Abstract
Purpose
Cerebral palsy is a common neurological disorder that involves spasticity of the extremities and can lead to lifelong disability. Selective dorsal rhizotomy (SDR) can improve spasticity and quality of life in these patients, but it may be associated with the development of spinal deformity. Risk factors for spinal deformity after SDR have not yet been systematically examined.
Methods
Medline, Embase, and Web of Science databases were queried for clinical studies reporting incidence of new or worsening spinal deformity, including scoliosis, after SDR. Variables that represent possible risk factors for deformity were correlated with reported incidence of deformity.
Results
Twenty-two articles for a total of 1485 patients met the inclusion criteria for this study. Deformity occurs among all patients with a weighted mean incidence of 28.0%. Scoliosis appears to be the most common deformity occurring with a weighted mean incidence of 31.6%. There is substantial heterogeneity between studies, limiting our analysis. Significant positive correlation was found between percent of patients that developed any type of deformity and the ratio of female to male patients, p = 0.02. Significant positive correlation was also found between percent of patients that develop scoliosis and the ratio of female to male patients, p < 0.01, and between scoliosis and the number of years to follow-up, p < 0.01.
Conclusion
Spinal deformity is an important potential complication of SDR with scoliosis being the most common type of deformity. The major risk factor for postoperative deformity is female sex. Deformity was also found to significantly increase with extended follow-up, indicating a slow process that should be carefully monitored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral palsy is a neurological disorder with an incidence of about 1 in 500 births in North America [1]. Patients may have lifelong disabilities, including spasticity, deficits in ambulation, risk of orthopedic deformities, and painful muscle contractions [2,3,4,5]. A recognized treatment for long-term reduction of spasticity in this patient population is selective dorsal rhizotomy (SDR), a surgical procedure initially described in 1911 by Foerster [6].
Trials studying outcomes of SDR have been published since the 1980s, with varying levels of success and complications. Importantly, the rate of long-term complications, such as spinal deformity, including scoliosis, kyphosis, lordosis, and spondylolysis or spondylolisthesis, frequently differs between studies, and a true rate is unknown. Risk factors that may contribute to a higher chance of deformity after surgery are similarly unknown and important for surgeons and providers to know when discussing risks and benefits of the procedure with individual patients.
Children who undergo vertebral surgery are especially susceptible to deformity due to the mechanics of their still-developing bone tissue, as seen in patients undergoing laminectomy for treatment of spinal tumors [7]. Furthermore, prior studies have noted that spinal deformity is common in patients with cerebral palsy, even in the absence of previous operative intervention [3, 4, 7,8,9,10]. Thus, differentiating an accelerated progression and increased incidence of spinal deformity due to surgical intervention, as opposed to deformity secondary to the natural history of cerebral palsy, is important, but difficult. Additionally, in order to protect patients from potentially avoidable complications, it is essential to identify variables that may increase the risk of deformity. This systematic review focuses on the long-term incidence of spinal deformities after SDR, with a primary interest in the development of scoliosis. The aims of this systematic review are to describe the progression of spinal deformities in the postoperative SDR patient and to identify risk factors that correlate with increased rates of spinal deformity.
Methods
Search strategy
A systematic review was conducted during December 2017 according to PRISMA 2015 guidelines [11]. Papers were found by querying Medline, Embase, and Web of Science with the following search terms: “selective dorsal rhizotomy” AND “complication,” “selective dorsal rhizotomy” AND “scoliosis,” “selective dorsal rhizotomy” AND “follow up,” “selective dorsal rhizotomy” AND “long term,” “selective dorsal rhizotomy” AND deformity,” and “selective dorsal rhizotomy” AND “spine.” Relevant review articles found during this process were examined, and individual references from those articles were manually searched using PubMed or Google Scholar.
All articles that reported spinal deformity outcomes after surgery, including scoliosis, kyphosis, lordosis, hyperlordosis, or spondylolysis or spondylolisthesis, were included. New onset as well as progression of deformity were included. Papers that reported incidence of spinal corrective surgery, but not incidence of all spinal deformity, were not included. Papers were excluded when it was explicitly stated that the patients were included in a cohort analyzed in a different paper that was already included. All articles included were retrospective level III evidence studies.
