Abstract
Introduction
Arachnoid cysts are benign developmental anomalies of arachnoid membrane origin that can occur anywhere along the neuro-axis. They are believed to develop from the splitting or duplication of the arachnoid membrane by CSF that is trapped by a ball-valve mechanism. Intracranial arachnoid cysts have only been described as intradural lesions while spinal arachnoid cysts can be both intradural or extradural.
Case Report
After an extensive literature review, we report the first case of an intracranial, extradural arachnoid cyst in a 5-yearold girl. The child presented with a 2-week history of suspected seizure-like activity and imaging revealed a large midline extradural CSF-containing arachnoid cyst causing severe compression of the superior sagittal sinus and underlying brain. Venous flow through the sagittal sinus was nearly obliterated. Osseous changes and bone growth adjacent to the cyst was also noted on imaging and intraoperatively. She underwent a bifrontal craniotomy and cyst excision with decompression of underlying brain and reestablishment of venous flow through the sagittal sinus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachnoid cysts are benign developmental anomalies of arachnoid membrane origin that can occur anywhere along the neuro-axis [2, 3] and account for approximately 1% of all intracranial lesions [7]. Arachnoid cysts can be asymptomatic, but may cause symptoms, such as headache, motor/sensory deficits, and seizure via local mass effect or obstruction of CSF flow [10, 11]. Intracranial arachnoid cysts are extra-parenchymal with intra-arachnoid CSF beneath the dural membrane [18, 20]. Here we report the first case of an intracranial extradural arachnoid cyst, which occurred in a 5-year-old girl, causing significant compression of the superior sagittal sinus and underlying brain.
Case
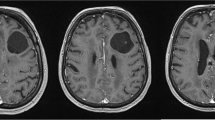
A 5-year-old girl without significant past medical history presented with a 2-week history of intermittent staring spells and unbalanced gait. These spells lasted for minutes and were concerning for seizures as the patient was unresponsive to external stimuli during each event. EEG identified focal epileptiform abnormalities in the right centrotemporal region but did not record any clinical seizures. Brain MRI revealed a midline non-enhancing extradural CSF-containing cyst near the junction of the coronal and sagittal sutures with significant mass effect on the sagittal sinus and bilateral frontal lobes (Fig. 1 ). The arachnoid cyst appeared extradural in origin. An MRI of the brain 2 years prior did not reveal this cyst, suggesting rapid growth over this time period (Fig. 2 ).
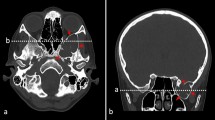
MR and CT imaging revealing an intracranial, extradural arachnoid cyst in a 5-year-old-girl. Pre-operative coronal (a) and sagittal (b) post-contrast T1-weighted MRI images demonstrating a 1.7 cm × 3 cm × 3.6 cm extradural arachnoid cyst (white arrow) near the junction of the coronal and sagittal sutures with mass effect on the underlying superior sagittal sinus brain parenchyma. Sagittal post-contrast T2- weighted MRI image (c) reveals the non-enhancing hyper-intense cystic lesion (black arrow) located in the extradural space. MR venography of the brain (d) shows compression of the superior sagittal sinus by the extradural arachnoid cyst and almost complete obliteration of venous flow through that portion of the superior sagittal sinus. Non-contrast axial CT images (e) and (f) demonstrate calvarium bone growth along the cyst wall (white arrow head)
Due to rapid growth of the cyst and significant brain and superior sagittal sinus compression, the child underwent a bifrontal craniotomy with resection of the extradural cyst and decompression of the brain and sagittal sinus. Intraoperatively, the dura and cyst was found to be closely adherent to the bone of the craniotomy, and the arachnoid cyst ruptured as the bone was reflected off the dura. The underlying brain and sagittal sinus were found to be severely depressed. Over the next 20 min, the brain and sagittal sinus gradually rose to fill the empty space. The inner table of the bone flap had osseous overgrowth with invaginations and spicules, which were drilled to flatten the bone with the remaining skull (Fig. 3 ). The area in the bone where the cyst resided was also drilled to ensure no cyst membrane remained. The edges of the arachnoid cyst wall circumscribing the sagittal sinus were excised and the edges cauterized to eliminate any dural defect and communication with the subarachnoid space. No definitive pathologic diagnosis could be obtained because of the small amount of tissue excised. However, intraoperatively, this arachnoid cyst wall appeared to be leptomeningeal in origin. The bone flap was then replaced and secured with titanium plates. The patient had an uncomplicated post-operative course and left the hospital on post-operative day four.
