks were assessed using the Framingham Risk Score (FRS), which were divided by tertile. Predictors of higher shift of FRS tertile by sex were calculated by stratified ordinal logistic regression analyses.
Results
Proportions of sarcopenia were 24.2% in males and 22.5% in females. Sarcopenic survivors were more likely to have a higher body mass index, waist circumference, blood pressure and fasting glucose level, and a lower high-density lipoprotein compared to those without sarcopenia. Sarcopenia was associated with a higher shift of FRS tertile (common odds ratio, 2.67; 95% confidence interval, 1.18–6.52, P < 0.001) in males. However, this association was not significant in female survivors.
Conclusions
Sarcopenia was associated with an increased CVD risk in Korean male cancer survivors. Interventions to prevent sarcopenia may be necessary to improve cardiovascular burden in cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increase in cancer survivors has generated considerable interest in long-term and late effects resulting from a variety of cancer therapies, chronic disease management strategies, and quality of life and psychosocial issues that are associated with long-term survival [1, 2]. The American Society of Clinical Oncology’s National Coalition for Cancer Survivorship has emphasized the need for an individualized comprehensive approach to survivorship care to include smoking cessation, nutrition and exercise to prevent cancer recurrence, cardiovascular disease (CVD), and mortality [3]. In particular, CVD is the most common cause of illness and death worldwide in the general population and has recently attracted attention as a major health problem for cancer survivors. One out of four cancer survivors had cardiovascular disease, and the rate of first hospitalization for cardiovascular disease was 30% higher than that of the general population, and these associations remained significant until 70 years of age [4]. In cancer patients, cardiac dysfunction, including hypertension, can be caused by anthracycline-based chemotherapy [5], radiation neat the mediastinum, and monoclonal antibodies such as trastuzumab and bevacizumab [6,7,8]. In addition, hormonal therapy such as androgen deprivation therapy or aromatase inhibitor-based therapy can increase the risk of developing CVD by exacerbating the lipid profile and increasing the incidence of hypercholesterolemia [9]. Aging, cigarette smoking, physical inactivity, overweight and obesity, hypertension, dyslipidemia, and hyperglycemia are well-known risk factors in the development of CVD. However CVD can be prevented by identifying and controlling risk factors. Therefore, it is important to evaluate the risk of developing CVD and to control risk factors according to one’s risk profile. Several CVD risk assessment methods have been developed to predict the risk of CVD, and the Framingham Risk Score (FHS) calculation classifies those with a risk of CVD greater than 20% within 10 years as a high-risk group [10].
Sarcopenia was once considered to be a decrease in muscle mass and function related to aging [11]; more recently it has been defined as a decrease in muscle strength and muscle mass due to chronic illness, chronic inflammation, or improper nutrition [12]. Sarcopenia prevalence estimates range from 16 to 23% among cancer survivors [13, 14]. Cancer patients with sarcopenia are more likely to have increased chemotherapy toxicity [15], shorter time to tumor progression, and more serious complications and mortality after cancer surgery than those without sarcopenia [16]. In cancer survivors, sarcopenia is a growing concern as a factor that may lead to treatment complications and poor prognosis. Several studies in the general population have reported that sarcopenia is associated with functional limitation, physical disability, poor quality of life; and that the lower the muscle mass, the higher the risk of morbidity and mortality [17, 18]. In addition, sarcopenia is a risk factor for hyperlipidemia and insulin resistance, which increases the risk of CVD [19]. However, it is not known whether these results observed in the general population apply equally to cancer survivors. Thus, this study aims to investigate the relationship between sarcopenia and CVD, considering that CVD represents a major health problem for cancer survivors.
