Abstract
Arachnoid cysts (ACs) are malformations that account for about 1% of all intracranial lesions. The aetiology and progression of these lesions have been debated, with one possible explanation being the production of cerebro-spinal fluid (CSF) by ectopic choroid plexus (CP). To our knowledge, only seven cases of ACs incorporating CP have been reported, and we believe this to be the first reported case of a suprasellar AC containing ectopic CP. A 1-year-old boy presented with developmental delay and macrocephaly. MRI scan revealed hydrocephalus due to a suprasellar AC. An endoscopic ventriculocisternostomy was undertaken. Intra-operatively, intra-cystic, pink frond-like tissue resembling choroid plexus was identified. Histologically, the cyst wall was composed of fibrous tissue, with layered arachnoid cells, while the frond-like tissue was composed of papillary structures in keeping with normal choroid plexus tissue. We postulate that the rest of the ectopic CP may have been trapped within the double layered arachnoid fold of the diencephalic leaf of Liliequist’s membrane which may drive the formation and development of certain suprasellar ACs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachnoid cysts (ACs) are malformations that account for about 1% of all intracranial lesions. The aetiology and progression of these lesions have been debated, with the production of cerebro-spinal fluid (CSF) by ectopic choroid plexus (CP) proposed as a possible mechanism. This is, to our knowledge, the first reported case of a suprasellar arachnoid cyst containing ectopic CP. Herein, we present our case and review the literature to discuss the pathophysiological mechanisms that are thought to lead to this rare occurrence.
Case report
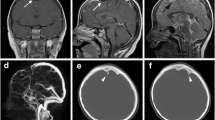
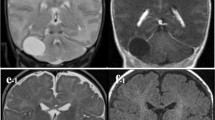
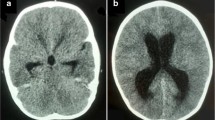
A 1-year-old boy presented with delayed neurological development and macrocephaly. There was no history of preceding trauma or infection. Visual testing was normal. Endocrine evaluation revealed hypothyroidism. MRI scan revealed hydrocephalus due to a suprasellar mass. The mass was in keeping with a suprasellar AC, measuring 3.45 (AP) by 3 cm (TV) by 3 cm (CC). The cyst extended into the base of the sella turcica. T1 and T2 MRI sequences (Fig. 1) were in keeping with that of CSF signal. The anterior wall of the cyst demonstrated a “nodule” of hypodense, non-enhancing tissue on both T1 and T2 sequences. Given the presence of hydrocephalus and endocrine abnormalities, an endoscopic ventriculocisternostomy was proposed and undertaken. Intra-operatively, the cyst roof was bulging through the foramen of Monroe. Following fenestration of the roof of the cyst, intra-cystic, pink frond-like tissue resembling CP was identified. The floor of the cyst was then widely fenestrated, caudal to which an incomplete/perforated mesencephalic leaf of Liliequist’s membrane could be identified along with neurovascular structures coursing through the prepontine cistern (see Fig. 2). The cyst wall and ectopic tissue were biopsied. Histologically the cyst wall was composed of fibrous tissue, with layered arachnoid cells, while the frond-like tissue was composed of papillary structures lined by exuberant cuboidal epithelium, in keeping with normal CP tissue. (See Figs. 3 and 4) The child had an uneventful post-operative course. Follow-up MRI revealed resolution of his hydrocephalus with partial collapse of the cyst. The child displayed significant improvement in his neurodevelopment. His endocrine function however did not improve.
Left: Endoscopic view from within lateral ventricle revealing roof of arachnoid cyst. Middle: following fenestration of both the roof and floor of the cyst. Asterisk: contents of prepontine cistern. Arrowhead: incomplete mesencephalic leaf of Lilliquist’s membrane. Small arrow: basilar tip behind the posterior wall of the cyst. Large arrow: septostomy in the floor of the cyst. Right: visualization of contents of cysts. Arrow: large tuft of normal appearing choroid plexus. Asterisk: posterior clinoid process. Arrowhead: floor of suprasellar cyst
Discussion
The majority of ACs are primary lesions, of congenital origin as a result of anomalous splitting and duplication of the endomenix during the early stages of embryonal development of the arachnoid pathways [3, 15]. Histological findings in these lesions demonstrate splitting or duplication of the arachnoid membrane into an inner and outer leaflet at the edge of the cyst cavity [5, 28].
