Abstract
Background
The relationship between computed tomography (CT)-assessed sarcopenia and colorectal cancer (CRC) prognosis varies in different studies. This systematic review aimed to examine the impact of preoperative CT-assessed sarcopenia on complications and long-term survival in CRC patients.
Methods
The PubMed, Web of Science, Cochrane Library, and Embase databases were searched for relevant literature up to September 10, 2020. Data and characteristics for each study were extracted. Long-term outcomes were assessed using a comprehensive HR with a 95% CI. Complications were assessed using a comprehensive OR with 95% CI. The heterogeneity and publication bias were also investigated, and subgroup and sensitivity analyses were performed.
Results
A total of 19 studies comprising 15,889 patients were included. The comprehensive results demonstrated that sarcopenia is significantly associated with overall survival of CRC patients (HR = 1.40, 95% CI = 1.25–1.58, p < 0.001). Patients with sarcopenia have a higher risk of complications compared to those without sarcopenia. In addition, sarcopenia is strongly associated with poor cancer-specific survival (HR = 1.49, 95% CI = 1.32–1.68, p < 0.001) and disease-free survival (HR = 1.59, 95% CI = 1.32–1.92, p < 0.001) in CRC patients. There is no significant relationship between sarcopenia and recurrence-free survival (HR = 1.32, 95% CI = 0.92–1.89, p = 0.126).
Conclusions
Preoperative CT-assessed sarcopenia can be employed as an effective predictor of complications and long-term prognosis in CRC patients. Standardization of CT-assessed sarcopenia requires comprehensive consideration of race, muscle mass index, body mass index, and gender.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common gastrointestinal cancers with a high incidence and mortality rate. CRC had a global estimated incidence of 1.8 million cases (10.2% of all new cases) and mortality of 861,000 cases (9.2% of all cancer deaths) in 2018 [1]. According to the latest 2020 statistics, CRC has become the second most common cancer and second leading cause of all cancer deaths in the USA [2]. Surgical treatment and radiochemotherapy are still the most effective means to improve the CRC patient survival rate. Recently, the rapid development of gene detection and biological targeting therapy has played a positive role in the treatment of CRC. However, short- and long-term outcomes in CRC patients are still unsatisfactory [3]. Therefore, it is necessary to carry out research on factors that may alter the prognostic stratification of CRC patients.
At present, most studies focus on tumor pathology itself, while the influence of host-related factors on CRC patient prognosis is often ignored. Clinically, the Tumor Node Metastasis (TNM) classification of American Joint Committee on Cancer has been regarded as an important prognostic tool for CRC. However, tumor characteristics are not the only factor influencing prognosis. Many other factors, such as nutritional and immune status, also play an important role in tumor progression and are associated with patient prognosis. More and more studies in recent years have reported that host-related sarcopenia is a risk factor affecting the prognosis of various cancers, such as gastric [4], pancreatic [5], and lung [6] cancers. Sarcopenia is a syndrome characterized by progressive and systemic skeletal muscle mass loss [7]. Sarcopenia has been reported to be associated with numerous causes, such as insulin resistance, anabolic resistance, anorexia, and systemic inflammation [8, 9]. Muscle mass quantification by computed tomography (CT) is a broad and accurate method for assessing sarcopenia. At present, the most commonly used measurement method is to calculate the total skeletal muscle area (cm2) at the level of the third lumbar vertebra, including the psoas muscle, lumbar muscle, erector spinae, transversus abdominis muscle, internal and external oblique muscles, and rectus abdominis, and the third lumbar vertebra skeletal mass index (L3SMI) by dividing by the height squared (m2).