Methods
Study design and participants
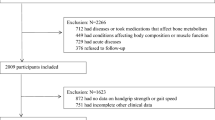
This study was conducted as a cross-sectional analysis of the fourth period (2007–2009) and the fifth period (2010–2011) of the Korea National Health and Nutrition Examination Survey (KNHANES) conducted by the Korea Center for Disease Control. This study was conducted after approval of exemption from review by the Institutional Board and Ethics Committee (IRB number 2017-23) The KNHANES is conducted nationwide between January and December of each year. The survey consists of a health interview, a nutrition survey, and a health examination. Following study enrollment, health questionnaires are administered by an interviewer; and screening is performed by direct measurement, observation, and lab samples. The subjects were stratified according to the characteristics of city, province, and region and were extracted using a two-step random sampling method on the basis of population ratio by sex and age group. In the fourth period (2007–2009), surveys were conducted in 200 population sites and 4600 households nationwide. In the fifth period (2010–2011), the surveys were performed in 192 population sites and 3800 households with the resident population in 2009 as the standard sample. The survey response rate was 78.4% in the fourth period and 80% in the fifth period. In 2008–2011, the total number of participants was 37,753, of whom 28,377 were over 19 years of age. Muscle mass was measured using dual energy X-ray absorptiometry (DEXA), which was introduced from July 2008 to May 2011. Participants without DEXA (n = 9271) and missing clinical information (n = 306) were excluded, and the remaining 28,377 subjects were screened from the KNHANES data. Among the 798 respondents who answered “Yes” to the question “Do you have a diagnosis of cancer from a doctor?”, 115 subjects were excluded who had missing blood tests, resulting in a final sample size of 683 subjects for the current study analysis.
Demographic and clinical characteristics
Gender of the subjects, age, income level, marital status, years of education, exercise level, and drinking and smoking habits were included. Income level was defined as the average monthly household income (monthly household income√number of households), and divided into four quartiles (lower, lower middle, upper middle, upper). Marital status was classified as “living with a spouse or domestic partners” or as “single, divorced, or widowed.” Years of education was categorized as 6 years or less, 7 to 9 years, 10 to 12 years, or 13 years or more. Regular exercise was defined as performing at least 30 min of moderate-intensity aerobic activity at least 5 days per week for a total of 150 min, or at least 25 min of vigorous aerobic activity at least 3 days per week for a total of 75 min [20]. Current drinker was defined as a person who drank one or more drinks/month for the past year [21]. Type of cancer was classified as gastric, liver, colon, breast, cervical, lung and other cancers. Survival time, calculated as the difference between the current age at the completion of the questionnaire and the age at which the cancer was diagnosed, was classified as ≥ 6 or < 6 years of duration.
Anthropometric characteristics
Health screening for the KNHANES was conducted by direct measurement, observation, and sample analysis. Direct measurements of height, weight, waist circumference, and blood pressure (average systolic and diastolic) were performed in this study. Body measurements were recorded by a trained examiner who had participants take off shoes while wearing the examination clothes. Waist circumference was measured from the midpoint between the lowest rib and the pelvic iliac crest after the subject relaxed both arms comfortably in the normal exhalation state. Systolic and diastolic blood pressures were measured three times after a 5-min rest in the sitting position by the trained person and the averaged value was used.
Biochemical measurements
Blood samples were collected after fasting for at least 8 h. Serum total cholesterol, triglyceride, high-density lipoprotein cholesterol, and fasting blood glucose were measured by enzymatic methods using a Hitachi automatic analyzer 7600 (Tokyo, Japan).
Measurement of sarcopenia
Bone mineral contents (g), fat mass (g), and regional lean mass (g) of each body part were measured using dual energy X-ray absorptiometry (DEXA). The appendicular skeletal muscle mass (ASM) was calculated as the sum of upper and lower muscles minus bones and fat [11]. Muscle mass was calculated as the weight of the ASM divided by the body weight (ASM/total body weight (%)), and sarcopenia was defined as values less than 1 standard deviation of the reference group of 20–39 years old [22, 23]. We excluded subjects with certain diseases (n = 1623) such as hypertension, dyslipidemia, stroke, myocardial infarction, angina pectoris, osteoarthritis, rheumatoid arthritis, pulmonary tuberculosis, asthma, diabetes, thyroid disease, and cancer from the adult reference group because these chronic wasting condition can affect the muscle mass of the individual subject. Among the 8961 subjects aged 20–39, 7338 subjects were the final adult reference group. The cut-off value for sarcopenia was 30.2% for males and 23.8% for females.