Secondary, or acquired, ACs are less common, and occur [3, 25] as a result of trauma, surgery, infection, or intracranial haemorrhage [16, 20, 25]. Suprasellar ACs in particular have been associated with trauma in infancy [14]. Fox and Al-Mefty [7] were the first to propose that ACs may develop in the suprasellar area due to the occlusion of the normal perforations in Liliequist’s membrane, “by adhesions after subarachnoid haemorrhage, infection and other causes of inflammation”. Choi and Kim [3] speculate that trauma may rather induce an abnormal splitting or duplication of the immature endomenix in infancy, given that the subarachnoid space is not yet fully developed, with areas still “closed without cerebrospinal fluid”.
The mechanism by which ACs expand is poorly understood [15], with several postulates, including osmotic gradients caused by higher protein or sodium content in the cyst, [23] one way valve mechanisms, [11, 13, 29] direct secretion of fluid by arachnoidal cells lining the cyst wall [8], or by fluid production by ectopic CP [3, 12].
To our knowledge, only seven cases of ACs incorporating CP have been reported [12, 26, 27]. In Schummann et al.’s [26] review of these cases, however, they noted that four of these described cysts were possibly lined with epithelium of ependymal origin, thus making the present case the fourth reported case of an AC arising due to entrapped CP, and the first case description of a suprasellar AC containing ectopic CP.
As postulated by Schummann et al., [26] two scenarios exist to explain this unique situation: either the ectopic CP is incorporated into an already developing AC, or the CSF production from the ectopic CP becomes entrapped between two layers of arachnoid which then induces membrane splitting and cyst growth.
The diencephalic leaf of Liliequist’s membrane has been shown by Zhang [30] to be a double layered fold of arachnoid mater, which can allow for the formation of a truly intra-arachnoid diverticulum [17]. We postulate that the rest of the ectopic CP may have been trapped within this intra-arachnoidal diverticulum and, following CSF production by the ectopic tissue, formed a truly intra-arachnoid cyst. This morphology would be in keeping with a suprasellar type 1 AC according to the classification of Andre et al. [1]. The position of the basilar artery bifurcation behind the posterior wall of the cyst and the clinical presentation of hydrocephalus are typical for the type 1 suprasellar AC [1, 21].
The presence of CP in an extra-ventricular location is thought to occur as a result of an abnormality in embryonic development from either anomalous migration of CP tissue or metaplasia from ependymal rests [2], and has been used to explain the occurrence of extra-ventricular CP cysts [9], choroid papillomas [2, 18, 24], and ACs [26].
In the current case, given the hydrocephalus and neuro-developmental delay, surgery would generally be indicated. In asymptomatic cases, however, or cases in which there is only endocrine dysfunction or isolated gaze disturbances, several authors [4, 10] have suggested a conservative approach as many of these cysts will not evolve and may even resolve spontaneously [6, 19, 22]. We postulate that given the presence of CSF producing CP within the cyst, progression is likely and that as suggested by Shuman et al. [26] in cases of reclosure of a fenestrated cyst, ectopic CP tissue would predispose to a faster recurrence.
Conclusions
The mechanism by which ACs expand is poorly understood, and fluid production by ectopic CP should be considered as an aetiological factor. We suggest that during the endoscopic treatment of suprasellar ACs, a careful inspection of the cyst cavity is performed as even small pieces of ectopic choroidal tissue may drive the progression or rapid recurrence of suprasellar ACs.