Patients with gastrointestinal tumors are commonly malnourished and are more prone to sarcopenia. This may be the result of the combined effects of malignant disease progression, host tumor response, anti-cancer treatment, and special comorbidities of gastrointestinal tumors (obstruction, bleeding, and perforation). Feliciano et al. conducted a study based on 2470 patients that showed that the combination of CT-assessed sarcopenia and inflammation indicators can effectively predict CRC patient prognosis [10]. Similarly, Dolan et al. proposed that CT-assessed sarcopenia is an important factor affecting long-term survival in CRC patients [11]. In addition, our previous studies confirmed that preoperative CT-assessed sarcopenia is an independent factor affecting complications and long-term prognosis in CRC patients, which can be used to assist the preoperative nutritional assessment of CRC patients [12, 13]. However, Vugt et al. believed that preoperative CT-assessed sarcopenia can be used to assess the risk of complications in CRC patients, but not to assess long-term efficacy [14]. Due to the heterogeneity of different studies, the role of CT-assessed sarcopenia in CRC patient outcomes in diverse populations remains controversial. Sun et al. [15] conducted a meta-analysis in 2018 to explore the prognostic value of CT-assessed sarcopenia in CRC patients. However, there were some limitations due to the small number of studies included. Moreover, many new studies on the relationship between CT-assessed sarcopenia and CRC have emerged in the past 2 years. Therefore, it is necessary to conduct the latest meta-analysis on the basis of existing evidence to investigate the value of preoperative CT-assessed sarcopenia in assessing complications and long-term prognosis in CRC patients.
Materials and methods
Data sources and search
This meta-analysis was strictly based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16]. A systematic search was conducted on the value of preoperative CT-assessed sarcopenia in complications and long-term prognosis in CRC patients in the PubMed, Web of Science, Cochrane Library, and Embase databases using a combination of keywords and free words. The search was restricted to English-language publications up to September 10, 2020. The search terms were “sarcopenia”, “muscle depletion”, “muscle mass” AND “rectal neoplasms”, “colonic neoplasms”, “colon”, “rectum” AND “outcomes”, “survival”, “complications”, "comorbidity", and "prognosis". In addition, potential reviews and meta-analyses were manually examined to identify any other relevant literature that could be included in this study. The complete search strategy is detailed in the supplementary appendix.
Inclusion and exclusion criteria
The eligibility of each study was assessed based on the PICOS framework. Studies meeting the following criteria were included: (1) patients underwent CRC resection, and no other combined tumors and distant metastasis were present; (2) studies reported the prognostic value of preoperative CT-assessed sarcopenia on complications, overall survival (OS), cancer-specific survival (CSS), disease-free survival (DFS), and recurrence-free survival (RFS); (3) the dichotomy cut-off value for L3SMI was reported; (4) the HR and corresponding 95% CI were provided, or can be estimated from Kaplan–Meier survival curve; (5) the study design was limited to comparative studies (randomized controlled trials, case–control studies, retrospective studies, and prospective studies). Studies meeting the following criteria were excluded: (1) CRC patients with other combined tumors or metastases; (2) sarcopenia was not defined using preoperative CT-measured L3SMI; (3) insufficient data or no goal outcomes; (4) sample size < 100; (5) animal studies, reviews, conference abstracts, or letters. When studies were performed at the same center and during the same period, the study with the largest sample size was selected. OS was defined as the time from the diagnosis to death or last follow-up. CSS was defined as the time from the diagnosis to death of CRC or last follow-up. DFS was defined as the time from radical surgery (when there is no tumor lesion in the body) to recurrence, metastasis, or death. RFS was defined as the time from removal of the lesion to recurrence or death.
Literature selection and data extraction
According to the established inclusion and exclusion criteria, two evaluators (H.X. and L.W.) independently screened the literature to select possible eligible studies. Any differences between evaluators were discussed with a third party until agreement was reached. The following information was extracted from each included study: general characteristics, including the first author’s surname, year, country, study design, sample, age, gender ratio, analysis methods, and TNM stage; sarcopenia characteristics, including percent of sarcopenia, cut-off of male and female, sarcopenia prevalence, method, and definition; outcome, including primary (OS and complications) and secondary (CSS, DFS, and RFS) outcomes. The complication outcome in this study refers to total complications. The outcome evaluation of studies that only provided survival curves was completed using Engauge Digitizer v.4.1 software [17]. In addition, two independent evaluators used the Newcastle–Ottawa Scale (NOS) to evaluate the methodological quality of included studies. The NOS score ranged from 0 to 9, and a study with NOS score ≥ 6 was considered to be of high quality.