Cardiovascular disease risk
The CVD risk assessment tool used was the FHS, which was introduced in 2008 as a general CVD risk profile for use in primary care [10]. Total risk scores were calculated by gender and points assigned for each of the following risk factors which are age (0 to 12 for women; 0 to 15 for men), total cholesterol (0 to 5 for women; 0 to 4 for men), high-density lipoprotein cholesterol (HDL-C, − 2 to 2 for women and men), smoking status (0 to 3 for women; 0 to 4 for men), diabetes (0 to 4 for women; 0 to13 for men), and systolic blood pressure by the degree of hypertension control (− 3 to 7 for women; − 2 to 5 for men). The risk of developing CVD for each subject in the next 10 years was calculated according to this risk score [10]. Low risk was defined as less than 10% risk of CVD in the next 10 years, moderate risk as 10–20%, and high risk as 20% or higher [24].
Statistical analysis
The baseline characteristics were described in terms of gender and how the anthropometric, clinical, and cancer-related characteristics can differ by the presence of the sarcopenia. Binary variables were compared with Pearson’s chi-square or Fisher’s exact test and continuous variables with Student’s t test or Mann-Whitney U test, respectively. Because FRS is already known to have a non-normal distribution, we divided these scores into tertiles. We performed analysis of variance or Kruskal-Wallis test and Chi-square test to compare the baseline characteristics according to FRS tertile. We analyzed which variables were associated with the higher shift of FRS tertile with a multivariable ordinal logistic regression analysis, and these associations were expressed as a common odds ratio (cOR). All variables with a P value < 0.10 in univariable ordinal logistic regression analysis were entered into the multivariable model. A two-sided P value of < 0.05 was considered statistically significant. We performed these analyses using IBM SPSS Statistical Software 22.0 (IBM SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical characteristics
Table 1 displays the demographics and clinical characteristics of the study population by gender and sarcopenia status. Of the 683 subjects, 248 (36.3%) were males and 435 (63.7%) were females. The mean age of the males and females were 64.6 (± 11.7) and 58.5 (± 12.6) years, respectively. The proportion of gastric cancer was 22.0% (150 out of 683), followed by cervical (15.7%), breast (13.6%), colorectal (8.8%), liver (3.1%), and lung (2.5%) cancer.
Demographic and clinical characteristics of cancer survivors with sarcopenia
Sixty (24.2%) of the males and 98 (22.5%) of the females were sarcopenic. The mean age, body mass index, waist circumference, systolic, diastolic blood pressure, and fasting blood glucose were higher, and HDL cholesterol level was lower in subjects who had sarcopenia compared to those without sarcopenia, irrespective of gender. In males, 28.3% of those with sarcopenia had a diabetes diagnosis, which was higher than for those without sarcopenia (17.0%); but prevalence of hyperlipidemia was not different between the two groups. On the other hand, 23.5% of the sarcopenic females had a diagnosis of hyperlipidemia, which was higher compared to non-sarcopenic females (14.2%). Additionally, females with sarcopenia had a statistically significant difference in total cholesterol, triglyceride, and high density cholesterol, compared to non-sarcopenic females. There were no statistically significant differences between the overall sarcopenic and non-sarcopenic groups in terms of income level, marital status, educational status, regular exercise, smoking status, drinking status, and time since cancer diagnosis.
Cardiovascular disease risk
Table 2 presents the characteristics of the study sample according to FRS tertile. The proportion of CVD risk in male cancer survivors was 156 (62.9%) in the high risk group, 50 (20.2%) in the moderate risk, and 42 (16.9%) in the low risk group. Among females, 50 were high risk (11.5%), 101 were moderate risk (23.2%), and 284 were low risk (65.3%). The lower the household income and educational status, the higher the observed risk of CVD. The risk for CVD was highest among gastric and colorectal cancer compared to other cancer types.
Sarcopenia and cardiovascular disease risk
Table 3 displays predictors of being in a higher FRS tertile from the univariable ordinal logistic regression analyses. Sarcopenia and age were positively associated with being in a higher FRS tertile in both gender groups. In males, household income, being married/living with a partner, and regular exercise were both associated with being in a higher FRS tertile. In females, BMI and cancer duration increased the risk, and household income, educational years, being married/living with a partner, and current drinking decreased the risk of being in a higher FRS tertile. Type of cancer was not associated with FRS tertile. In Table 4, sarcopenia was a significant predictor of being in a higher FRS tertile, adjusting for age, household income, years of education, being married/living with a partner, regular exercise, and cancer duration. In multivariable ordinal logistic analysis, sarcopenia in males was associated with an increased cOR (2.67; 95% confidence interval, 1.18–6.52, P < 0.001) of being in a higher FRS even after adjusting duration of cancer. However, sarcopenia was not associated with an increased cOR of being in a higher FRS tertile among the female group in a multivariable model.