Two authors (MW and PJS) independently performed database searches and reviewed abstracts and full texts of relevant manuscripts. The third author (AT) reviewed the two lists of included manuscripts and resolved any differences, combining them into a single list.
Data extraction
Two authors (MW and PJS) independently reviewed the full text of each paper and recorded spinal deformity incidence, as well as variables reported by the individual study. The third author (AT) reviewed all extracted data and reconciled any differences. Recorded variables included number of patients, gender ratio, years during which surgeries took place, duration of follow-up after surgery, average baseline GMFCS scores [12], age at initial surgery, levels of laminectomy, levels of rhizotomy, type of deformity, and percentage of rootlet cut.
Statistical analysis
Statistical analysis was performed, and plots were created using R (R Foundation for Statistical Computing, Vienna, Austria). Means were weighted by variance. Correlation of incidence of spinal deformity to single variables was done using linear regression analysis, weighted by variance. Funnel plots were constructed by plotting incidence of deformity against number of patients [13]. I2 statistic was calculated according to Higgins et al. [14].
Results
Search results
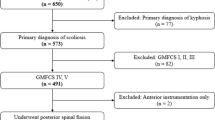
Initial search results returned 760 published articles, including identified search terms. Of these, 39 articles reported spinal deformity as an adverse outcome following rhizotomy surgery. Of the 39 articles, 5 were excluded for not quantifying deformities, 3 were excluded as case reports, and 4 were excluded due to reporting outcomes of patients that belonged to a cohort that had been reported in a separate, included article. Altogether, 22 articles with a total of 1485 patients were included in this review (Fig. 1) [3, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
Several variables were virtually identical between studies, notably those relating to surgical technique, such as the use of electrophysiology recordings during surgery, as well as the initial diagnosis (spastic cerebral palsy predominating over other conditions). Several variables were reported in only a few studies and were not useful to the review—these included GMFCS score preoperatively and postoperatively, the presence of truncal or upper extremity involvement, and preoperative deformity angles. Importantly, some variables, including postoperative deformity angles, were not reported in a consistent manner between studies, making direct comparisons impossible. The more frequently reported variables are shown in Table 1.
Incidence of spinal deformity
The mean incidence of spinal deformity at follow-up is 28.0 ± 9.1% (Fig. 2a). A funnel plot of incidence against number of patients shows significant asymmetry (Fig. 2b, rank correlation test of asymmetry p = 0.28). I2 statistic is calculated to be 94.2, suggesting substantial heterogeneity between studies.
Incidence of spinal deformity at follow-up as a percentage of patients at follow-up was plotted against several variables. Correlation coefficients were calculated, weighted by variance (Table 2). A significant positive correlation was found between the incidence of spinal deformity and the ratio of female to male patients in the study (Fig. 3a, p = 0.02). There was a strong trend towards a positive correlation between incidence of spinal deformity and the number of patients in the study (Fig. 3b, p = 0.09).
a Plot of incidence of spinal deformity against ratio of female to male patients by study, with regression line. Error bars represent 95% confidence intervals. b Plot of incidence of spinal deformity against number of patients by study, with regression line. Error bars represent 95% confidence intervals
Types of spinal deformity
Of the 22 articles, 19 (1406 patients total) broke down total incidence of spinal deformity into types of spinal deformity, which were placed into categories of scoliosis (lateral curvature of the spine), kyphosis (excessive concave curvature), lordosis (excessive convex curvature), and spondylolysis or spondylolisthesis (defect or slippage in vertebra) (Table 3) [3, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The majority of studies had patients who developed new or worsening scoliosis; 17 of 19 reported scoliosis and 12 studies had patients who developed spondylolysis or spondylolisthesis. Fewer articles reported kyphosis (7 studies) or lordosis (7 studies).