Statistical analysis
The comprehensive OR and 95% CI were used to evaluate the role of preoperative CT-assessed sarcopenia when assessing the risk of complications in CRC patients. The comprehensive HR and 95% CI were used to estimate the long-term prognostic effect of CT-assessed sarcopenia in CRC patients, including OS, CSS, DFS, and PFS. Heterogeneity between studies was tested using Higgins I2 statistic and Cochran’s Q test. If I2 ≥ 50% or PQ < 0.05, the random effects model was used for statistical analysis. Otherwise, the fixed-effects model was utilized. To explore the source of potential heterogeneity, subgroup and meta-regression analyses were performed. Sensitivity analysis assessed study reliability by omitting one study at a time and examining the impact of each study on the comprehensive results. Potential publication bias was evaluated using Begg’s and Egger’s tests. If publication bias was present, the trim-and-fill method was used to further evaluate the stability of the results. A two-sided p value < 0.05 was considered significant. All statistical analyses were carried out using Stata 12.0 software (Stata Corp, College Station, TX, USA).
Results
Description of included studies
The PRISMA diagram for the study selection is represented in Fig. 1. According to the established search strategy, 1105 studies were initially evaluated, including 166 studies from PubMed, 430 from Web of Science, 137 from Cochrane Library, and 372 from Embase. After deleting duplicates and reviewing titles and abstracts, most of the irrelevant studies were excluded, leaving 53 to be further screened. After carefully reading the full text, 34 studies that did not meet the inclusion requirements were excluded. Ultimately, a total of 19 studies involving 15,889 cases were identified through systematic search [11, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
Study characteristics
Characteristics of the included studies are summarized in Table 1. These studies were published between 2015 and 2020, of which four were prospective comparative studies and 15 were retrospective comparative studies. Of these studies, ten were from Asia (five from China, three from Japan, and two from South Korea) and nine were from outside Asia (two from the UK, one from the USA, three from Canada, one from Finland, one from Sweden, and one from the Netherlands). The sample size median was 494, ranging from 142 to 3262. In addition, the NOS score for these 19 studies ranged from 6 to 8.
Assessment of sarcopenia prevalence
The description of sarcopenia from each study is presented in Table 2. In the present study, the median incidence of sarcopenia was 39.75%, ranging from 11.97 to 68.21%. Among Asian countries, the median prevalence of sarcopenia was 29.09%, ranging from 11.97 to 60.32%. The median prevalence was 43.19% in non-Asian countries, ranging from 27.48 to 59.77%.
Meta-analysis for OS
A total of 14 studies with 14,100 patients explored the prognostic value of CT-assessed sarcopenia for OS in CRC patients (Fig. 2). The comprehensive results showed that sarcopenia was significantly associated with OS in CRC patients (HR = 1.40, 95% CI = 1.25–1.58, p < 0.001). In other words, when compared to patients without sarcopenia, patients with sarcopenia have a worse OS. A random-effects model was used due to apparent heterogeneity (I2 = 54.3%, PQ = 0.008). The sources of heterogeneity were further analyzed based on study type, publication time, country, sample, sarcopenia criterion, and NOS score (Table 3). The results indicated that sarcopenia is an independent influencing factor for poor OS in each subgroup and there was no significant heterogeneity between the sample subgroup. Furthermore, meta-regression analysis indicated that the sample subgroup may be the source of potential heterogeneity (Pdesign = 0.160, Ppublication time = 0.349, Pcountry = 0.476, Psample = 0.047, Psarcopenia criterion = 0.667, and PNOS score = 0.181). Based on the subgroup and meta-regression analyses results, it was speculated that OS meta-analysis heterogeneity might be due to the different sample sizes of each study. In order to verify the stability of the present study, sensitivity analysis was conducted by removing one study at a time (Fig. 3). The results showed that removing any one study has little effect on the comprehensive results, indicating that the present results are reliable. In the analysis of publication bias, research using visual funnel plots was basically symmetrical without obvious publication bias in the Begg’s (p = 0.125; Fig. 4a) and Egger’s (p = 0.115; Fig. 4b) tests.