a Intraoperative photograph reveals bony overgrowth with large spicules and invaginations (white arrow) on the inner table of the bone flap. b Intraoperative photograph, taken 20 min after cyst drainage, shows remnants of cyst wall (dashed line) and decompression of superior sagittal sinus (asterisk) and underlying brain parenchymal. A anterior; P posterior; R right; L left
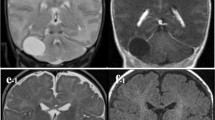
At 3 months post-operatively, the patient was doing well and neurologically at her baseline. MRI of the brain showed complete resection without any residual arachnoid cyst and reestablished superior sagittal sinus venous flow (Fig. 4 ).
Discussion
Arachnoid cysts are congenital, extra-axial, and intra-arachnoid lesions filled with CSF. Intracranial arachnoid cysts have been described as intradural lesions while spinal arachnoid cysts may present as intradural or extradural lesions [4]. Ninety percent of arachnoid cysts exist in the supratentorial space with majority in the sylvian fissure. After an extensive literature review, we report the first case of an intracranial, extradural arachnoid cyst.
The case presented here is similar to intradiploic arachnoid cysts, which are osteolytic skull lesions that can also present as an extradural cyst containing CSF-like secretions [19]. Continuous pulsations of arachnoid matter through dural and inner calvarial table defects—trauma or congenital in origin—result in erosion into the intradiploic space [5, 8, 17]. Over time, expansion of the arachnoid diverticulum within the diploic space undermines both inner and outer tables of the calvaria. In contrast, in the patient reported here, we observed inner calvarial bone growth along the cyst instead of a lytic skull lesion. Instead of erosion through the inner table of the calvaria, the arachnoid diverticulum expanded within the extradural space, resulting in an extradural arachnoid cyst with brain compression, osseous overgrowth, and osseous changes adjacent to the cyst. Our case resembles the cranial equivalent of extradural spinal arachnoid cysts, which account for 35% of all spinal arachnoid cysts in one series [2]. Most extradural spinal arachnoid cysts have intradural arachnoid communications. Expansion of arachnoid cysts in the extradural space is likely multifactorial. The ball-valve mechanism has been observed in multiple cases, in which the stalk communicating between cyst and subarachnoid space acts as a one-way valve [13, 14]. Active secretions by cells lining the cyst wall may also contribute to cyst expansion [6]. The actual mechanism is likely heterogeneous, involving either or both processes.
Arachnoid cysts are often found incidentally and therefore asymptomatic. They become symptomatic via local mass effect, obstruction of CSF outflow tract, or hemorrhage into the cyst. Surgical treatments of arachnoid cysts include craniotomy and cyst excision, cyst-peritoneal and/or ventriculoperitoneal shunt placement, and endoscopic cyst fenestration. Location and size of symptomatic arachnoid cysts are major determinants of treatment strategy. Craniotomy and cyst marsupialization remains the most favored treatment option for sylvian fissure cysts and other convexity cysts with a reported success rate up to 76% in one series [12, 16]. With the advancement of neuro-navigation, endoscopic cyst fenestration has become increasingly popular [15]. Arachnoid cysts located in the suprasellar region and the ventricles are treated with endoscopic fenestration with good outcome [9]. Compared to cyst fenestration, shunting is the less preferred due to its well-known complications and high rate of malfunction [12]. However, surgical excision or cyst fenestration may not always be successful. Infants presenting with macrocephaly or ventriculomegaly are significantly more likely to require a shunt placement despite excision of the cyst [21]. Ali et al. found that patient’s overall quality of life is comparable among these three types of procedures. [1].