Discussion
This study was conducted to analyze the effect of sarcopenia on CVD risk in cancer survivors. The CVD risk was assessed using the FRS. A number of CVD risk assessment methods have been developed to predict the risk of developing CVD. Metabolic syndrome and FRS are widely used risk predictors in clinical practice, and metabolic syndrome status is highly correlated with the risk of CVD. However, recent large-scale prospective studies have shown that the FRS is superior to the metabolic syndrome in predicting CVD, including coronary artery disease [25].
In this study, sarcopenia was associated with an increase in FRS tertile. In particular, in male cancer survivors, sarcopenia was a significant predictor of increased 10-year CVD risk predicted by the FRS, even when adjusted for demographic variables such as sex, household income, educational year, and marital status. However, this relationship was not statistically significant in female cancer survivors. In this study, obese subjects with sarcopenia had a higher risk of developing CVD in the next 10 years than those without sarcopenia. Interestingly, the FRS was significantly higher in the male group than in the female group (Fig. 1). In the previous study, gender was reported to be an important risk factor in the development of hypertension [26]. Activation of the renin-angiotensin-aldosterone system (RAAS) is associated with elevated blood pressure and the development of hypertension, and estrogen has a beneficial effect on cardiovascular function by acting directly on RAAS [27]. In addition, premenopausal women have a lower blood pressure than men of the same age group, and these differences between men and women gradually decrease after menopause [28].
In this study, the difference in the FRS risk distribution by gender was considered to be due to the fact that males were more likely to be older, have higher systolic blood pressure, and be smokers, compared to females. In a number of studies investigating smoking status in cancer survivors, 59.6% of Koreans [29] continue to smoke after cancer diagnosis, and prevalence of smoking is higher in men than in women [30]. In the current study, 52% of male cancer survivors and 4.7% of female cancer survivors continued to smoke after a cancer diagnosis. These factors may help to explain the difference in CVD risk distribution by gender. In our study, 81.7% of male cancer survivors with sarcopenia were in the high-risk group for CVD. Sarcopenia is associated with daily lifestyle habits such as low physical activity, inadequate nutrition, smoking, and drinking [14]. Cancer survivors are at risk for sarcopenia due to side effects such as inadequate nutrition due to chemotherapy- and radiotherapy-induced gastrointestinal side effects [31], and physical inactivity and mobility difficulty are higher in cancer patients than in the general population [32]. Moreover, interleukin-1, interleukin-6, and Tumor necrosis factor-α, which are pro-inflammatory cytokines secreted from tumors, may cause loss of appetite [33] and promote the degradation of myofibrillar protein and reduce protein synthesis, resulting in muscle wasting [34]. In this study, the FRS was higher in male cancer survivors with sarcopenia, and 24.2% of men and 22.5% of women had sarcopenia. In particular, the difference in CVD risk by gender may be caused by the difference in muscle mass between men and women. Jassen et al. studied the cross-sectional relationship between aging and change of skeletal muscle mass, which was measured by magnetic resonance image. They showed that there was a decrease in absolute skeletal muscle mass after the end of the fifth decade, and the decrease of muscle mass were 1.9 and 1.1 kg/decade in the men and women, respectively [35]. Additionally, in terminal cancer patients, 59% of males and 28% of females were sarcopenic, and the decrease of muscle mass in males was relatively higher than in females [36]. Insulin, growth hormone, insulin-like growth factor-1, and testosterone (relevant to males) also affect sarcopenia, as they are involved in protein metabolism and catabolism and affect muscle mass and muscle strength, potentially increasing the risk of dyslipidemia and increasing the risk of CVD [37].