Meta-analysis for complications
The relationship between preoperative CT-assessed sarcopenia and complications was reported in six studies with 3419 cases (Fig. 5a). According to a fixed effects model (I2 = 50.0%, PQ = 0.075), the combined results showed that sarcopenia was significantly associated with complications (OR = 1.82, 95% CI = 0.36–2.44, p < 0.001). Thus, patients with sarcopenia have a higher risk of complications compared to patients without sarcopenia. Subgroup analysis results further demonstrated that sarcopenia is an independent factor affecting complications in CRC patients independent of all subgroup factors (Table 4). In addition, sensitivity analysis was performed by removing each included study one at a time (Fig. 6a). The results showed that ignoring any included study does not change the comprehensive effect of sarcopenia on complications. Since a large asymmetry is observed in the funnel plot and Begg’s (p = 0.009; Fig. 7a) and Egger’s (p < 0.001; Fig. 7c) tests are both < 0.05, a potential publication bias is implied. Three estimation studies were supplemented with the trim-and-fill method, resulting in a symmetric funnel plot (Fig. 7b) with an adjusted OR = 1.47 and 95% CI = 1.09–1.99 (p = 0.012), suggesting that correcting potential publication bias does not alter the significant association of sarcopenia with complications.
Meta-analysis for CSS/DFS/RFS
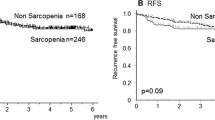
The present study also investigated the effect of CT-assessed sarcopenia on CSS/DFS/RFS prognosis in CRC patients. Seven studies comprising 8669 patients reported the prognostic value of sarcopenia for CSS (Fig. 5b). Comprehensive results suggested that sarcopenia is strongly associated with poor CSS in CRC patients (HR = 1.49, 95% CI = 1.32–1.68, p < 0.001). A fixed-effects model was used to assess heterogeneity (I2 = 0.0%, PQ = 0.705). Five studies involving 2446 patients examined the relationship between sarcopenia and DFS (Fig. 5c). Since no heterogeneity was present, the fixed-effects model was adopted (I2 = 46.4%, PQ = 0.114). The comprehensive results showed that sarcopenia patients had poorer DFS when compared to patients without sarcopenia (HR = 1.59, 95% CI = 1.32–1.92, p < 0.001). However, the comprehensive results from three studies involving 2572 patients suggested that sarcopenia is not an independent factor for adverse RFS in CRC patients (HR = 1.32, 95% CI = 0.92–1.89, p = 0.126; Fig. 5d). Sensitivity analysis showed that omitting any included studies did not change the outcome of sarcopenia’s comprehensive meta-analysis for CSS (Fig. 6b), DFS (Fig. 6c), and RFS (Fig. 6d), suggesting that the present findings are reliable.