References
Ali ZS, Lang SS, Bakar D, Storm PB, Stein SC (2014) Pediatric intracranial arachnoid cysts: comparative effectiveness of surgical treatment options. Childs Nerv Syst 30:461–469. doi:10.1007/s00381-013-2306-2
Bond AE, Zada G, Bowen I, McComb JG, Krieger MD (2012) Spinal arachnoid cysts in the pediatric population: report of 31 cases and a review of the literature. J Neurosurg Pediatr 9:432–441. doi:10.3171/2012.1.PEDS11391
Cincu R, Agrawal A, Eiras J (2007) Intracranial arachnoid cysts: current concepts and treatment alternatives. Clin Neurol Neurosurg 109:837–843. doi:10.1016/j.clineuro.2007.07.013
de Oliveira RS, Amato MC, Santos MV, Simao GN, Machado HR (2007) Extradural arachnoid cysts in children. Childs Nerv Syst 23:1233–1238. doi:10.1007/s00381-007-0414-6
Garg K, Sinha S, Kale SS, Kumar R, Sharma BS (2013) A rare case of non-traumatic intradiploic arachnoid cyst. Neurol India 61:446–447. doi:10.4103/0028-3886.117605
Go KG, Houthoff HJ, Blaauw EH, Havinga P, Hartsuiker J (1984) Arachnoid cysts of the sylvian fissure: evidence of fluid secretion. J Neurosurg 60:803–813. doi:10.3171/jns.1984.60.4.0803
Harsh GR, Edwards MS, Wilson CB (1986) Intracranial arachnoid cysts in children. J Neurosurg 64:835–842. doi:10.3171/jns.1986.64.6.0835
Hasegawa H, Bitoh S, Koshino K, Obashi J, Iwaisako K, Fukushima Y (1992) Nontraumatic intradiploic arachnoid cysts—report of five cases. Neurol Med Chir (Tokyo) 32:887–890
Knie B, Morota N, Ihara S, Tamura G, Ogiwara H (2016) Pediatric intraventricular arachnoid cysts in the body of lateral ventricle: surgical outcome and its embryologic background. Childs Nerv Syst 32:2197–2204. doi:10.1007/s00381-016-3203-2
Koch CA, Voth D, Kraemer G, Schwarz M (1995) Arachnoid cysts: does surgery improve epileptic seizures and headaches? Neurosurg Rev 18:173–181
Martinez-Lage JF, Perez-Espejo MA, Almagro MJ, Lopez-Guerrero AL (2011) Hydrocephalus and arachnoid cysts. Childs Nerv Syst 27:1643–1652. doi:10.1007/s00381-011-1481-2
Raffel C, McComb JG (1988) To shunt or to fenestrate: which is the best surgical treatment for arachnoid cysts in pediatric patients? Neurosurgery 23:338–342
Rohrer DC, Burchiel KJ, Gruber DP (1993) Intraspinal extradural meningeal cyst demonstrating ball-valve mechanism of formation: case report. J Neurosurg 78:122–125. doi:10.3171/jns.1993.78.1.0122
Santamarta D, Aguas J, Ferrer E (1995) The natural history of arachnoid cysts: endoscopic and cine-mode MRI evidence of a slit-valve mechanism. Minim Invasive Neurosurg 38:133–137. doi:10.1055/s-2008-1053473
Tamburrini G, D'Angelo L, Paternoster G, Massimi L, Caldarelli M, Di Rocco C (2007) Endoscopic management of intra and paraventricular CSF cysts. Childs Nerv Syst 23:645–651. doi:10.1007/s00381-007-0327-4
Tamburrini G, Dal Fabbro M, Di Rocco C (2008) Sylvian fissure arachnoid cysts: a survey on their diagnostic workout and practical management. Childs Nerv Syst 24:593–604. doi:10.1007/s00381-008-0585-9
Verma SK, Satyarthee GD, Sharma BS (2014) Giant intradiploic arachnoid cyst for 13 years. J Pediatr Neurosci 9:139–141. doi:10.4103/1817-1745.139318
Wang PJ, Lin HC, Liu HM, Tseng CL, Shen YZ (1998) Intracranial arachnoid cysts in children: related signs and associated anomalies. Pediatr Neurol 19:100–104
Weinand ME, Rengachary SS, McGregor DH, Watanabe I (1989) Intradiploic arachnoid cysts: report of two cases. J Neurosurg 70:954–958. doi:10.3171/jns.1989.70.6.0954
Wetjen NM, Walker ML (2011) Arachnoid cysts. In: Youmans JR, Winn HR (eds) Youmans neurological surgery, 6th edn. Saunders/Elsevier, Philadelphia
Zada G, Krieger MD, McNatt SA, Bowen I, McComb JG (2007) Pathogenesis and treatment of intracranial arachnoid cysts in pediatric patients younger than 2 years of age. Neurosurg Focus 22:E1. doi:10.3171/foc.2007.22.2.1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, L., Ali, M., Menezes, A.H. et al. Intracranial extradural arachnoid cyst in a child. Childs Nerv Syst 33, 2201–2204 (2017). https://doi.org/10.1007/s00381-017-3556-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3556-1