The relationship between socioeconomic status and CVD is well-known. The lower the income level, the higher the prevalence of smoking, hypercholesterolemia, hypertension, diabetes mellitus, and the risk of ischemic heart disease in males. Degree of educational attainment has also been reported to be associated with a higher risk of CVD [38], which mirrors our study results. In addition, the risk of CVD was higher in gastric and colorectal cancer survivors than those with the other type of cancer in our study. The development of gastric cancer is related with eating habit in Koreans, especially high salt intake which is a cause of both CVD and gastric cancer [39]. In a previous study of the beneficial effect of salt intake restriction on blood pressure, the low-salt diet group had lower systolic and diastolic blood pressures than the control group [40]. Moreover, the toxicity of anti-cancer drugs, which are commonly used in the treatment of gastric cancer such as fluoropyrimidine, has also been reported [41]. However, the high risk of CVD in the gastric cancer survivors is attributed to the male predominance (65.2%) in these cancer groups.
These findings suggest that sarcopenia is a risk factor for CVD risk, especially in male cancer survivors, and sarcopenia must be addressed as a health issue for cancer survivors. In most previous studies, DEXA, bioelectrical impedance analysis, and computed tomography were used to diagnose sarcopenia [42]. However, use of these diagnostic methods is not widely used in the early diagnosis of sarcopenia due to cost and the need for special equipment in clinical practice. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) presented a simple two-step algorithm to diagnose sarcopenia using gait speed assessment and handgrip strength. If the subject’s gait speed is 0.8 m/s or faster, handgrip strength is measured, and in subjects with low grip strength, muscle mass is then measured. In the second step, muscle mass is measured in those subjects with gait speed of slower than 0.8 m/s. If the handgrip strength is low, a muscle mass test is recommended to diagnose sarcopenia [43]. Health care managers who care for cancer survivors should be able to easily screen for sarcopenia using the above criteria.
This study has several limitations. First, our study is a retrospective case-control study using KNHANES data; therefore, these results can be used to evaluate the association between sarcopenia and CVD, but not to establish a causal relationship. Second, the risk stratification of CVD assessed by the FRS was developed based on studies of Caucasians, and so the relationship between sarcopenia and CVD risk may be different in other racial groups. Third, muscle strength and muscle mass should be considered together in evaluating sarcopenia, but data on muscle strength were not available within the context of this study. In cancer survivors, the degree and severity of adverse effects of cancer treatments such as chemotherapy, radiotherapy, hormone therapy, and steroid may influence the extent of muscle mass, but data on these variables were not available for analysis in this study. Despite these limitations, this study is meaningful because CVD risk associated with sarcopenic cancer survivors was quantified according to the FRS, and thus it is possible to select a high-risk sarcopenic group for future CVD in cancer survivors.
In summary, there was a difference in the CVD risk distribution by sex in cancer survivors, with men having a greater risk than women. Among men, BMI, waist circumference, blood pressure, and fasting blood glucose were higher, and HDL cholesterol was lower in men, as compared to women. Of important note, being a male cancer survivor with sarcopenia is associated with an increased risk of developing CVD within 10 years. In order to prevent CVD in cancer survivors, it is necessary to emphasize the need to screen for high CVD risk and to provide dietary control and exercise intervention programs to those with elevated risk to prevent the development of sarcopenia. Prospective studies are needed to determine whether prevention and intervention to address sarcopenia in cancer survivors will reduce the development of CVD and improve the long-term prognosis in cancer survivors.