Discussion
There is growing evidence that various changes occur in the body composition of cancer patients, including muscle, fat, and bone. Therefore, body composition has become an increasingly important prognostic factor for cancer patients [36]. In recent years, it has been observed that sarcopenia is a common pathological body composition change in cancer patients, which has gradually attracted more and more attention. Sarcopenia was first proposed by Rosenberg et al. in 1989 as an evaluation of skeletal muscle degeneration in the elderly [37]. Recently, sarcopenia has been found to be an adverse factor affecting postoperative complications and long-term prognosis of many malignancies [38, 39]. Currently, there are many methods for assessing sarcopenia, including CT, magnetic resonance imaging, and dual energy X-ray absorptiometry. However, the recent study by Simonsen et al. showed that the incidence of sarcopenia defined by different methods varies greatly and is less consistent [40]. CT is considered the gold standard for muscle mass measurement because it can provide important quantitative information about muscle composition and distribution [41, 42]. In addition, routine use of CT imaging in preoperative evaluation of CRC patients provides an easily available and cost-free resource for sarcopenia identification.
This systematic review discussed the prognostic implications of CT-assessed sarcopenia in CRC patients by including 19 studies involving 15,889 cases. The results suggested that CT-assessed sarcopenia is an important independent risk factor for OS in CRC patients. Subgroup and meta-regression analyses showed that the reason for the heterogeneity in the comprehensive results may be explained by different sample sizes in each study (sample sizes ranged from 142 to 3262). Despite the heterogeneity, sensitivity analysis results still suggested that the present research is trustworthy. In addition, sarcopenia may increase the risk of total complications by 1.36–2.44-fold in CRC patients, which may be due to the fact that sarcopenia patients may feel weak, have limited mobility, and have greater difficulty recovering from major surgical trauma, thereby affecting the postoperative recovery process and leading to the occurrence of adverse complications. The consistent sensitivity and subgroup analyses results showed that our results are reliable and robust. Although publication bias was detected, it was modified using trim-and-fill method, and there was no significant change in the merged results, indicating that our conclusions were reliable. Moreover, sarcopenia was also associated with poor CSS and DFS in CRC patients. In summary, CT-assessed sarcopenia is an effective predictor of short- and long-term prognosis in CRC patients.
Studies have shown that sarcopenia is most likely to operate through physiological and metabolic pathways (such as systemic inflammatory response) as well as behavioral pathways (such as reduced physical activity due to dehydration and fatigue). Systemic inflammatory response plays a major role in the occurrence and development of sarcopenia [43]. Dodson et al. suggested that sarcopenia may be associated with increased metabolic activity in tumor patients, which leads to systemic inflammation and muscle wasting [44]. Richards et al. demonstrated a significant correlation between sarcopenia and systemic inflammatory response [45]. In addition, studies have suggested that inflammatory cytokines may be involved in sarcopenia by interfering with insulin-like growth factor-I signaling in skeletal muscle [46]. CRC patients often suffer from malnutrition, weight loss, and sarcopenia. This not only increases hospitalization time and costs, but also affects patient quality of life and survival. Studies have demonstrated that endurance and resistance training for cancer patients can effectively improve or maintain the quality and function of skeletal muscle [47, 48]. A high-protein diet and certain nutritional supplements (melanocortin-4 receptor antagonists and IL-6 antagonists) can increase or prevent further loss of muscle mass [49, 50]. Therefore, early nutritional support therapy and muscle mass maintenance exercise may help to improve the outcome in sarcopenia patients during treatment.