Incidence of scoliosis
Of 19 articles that reported type of spinal deformity, 17 of them had patients who developed new-onset or worsening scoliosis at follow-up (337 of the 1406 patients), while 2 studies reported only non-scoliosis deformities. The mean weighted incidence of scoliosis at follow-up is 31.6 ± 8.4% (Fig. 4a). A funnel plot of incidence against number of patients shows significant asymmetry (Fig. 4b, rank correlation test of asymmetry p = 0.25). I2 statistic is calculated to be 47.9, suggesting moderate heterogeneity between studies.
We correlated the incidence of scoliosis in all 19 articles against all other variables, weighted by variance (Table 4). There is a significant positive correlation between the incidence of scoliosis and the ratio of female to male patients in each study (Fig. 5a, p < 0.01). There is also a significant positive correlation between incidence of scoliosis and the number of years to follow-up (Fig. 5b, p < 0.01).
a Plot of incidence of scoliosis against ratio of female to male patients by study, with regression line. Error bars represent 95% confidence intervals. b Plot of incidence of scoliosis against number of years to follow-up by study, with regression line. Error bars represent 95% confidence intervals
Discussion
Overall incidence of deformity after SDR
In this review, we have examined rates of spinal deformity after selective dorsal rhizotomy, as reported in a large number of clinical studies. We found that the incidence of spinal deformity after SDR is highly variably reported, with some studies reporting incidence as high as 88% [21], some as low as 0% [15], and an average incidence of 28%. It is important to note that this incidence is very close to, or slightly higher than, the generally accepted rate of 20–25% that occurs as part of the natural history of cerebral palsy [10].
In studies that differentiate between different types of spinal deformity (scoliosis, kyphosis, lordosis, spondylolysis/spondylolisthesis), scoliosis appears to be the most common complication, with an average incidence 31.6%. Kyphosis appears with an average incidence of 6.8%, lordosis 18.6%, and spondylolysis/spondylolisthesis 10.6%. However, there is significant variation between studies in these rates. For example, one study reported an incidence of kyphosis of 47% [17] and 12 studies reported an incidence of 0% [3, 15, 16, 20, 22, 24,25,26,27,28,29,30,31]. This discrepancy could reflect differing definitions of what angle constitutes kyphosis, methods of interpreting radiographic data, or incidence in reporting kyphosis/lordosis. Alternatively, it is possible that patients with scoliosis were more likely to present for follow-up than patients with other types of deformity, pointing to possible selection bias in studies that report 0% prevalence of other types of deformity.
Risk factors for spinal deformity
There are many variables, including patient characteristics and surgical techniques, that are likely predictors of an increased risk of spinal deformity. Based on the variables that did have significant correlations, or correlations trending towards significance, we conclude that being female is likely a risk factor for postoperative deformity, which is in line with previous studies. Female cerebral palsy patients are more likely to develop scoliosis than male patients in the natural history of the disease, although the cause for this is not entirely clear [14, 36,37,38]. It has been suggested that females may present with deformity at an earlier age due to faster spinal maturation [37]. It is possible that in the SDR population, males develop deformity at a slower rate, and so corroborate our finding of longer follow-up being a predisposing factor to deformity. In the end, due to a lack of control groups in these retrospective studies, it is impossible to determine that attributable risk of being female or of having surgery, to the development of scoliosis. It is reasonable to conclude that laminectomy for SDR may not pose any increased risk for the development of spinal deformity beyond the natural history of the disease.
Studies with longer time to follow-up have higher incidence rates, indicating studies with short follow-up periods may not have allowed enough time to pass to adequately assess long-term complication rates. This is an important finding that future studies should take into consideration, in order to avoid premature follow-up and reporting of data. Furthermore, it suggests that clinicians should take care to monitor their patients for onset of deformity for potentially decades after surgery and that adult neurologists and neurosurgeons should be trained to watch for deformity in patients that they inherit from their pediatric counterparts.
We note that publications with higher patient sample sizes report higher rates of spinal deformity. This most likely reflects reporting bias in the current literature. It is possible that larger studies are better representations of all the surgeries that have been performed at a center and their outcomes and that smaller studies are more similar to case series, published by authors who are more optimistic about outcomes and the usefulness of the procedure.