References
Adams E, Boulton M, Horne A et al (2014) The effects of pelvic radiotherapy on cancer survivors: symptom profile, psychological morbidity and quality of life. Clin Oncol 26(1):10–17. https://doi.org/10.1016/j.clon.2013.08.003
Moser EC, Meunier F (2014) Cancer survivorship: a positive side-effect of more successful cancer treatment. EJC Suppl 12(1):1–4. https://doi.org/10.1016/j.ejcsup.2014.03.001
McCabe MS, Bhatia S, Oeffinger KC et al (2013) American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol 31(5):631–640. https://doi.org/10.1200/JCO.2012.46.6854
Rugbjerg K, Mellemkjær L, Boice JD, Køber L, Ewertz M, Olsen JH (2014) Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943–2009. J Natl Cancer Inst 106(6). https://doi.org/10.1093/jnci/dju110
Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB (2008) Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 26(22):3777–3784. https://doi.org/10.1200/JCO.2007.14.9401
Ewer SM, Ewer MS (2008) Cardiotoxicity profile of trastuzumab. Drug Saf 31(6):459–467. https://doi.org/10.2165/00002018-200831060-00002
Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, Bellmunt J, Burstein HJ, Schutz FAB (2011) Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol 29(6):632–638. https://doi.org/10.1200/JCO.2010.31.9129
Okwuosa TM, Anzevino S, Rao R (2017) Cardiovascular disease in cancer survivors. Postgrad Med J 93(1096):82–90. https://doi.org/10.1136/postgradmedj-2016-134417
Mouridsen H, Keshaviah A, Coates AS, Rabaglio M, Castiglione-Gertsch M, Sun Z, Thürlimann B, Mauriac L, Forbes JF, Paridaens R, Gelber RD, Colleoni M, Smith I, Price KN, Goldhirsch A (2007) Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: safety analysis of BIG 1-98 trial. J Clin Oncol 25(36):5715–5722. https://doi.org/10.1200/JCO.2007.12.1665
D’Agostino RB, Vasan RS, Pencina MJ et al (2008) General cardiovascular risk profile for use in primary care. Circulation 117(6):743–753. https://doi.org/10.1161/CIRCULATIONAHA.107.699579
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763. https://doi.org/10.1093/oxfordjournals.aje.a009520
Muscaritoli M, Anker S, Argiles J et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by special interest groups (SIG)“cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 29(2):154–159. https://doi.org/10.1016/j.clnu.2009.12.004
Lee SJ, Kim NC (2017) Association between sarcopenia and metabolic syndrome in cancer survivors. Cancer Nurs 40(6):479–487. https://doi.org/10.1097/NCC.0000000000000454
Villaseñor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, Neuhouser ML (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL study. J Cancer Surviv 6(4):398–406. https://doi.org/10.1007/s11764-012-0234-x
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15(8):2920–2926. https://doi.org/10.1158/1078-0432.CCR-08-2242
Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, Tanikawa S, Maeda S, Uemura M, Miyake M, Hama N, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M, Fujitani K, Tsujinaka T (2016) Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 19(3):986–993. https://doi.org/10.1007/s10120-015-0546-4
Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L (2003) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 95(5):1851–1860. https://doi.org/10.1152/japplphysiol.00246.2003
Srikanthan P, Karlamangla AS (2011) Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and nutrition examination survey. J Clin Endocrinol Metab 96(9):2898–2903. https://doi.org/10.1210/jc.2011-0435
Karakelides H, Nair KS (2005) Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol 68:123–148. https://doi.org/10.1016/S0070-2153(05)68005-2
Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A, American College of Sports Medicine, American Heart Association (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116(9):1081–1093. https://doi.org/10.1161/CIRCULATIONAHA.107.185649
Schoonen WM, Salinas CA, Kiemeney LA et al (2005) Alcohol consumption and risk of prostate cancer in middle-aged men. Int J Cancer 113(1):133–140. https://doi.org/10.1002/ijc.20528
Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, Song G, Kim HJ, Choi YJ, Kim KM Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the fourth Korean National Health and nutritional examination surveys. J Gerontol A Biol Sci Med Sci 2012;67(10):1107–1113, DOI: https://doi.org/10.1093/gerona/gls071
Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC (2010) Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean longitudinal study on health and aging (KLoSHA). Diabetes Care 33(7):1652–1654. https://doi.org/10.2337/dc10-0107