At present, there is no uniform standard for the diagnosis of CT-assessed sarcopenia. The diagnostic rate of sarcopenia largely depends on how to determine the diagnostic threshold. In our systematic review, 11 criteria were used to define sarcopenia. When Western standards (Prado et al. and Martin et al.) were applied in the Asian population, the diagnostic rate of sarcopenia was consistently higher than that of some Asian standards (Zhuang et al. and Iritani et al.). There are differences in body composition and muscle mass among different races. Studies have demonstrated that Asians have significantly lower muscle mass than Westerners by about 17% [51]. The mixed use of diagnostic criteria based on different races may lead to research heterogeneity. In addition, studies combining muscle mass and body mass indices as criteria for sarcopenia had less fluctuation in the diagnostic rate of sarcopenia than studies using muscle mass index alone, suggesting that a combination of muscle mass and body mass indices may help to reduce heterogeneity and standardize the criteria for sarcopenia. Gender is also one of the main factors that affect the diagnostic criteria for sarcopenia. The different physique of male and female often cause the threshold of sarcopenia in male to be higher than that in female. In this study, the difference in sarcopenia thresholds between male and female ranged from 2 to 15.2 (median of 12). Based on current evidence, standardized sarcopenia should be considered in terms of race, muscle mass index, body mass index, and gender. Our meta-analysis demonstrated that CT-assessed sarcopenia is an effective predictor of poor outcomes for CRC patients. Early nutritional intervention may help improve the prognosis of these sarcopenia patients. Therefore, the development of standardized sarcopenia is conducive to more accurate and convenient identification of patients with poor prognosis in sarcopenia state, thereby timely intervention can be conducted to improve the prognosis of these patients.
Some study limitations still need to be resolved. First, there was significant heterogeneity in the analysis of the relationship between sarcopenia and OS. However, sensitivity analysis identified no significant change in the prognostic impact of sarcopenia on OS meta-analysis. Based on the subgroup and meta-regression analyses results, it was speculated that heterogeneity may be caused by the large difference in sample size among studies. Second, publication bias was present in the meta-analysis for complications. However, there were no significant differences between the results before and after the application of the trim-and-fill method, suggesting that publication bias did not change the significant correlation between sarcopenia and complications. In summary, despite these limitations, the present results provide valuable support for assessing the prognostic value of CT-assessed sarcopenia in CRC patients.
Conclusions
This study suggested that preoperative CT-assessed sarcopenia can be employed as an effective predictor of complications and OS/CSS/DFS in CRC patients. Early identification of preoperative sarcopenia and timely administration of nutritional intervention and exercise training may help to improve the adverse outcomes in CRC patients. In addition, the prevalence of sarcopenia correlates with the L3SMI threshold and standardization of CT-assessed sarcopenia requires comprehensive consideration of race, muscle mass index, body mass index, and gender.
Data availability
All datasets presented in this study are included in the article.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Siegel RL, Miller KD, Sauer AG, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A (2020) Colorectal cancer statistics, 2020. CA Cancer J Clin 70(3):145–164. https://doi.org/10.3322/caac.21601
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH et al (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66(4):271–289
Yang Z, Zhou X, Ma B, Xing Y (2018) Predictive value of preoperative sarcopenia in patients with gastric cancer: a meta-analysis and systematic review. J Gastrointest Surg 22(11):1890–1902
Bundred J, Kamarajah SK, Roberts KJ (2019) Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford) 21(12):1603–1612
Yang M, Shen Y, Tan L, Li W (2019) Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest 156(1):101–111
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39(4):412–423
Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR (2008) Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr 87(5):1562s-s1566