Finally, it is important to note that the SDR procedure has been found to have similar rates of spinal deformity when comparing to that of the natural disease progression in cerebral palsy. Thus, this argues that the SDR procedure only does not significantly increase the risk of spinal deformity compared to the natural process making it an effective and useful surgical procedure for those patients suffering from spasticity.
Other possible factors
There are other variables that have been hypothesized to contribute to increased risk of deformity that did not come out in our analysis. First, due to the continuous development of vertebral structural mechanics throughout childhood, it seems reasonable that older children are less likely to suffer from deformity than younger children and that studies with a younger population at the time of surgery would have higher incidence, as has been shown to be true in patients undergoing laminectomy for indications other than cerebral palsy [7]. However, we found no correlation between age and deformity rate. This is hard to draw conclusions from and may be due to the fact that the average age at surgery was very similar between studies. Second, GMFCS may be an important predictor of postoperative deformity, but too few papers reported it, and, so, analysis did not provide any meaningful information. However, investigation of GMFCS scores as a predictor of postoperative deformity would be valuable in a future prospective study. Finally, it is possible that laminoplasty may result in fewer complications than laminectomy, due to altered structural forces in the vertebral column [8, 39]. Unfortunately, only 6 of our included studies reported using laminoplasty, including one study that reported using both [19,20,21,22, 28, 30]. Furthermore, all studies that reported their techniques reported laminectomy of 4–6 vertebral levels, making it impossible to compare outcomes against operations involving only 1–2 levels. Improved reporting of surgical technique and reporting of individual patient data in future studies would allow this to be examined more thoroughly.
Our findings are in line with what is known about the pathophysiology of spinal deformity and agree with findings from other patient populations. For example, it is understood from studies involving laminectomy in pediatric cancer patients, cadaveric human spines, and porcine spines that laminectomy fundamentally alters the biomechanics of the spine, with laminectomy leading to increased spinal range of motion in response to flexion, extension, lateral bending, and rotation [7, 8, 39,39,41]. It is possible that operations involving greater numbers of laminectomy may lead to greater biomechanical instability, which may be a risk factor for development of deformity. The development of spinal deformity in this patient population likely corresponds to altered biomechanics and may indicate diminished functional reserve of the child’s musculoskeletal system to respond to laminectomy and prevent the development of spinal asymmetry [8, 9, 39]. A larger study with rigorous documentation of individual patient data may well be able to predict risk based on several variables including gender, GMFCS, and extent of surgery.
Limitations
Sources of weakness in this review include widely disparate incidence rates between studies, including rates as low as 0% in some studies, and lack of individual patient data, prohibiting the use of more advanced statistical techniques, such as multivariable linear regression. This is apparent in the funnel plots and I2 statistics, which show considerable heterogeneity and variability between studies. A lack of consistent reporting between studies is likely responsible for much of the differences between studies, and a dearth of information in some studies on how data was collected may be hiding real incidence values. Generally, the quality of the studies is low, as seen by the fact that all of the studies are retrospective level III evidence studies. Furthermore, differences in surgical techniques between institutions and individual surgeons, differences in measuring and reporting deformity angles, and differences in follow-up all likely contribute to disparities between studies. With increasing numbers of patients undergoing this surgery, future studies are likely to improve in consistent methods of reporting and protocols.
One limitation of our methodology is that we have only examined papers reporting incidence of spinal deformity in this population. However, there are many studies examining the outcomes of SDR in children with cerebral palsy that did not report spinal deformity. It is possible that studies with higher incidence rates of deformity are more likely to report it as an outcome than studies with low incidence, where the authors may feel it is unnecessary to report it. This may introduce an amount of selection bias into our analysis, again highlighting the importance of consistent reporting of results in future studies.