References
André A, Zérah M, Roujeau T, Brunelle F, Blauwblomme T, Puget S, Bourgeois M, Sainte-Rose C, Ville Y, Di Rocco F (2016) Suprasellar arachnoid cysts: toward a new simple classification based on prognosis and treatment modality. Neurosurgery 78(3):370–9; discussion 379–80. https://doi.org/10.1227/NEU.0000000000001049PMID: 26445374
Beskonakli E, Cayli S, Bostanci U, Kulaçoglu S, Yalçinlar Y. Choroid (1998) plexus papillomas of the posterior fossa: extraventricular extension, intraventricular and primary extraventricular location. Report of four cases. J Neurosurg Sci 42(1):37–40. PMID: 9766271
Choi JU, Kim DS (1998) Pathogenesis of arachnoid cyst: congenital or traumatic? Pediatr Neurosurg 29(5):260–266. https://doi.org/10.1159/000028733 (PMID: 9917544)
Crimmins DW, Pierre-Kahn A, Sainte-Rose C, Zerah M (2006) Treatment of suprasellar cysts and patient outcome. J Neurosurg 105(2 Suppl):107–114. https://doi.org/10.3171/ped.2006.105.2.107 (PMID: 16922071)
Cristobal R, Oghalai JS (2007) Peripetrosal arachnoid cysts. Curr Opin Otolaryngol Head Neck Surg 15(5):323–329. https://doi.org/10.1097/MOO.0b013e328270b8c5 (PMID: 17823548)
Dodd RL, Barnes PD, Huhn SL (2002) Spontaneous resolution of a prepontine arachnoid cyst. Case report and review of the literature. Pediatr Neurosurg 37(3):152–7. https://doi.org/10.1159/000064394PMID: 12187060
Fox JL, Al-Mefty O (1980) Suprasellar arachnoid cysts: an extension of the membrane of Liliequist. Neurosurgery 7(6):615–618. https://doi.org/10.1227/00006123-198012000-00016 (PMID: 6970903)
Go KG, Houthoff HJ, Blaauw EH, Havinga P, Hartsuiker J (1984) Arachnoid cysts of the sylvian fissure. Evidence of fluid secretion J Neurosurg 60(4):803–813. https://doi.org/10.3171/jns.1984.60.4.0803 (PMID: 6231356)
Gross E, Koplewitz BZ, Arbell D, Fellig J, Udassin R (2009) Ectopic presacral choroid plexus cyst in a neonate. J Pediatr Surg 44(5):e13–e15. https://doi.org/10.1016/j.jpedsurg.2009.01.015 (PMID: 19433153)
Gui SB, Wang XS, Zong XY, Zhang YZ, Li CZ (2011) Suprasellar cysts: clinical presentation, surgical indications, and optimal surgical treatment. BMC Neurol 18(11):52. https://doi.org/10.1186/1471-2377-11-52.PMID:21586175;PMCID:PMC3119168
Halani SH, Safain MG, Heilman CB (2013) Arachnoid cyst slit valves: the mechanism for arachnoid cyst enlargement. J Neurosurg Pediatr 12(1):62–66. https://doi.org/10.3171/2013.4.PEDS12609 (Epub 2013 May 10 PMID: 23662935)
Hamada H, Nonaka Y, Kusaka Y, Nakazaki H, Abdullah SH, Oi S (2006) Huge arachnoid cyst incorporating choroid plexus. Childs Nerv Syst 22(4):420–423. https://doi.org/10.1007/s00381-005-1174-9 (Epub 2005 Jun 3 PMID: 15933883)
Hornig GW, Zervas NT (1992) Slit defect of the diaphragma sellae with valve effect: observation of a “slit valve.” Neurosurgery 30(2):265–267. https://doi.org/10.1227/00006123-199202000-00022 (PMID: 1545899)
Kim TG, Kim DS, Choi JU (2010) Are arachnoid cysts localized hydrocephali? Pediatr Neurosurg 46(5):362–367. https://doi.org/10.1159/000321597 (Epub 2011 Mar 9 PMID: 21389748)
Krawchenko J, Collins GH (1979) Pathology of an arachnoid cyst. Case report J Neurosurg 50(2):224–228. https://doi.org/10.3171/jns.1979.50.2.0224 (PMID: 430135)
Little JR, Gomez MR, MacCarty CS (1973) Infratentorial arachnoid cysts. J Neurosurg 39(3):380–386. https://doi.org/10.3171/jns.1973.39.3.0380 (PMID: 4542573)
Lyu J (2014) Possible origins of suprasellar arachnoid cysts based on anatomical considerations. World Neurosurg 82(3–4):e570–3. https://doi.org/10.1016/j.wneu.2014.05.020Epub 2014 May 14. PMID: 24836579