Sayer AA, Dennison EM, Syddall HE, Jameson K, Martin HJ, Cooper C (2008) The developmental origins of sarcopenia: using peripheral quantitative computed tomography to assess muscle size in older people. J Gerontol A Biol Sci Med Sci 63(8):835–840
Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML et al (2017) Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol 3(12):e172319
Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC (2019) The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle 10(1):111–122
Xie H, Gong Y, Kuang J, Yan L, Ruan G, Tang S et al (2020) Computed tomography-determined sarcopenia is a useful imaging biomarker for predicting postoperative outcomes in elderly colorectal cancer patients. Cancer Res Treat 52(3):957–972
Wang S, Xie H, Gong Y, Kuang J, Yan L, Ruan G et al (2020) The value of L3 skeletal muscle index in evaluating preoperative nutritional risk and long-term prognosis in colorectal cancer patients. Sci Rep 10(1):8153
van Vugt JLA, Coebergh van den Braak RRJ, Lalmahomed ZS, Vrijland WW, Dekker JWT, Zimmerman DDE et al (2018) Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol 44(9):1354–1360
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X et al (2018) Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis 33(10):1419–1427
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16
Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF et al (2015) Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis 17(11):O256–O264
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J et al (2015) Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol 22(8):2663–2668
Malietzis G, Currie A, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R et al (2016) Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg 103(5):572–580
Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E et al (2017) Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev 26(7):1008–1015
Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML et al (2017) Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol 3(12):e172319
Chen W-Z, Chen X-D, Ma L-L, Zhang F-M, Lin J, Zhuang C-L et al (2018) Impact of visceral obesity and sarcopenia on short-term outcomes after colorectal cancer surgery. Dig Dis Sci 63(6):1620–1630
Nakanishi R, Oki E, Sasaki S, Hirose K, Jogo T, Edahiro K et al (2018) Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today 48(2):151–157
Sueda T, Takahasi H, Nishimura J, Hata T, Matsuda C, Mizushima T et al (2018) Impact of low muscularity and myosteatosis on long-term outcome after curative colorectal cancer surgery: a propensity score–matched analysis. Dis Colon Rectum 61(3):364–374
van Vugt JL, van den Braak RRC, Lalmahomed ZS, Vrijland WW, Dekker JW, Zimmerman DD et al (2018) Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol 44(9):1354–1360
Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB (2019) The impact of muscle and adipose tissue on long-term survival in patients with stage I to III colorectal cancer. Dis Colon Rectum 62(5):549–560
Yang J, Zhang T, Feng D, Dai X, Lv T, Wang X et al (2019) A new diagnostic index for sarcopenia and its association with short-term postoperative complications in patients undergoing surgery for colorectal cancer. Colorectal Dis 21(5):538–547
Aro R, Mäkäräinen-Uhlbäck E, Ämmälä N, Rautio T, Ohtonen P, Saarnio J, Meriläinen S (2020) The impact of sarcopenia and myosteatosis on postoperative outcomes and 5-year survival in curatively operated colorectal cancer patients – a retrospective register study. Eur J Surg Oncol 46(9):1656–1662. https://doi.org/10.1016/j.ejso.2020.03.206
Chen W, Huang Y, Xu L, Shi M, Chen X, Ye G et al (2020) Effects of sarcopenia, hypoalbuminemia, and laparoscopic surgery on postoperative complications in elderly patients with colorectal cancer: a prospective study. Neoplasma 67(4):922–932
Han JS, Ryu H, Park IJ, Kim KW, Shin Y, Kim SO et al (2020) Association of body composition with long-term survival in non-metastatic rectal cancer patients. Cancer Res Treatment 52(2):563
Lee CS, Won DD, Oh SN, Lee YS, Lee IK, Kim IH, Choi MH, Oh ST (2020) Prognostic role of pre-sarcopenia and body composition with long-term outcomes in obstructive colorectal cancer: a retrospective cohort study. World J Surg Oncol 18(1):230. https://doi.org/10.1186/s12957-020-02006-3