Ford ES, Giles WH, Mokdad AH (2004) The distribution of 10-year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol 43(10):1791–1796. https://doi.org/10.1016/j.jacc.2003.11.061
Wannamethee SG, Shaper AG, Lennon L, Morris RW (2005) Metabolic syndrome vs Framingham risk score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 165(22):2644–2650. https://doi.org/10.1001/archinte.165.22.2644
Bu S, Ruan D, Yang Z, Xing X, Zhao W, Wang N, Xie L, Yang W (2015) Sex-specific prevalence of diabetes and cardiovascular risk factors in the middle-aged population of China: a subgroup analysis of the 2007–2008 China National Diabetes and metabolic disorders study. PLoS One 10(9):e0139039. https://doi.org/10.1371/journal.pone.0139039
Xue B, Johnson AK, Hay M (2013) Sex differences in angiotensin II-and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Physiol Regul Integr Comp Physiol 305(5):R459–R463. https://doi.org/10.1152/ajpregu.00222.2013
Reckelhoff JF (2001) Gender differences in the regulation of blood pressure. Hypertension 37(5):1199–1208. https://doi.org/10.1161/01.HYP.37.5.1199
Kim H, Kim MH, Park YS, Shin JY, Song YM (2015) Factors that predict persistent smoking of cancer survivors. J Korean Med Sci 30(7):853–859. https://doi.org/10.3346/jkms.2015.30.7.853
Park SJ, Kim BC, Han HC, Kim SY, Gwak JI, Lee JK (2009) Effect of cancer diagnosis on smoking behavior. Korean J Fam Med 30(9):681–687. https://doi.org/10.4082/kjfm.2009.30.9.681
Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM (2016) Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc 75(2):199–211. https://doi.org/10.1017/S002966511500419X
Florin TA, Fryer GE, Miyoshi T, Weitzman M, Mertens AC, Hudson MM, Sklar CA, Emmons K, Hinkle A, Whitton J, Stovall M, Robison LL, Oeffinger KC (2007) Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. Cancer Epidemiol Biomark Prev 16(7):1356–1363. https://doi.org/10.1158/1055-9965.EPI-07-0048
Plata-Salamán CR, Oomura Y, Kai Y (1988) Tumor necrosis factor and interleukin-1β: suppression of food intake by direct action in the central nervous system. Brain Res 448(1):106–114. https://doi.org/10.1016/0006-8993(88)91106-7
Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC (2002) TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab 282(2):E336–E347. https://doi.org/10.1152/ajpendo.00366.2001
Janssen I, Heymsfield SB, Wang Z, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89(1):81–88. https://doi.org/10.1152/jappl.2000.89.1.81
Wallengren O, Iresjö B-M, Lundholm K, Bosaeus I (2015) Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer 23(1):79–86. https://doi.org/10.1007/s00520-014-2332-y
Walrand S, Guillet C, Salles J, Cano N, Boirie Y (2011) Physiopathological mechanism of sarcopenia. Clin Geriatr Med 27(3):365–385. https://doi.org/10.1016/j.cger.2011.03.005
Saidi O, Malouche D, O'Flaherty M, Ben Mansour N, A Skhiri H, Ben Romdhane H, Bezdah L (2016) Assessment of cardiovascular risk in Tunisia: applying the Framingham risk score to national survey data. BMJ Open 6(11):e009195. https://doi.org/10.1136/bmjopen-2015-009195
Kim YJ, Kim CH, Meta SSJ (2002) Analysis for the relation between Korean dietary factors and stomach cancer. J Korean Acad Fam Med 23(9):1098–1106
He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, Chen JC, Duan X, Huang JF, Chen CS, Kelly TN, Bazzano LA, Whelton PK, GenSalt Collaborative Research Group (2009) Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens 27(1):48–54. https://doi.org/10.1097/HJH.0b013e328316bb87
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K, ACTS-GC Group (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820. https://doi.org/10.1056/NEJMoa072252
Yu SC, Khow KS, Jadczak AD et al (2016) Clinical screening tools for sarcopenia and its management. Curr Gerontol Geriatr Res 2016:1–10. https://doi.org/10.1155/2016/5978523
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis report of the European working group on sarcopenia in older people. Age Ageing 39(4):412–423. https://doi.org/10.1093/ageing/afq034
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was conducted solely using a de-identified research dataset, with approval of exemption from review by the Institutional Board and Ethics Committee (IRB number 2017-23).
Informed consent
Informed consent was not required for this study, given the use of secondary data that contained no patient identifiers.
Rights and permissions
About this article
Cite this article
Lee, S.J., Park, Y.J. & Cartmell, K.B. Sarcopenia in cancer survivors is associated with increased cardiovascular disease risk. Support Care Cancer 26, 2313–2321 (2018). https://doi.org/10.1007/s00520-018-4083-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4083-7