Ma YH, Ye K, Zhan RY, Wang LJ (2008) Primary choroid plexus papilloma of the sellar region. J Neurooncol 88(1):51–55. https://doi.org/10.1007/s11060-008-9531-7 (Epub 2008 Jan 26 PMID: 18224277)
Moon KS, Lee JK, Kim JH, Kim SH (2007) Spontaneous disappearance of a suprasellar arachnoid cyst: case report and review of the literature. Childs Nerv Syst 23(1):99–104. https://doi.org/10.1007/s00381-006-0161-0 (Epub 2006 Aug 30 PMID: 16944178)
Mustansir F, Bashir S, Darbar A (2018) Management of Arachnoid Cysts: A Comprehensive Review. Cureus 10(4):e2458. https://doi.org/10.7759/cureus.2458.PMID:29888162;PMCID:PMC5991924
Ozek MM, Urgun K (2013) Neuroendoscopic management of suprasellar arachnoid cysts. World Neurosurg 79(2 Suppl):S19.e13–8. https://doi.org/10.1016/j.wneu.2012.02.011Epub 2012 Feb 10. PMID: 22381821
Peterson C, Lawless M, Sood S (2020) Spontaneous resolution of asymptomatic pediatric suprasellar arachnoid cysts: report of 2 cases and review of the literature. Pediatr Neurosurg 55(1):62–66. https://doi.org/10.1159/000504262 (Epub 2019 Nov 26 PMID: 31770757)
Pradilla G, Jallo G (2007) Arachnoid cysts: case series and review of the literature. Neurosurg Focus 22(2):E7. https://doi.org/10.3171/foc.2007.22.2.7 (PMID: 17608350)
Sameshima T, Tanikawa R, Sugimura T, Izumi N, Seki T, Maeda T, Tsuboi T, Hashimoto M, Kimura T, Nabeshima K (2010) Choroid plexus papilloma originating in the sella turcica–case report. Neurol Med Chir (Tokyo) 50(2):144–146. https://doi.org/10.2176/nmc.50.144 (PMID: 20185881)
Samii M, Carvalho GA, Schuhmann MU, Matthies C (1999) Arachnoid cysts of the posterior fossa. Surg Neurol 51(4):376–382. https://doi.org/10.1016/s0090-3019(98)00095-0 (PMID: 10199290)
Schuhmann MU, Tatagiba M, Hader C, Brandis A, Samii M (2000) Ectopic choroid plexus within a juvenile arachnoid cyst of the cerebellopontine angle: cause of cyst formation or reason of cyst growth. Pediatr Neurosurg 32(2):73–76. https://doi.org/10.1159/000028902 (PMID: 10838504)
Singleton WG, Lawrence T, Green AL, Jeans A, Kerr RS (2010) Cerebellopontine angle arachnoid cyst containing ectopic choroid plexus–case report. Acta Neurochir (Wien) 152(5):881–883. https://doi.org/10.1007/s00701-009-0516-x (Epub 2009 Oct 6 PMID: 19806308)
Vaquero J, Carrillo R, Cabezudo JM, Nombela L, Bravo G (1981) Arachnoid cysts of the posterior fossa. Surg Neurol 16(2):117–121. https://doi.org/10.1016/0090-3019(81)90110-5 (PMID: 6974408)
Yamakawa H, Ohkuma A, Hattori T, Niikawa S, Kobayashi H (1991) Primary intracranial arachnoid cyst in the elderly: a survey on 39 cases. Acta Neurochir (Wien) 113(1–2):42–47. https://doi.org/10.1007/BF01402113 (PMID: 1799142)
Zhang M, An PC (2000) Liliequist’s membrane is a fold of the arachnoid mater: study using sheet plastination and scanning electron microscopy. Neurosurgery 47(4):902–8; discussion 908–9. https://doi.org/10.1097/00006123-200010000-00021 PMID: 11014430
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The Human Research Ethics Committee of the University of the Witwatersrand approved the presentation of the case report.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Labuschagne, J., Mutyaba, D., Pillay, T. et al. Suprasellar arachnoid cyst due to ectopic choroid plexus: case report. Childs Nerv Syst 38, 1381–1384 (2022). https://doi.org/10.1007/s00381-021-05395-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05395-2