Shirdel M, Andersson F, Myte R, Axelsson J, Rutegård M, Blomqvist L, Riklund K, van Guelpen B, Palmqvist R, Gylling B (2020) Body composition measured by computed tomography is associated with colorectal cancer survival, also in early-stage disease. Acta Oncol 59(7):799–808. https://doi.org/10.1080/0284186X.2020.1744716
Xiao J, Caan BJ, Feliciano EMC, Meyerhardt JA, Peng PD, Baracos VE et al (2020) Association of low muscle mass and low muscle radiodensity with morbidity and mortality for colon cancer surgery. JAMA Surg 155(10):942. https://doi.org/10.1001/jamasurg.2020.2497
Wang S, Xie H, Gong Y, Kuang J, Yan L, Ruan G et al (2020) The value of L3 skeletal muscle index in evaluating preoperative nutritional risk and long-term prognosis in colorectal cancer patients. Sci Rep 10(1):1–11
Bianchini F, Kaaks R, Vainio H (2002) Overweight, obesity, and cancer risk. Lancet Oncol 3(9):565–574
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127(5 Suppl):990S–991S. https://doi.org/10.1093/jn/127.5.990S
Xie H, Gong Y, Kuang J, Yan L, Ruan G, Tang S, Gao F, Gan J (2020) Computed tomography–determined sarcopenia is a useful imaging biomarker for predicting postoperative outcomes in elderly colorectal cancer patients. Cancer Res Treatment 52(3):957–972. https://doi.org/10.4143/crt.2019.695
Zhuang Cheng-Le, Huang Dong-Dong, Pang Wen-Yang, Zhou Chong-Jun, Wang Su-Lin, Lou Neng, Ma Liang-Liang, Zhen Yu, Shen Xian (2016) Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine 95(13):e3164. https://doi.org/10.1097/MD.0000000000003164
Simonsen C, Kristensen TS, Sundberg A, Wielsøe S, Christensen J, Hansen CP et al (2021) Assessment of sarcopenia in patients with upper gastrointestinal tumors: prevalence and agreement between computed tomography and dual-energy x-ray absorptiometry. Clin Nutr 40(5):2809–2816
Goodpaster BH, Thaete FL, Kelley DE (2000) Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci 904(1):18–24
Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG et al (2015) Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 261(2):345–352
Wen M-J, Hsieh C-H, Wu C-Z, Hsiao F-C, Hsia T-L, Hung Y-J et al (2013) The adipokines and inflammatory marker in young type 2 diabetics with metabolic syndrome: a pilot study. Obes Res Clin Pract 7(3):e206–e210
Van Vledder M, Levolger S, Ayez N, Verhoef C, Tran T, Ijzermans J (2012) Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 99(4):550–557
Richards CH, Roxburgh CS, MacMillan MT, Isswiasi S, Robertson EG, Guthrie GK et al (2012) The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PloS one. 7(8):e41883
Alemán MR, Santolaria F, Batista N, Marıa J, González-Reimers E, Milena A et al (2002) Leptin role in advanced lung cancer. A mediator of the acute phase response or a marker of the status of nutrition? Cytokine 19(1):21–6
Stene GB, Helbostad JL, Balstad TR, Riphagen II, Kaasa S, Oldervoll LM (2013) Effect of physical exercise on muscle mass and strength in cancer patients during treatment—a systematic review. Crit Rev Oncol Hematol 88(3):573–593
Strasser B, Steindorf K, Wiskemann J, Ulrich CM (2013) Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc 45(11):2080–2090
Dallmann R, Weyermann P, Anklin C, Boroff M, Bray-French K, Cardel B et al (2011) The orally active melanocortin-4 receptor antagonist BL-6020/979: a promising candidate for the treatment of cancer cachexia. J Cachexia Sarcopenia Muscle 2(3):163–174
Berardi E, Annibali D, Cassano M, Crippa S, Sampaolesi M (2014) Molecular and cell-based therapies for muscle degenerations: a road under construction. Front Physiol 5:119
Lau EM, Lynn HS, Woo JW, Kwok TC, Melton LJ 3rd (2005) Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci 60(2):213–216
Author information
Authors and Affiliations
Contributions
J.L.G., S.Y.T., H.L.X., and L.S.W. designed this research; M.X.L., G.H.Y., and S.Y.T. performed the statistical analysis; H.L.X. and L.S.W. performed the data extraction, and drafted and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This article did not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Considering the nature of this study, informed consent was not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Research registration number
The protocol was registered at PROSPERO: CRD42020216306.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, H., Wei, L., Liu, M. et al. Preoperative computed tomography-assessed sarcopenia as a predictor of complications and long-term prognosis in patients with colorectal cancer: a systematic review and meta-analysis. Langenbecks Arch Surg 406, 1775–1788 (2021). https://doi.org/10.1007/s00423-021-02274-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02274-x