Conclusion
The incidence of spinal deformity after SDR is an important complication to consider when deciding whether to proceed with the procedure, and when educating patients about long-term outcomes. Spinal deformity can have a major impact on quality of life and should be weighed against the degree of spasticity and current quality of life. This review demonstrates that there are several possible predisposing factors that increase the risk of spinal deformity, including that males may be slightly less susceptible than females. Age at the time of surgery does not have a clear effect on long-term incidence, and studies that report longer time to follow-up report higher incidence of spinal deformity, which suggests that spinal deformity should be vigorously screened for throughout the life of the postoperative patient in order to provide timely intervention. Finally, we demonstrate that incidence of spinal deformity after SDR is likely no different, or only minimally higher, than in the natural history of cerebral palsy.
References
Oskoui M, Coutinho F, Dykeman J, Jette N, Pringsheim T (2013) An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol 55:509–519
Aquilina K, Graham D, Wimalasundera N (2015) Selective dorsal rhizotomy: an old treatment re-emerging. Arch Dis Child 100:798–802
Bolster E, van Schie P, Becher J, van Ouwerkerk W, Strijers R, Vermeulen R (2013) Long-term effect of selective dorsal rhizotomy on gross motor function in ambulant children with spastic bilateral cerebral palsy, compared with reference centiles. Dev Med Child Neurol 55:610–616
Carraro E, Zeme S, Ticcinelli V, Massaroni C, Santin M, Peretta P, Martinuzzi A, Trevisi E (2014) Multidimensional outcome measure of selective dorsal rhizotomy in spastic cerebral palsy. Eur J Paediatr Neurol 18:704–713
Loewen P, Steinbok P, Holsti L, MacKay M (1998) Upper extremity performance and self-care skill changes in children with spastic cerebral palsy following selective posterior rhizotomy. Pediatr Neurosurg 29:191–198
Foerster (1911) Resection of the posterior spinal nerve-roots in the treatment of gastric crises and spastic paralysis. Proc R Soc Med 4:254
De Jonge T, Slullitel H, Dubousset J, Miladi L, Wicart P, Illes T (2005) Late-onset spinal deformities in children treated by laminectomy and radiation therapy for malignant tumors. Eur Spine J 14:765–771
Bisschop A, van Engelen S, Kingma I, Holewijn R, Stadhouder A, van der Veen AJ, van Dieen JH, van Royen BJ (2014) Single level lumbar laminectomy alters segmental biomechanical behavior without affecting adjacent segments. Clin Biomech 29:912–917
Cobb M, Boop F (1994) Replacement laminoplasty in selective dorsal rhizotomy: possible protection against the development of musculoskeletal pain. Pediatr Neurosurg 21:237–242
Rutz E, Brunner R (2013) Management of spinal deformity in cerebral palsy: conservative treatment. J Child Orthop 7:415–418
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1–9
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B (1997) Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39:214–223
Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ (2014) In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 67:897–903
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Abbott R, Feldsten N, Wisoff J, Epstein (1992) Osteoplastic laminotomy in children. Pediatr Neurosurg 18:153–156
Hurvitz E, Marcinak C, Daunter A, Haapala H, Stibb S, McCormick S, Muraszko K, Gaebler-Spira D (2013) Functional outcomes of childhood dorsal rhizotomy in adults and adolescents with cerebral palsy. J Neurosurg 11:380–388
Langerak N, Vaughan C, Hoffman E, Figaji A, Fieggen A, Peter J (2009) Incidence of spinal abnormalities in patients with spastic diplegia 17 to 26 years after selective dorsal rhizotomy. Childs Nerv Syst 25:1593–1603
Li Z, Zhu J, Liu X (2008) Deformity of lumbar spine after selective dorsal rhizotomy for spastic cerebral palsy. Microsurgery 28:10–12
Golan J, Hall J, O’Gorman G, Poulin C, Benaroch T, Cantin M, Farmer J (2007) Spinal deformities following selective dorsal rhizotomy. J Neurosurg 106:441–449
Steinbok P, Hicdonmez T, Sawatzky B, Beauchamp R, Wickenheiser D (2005) Spinal deformities after selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg 102:363–373
Johnson M, Goldstein L, Thomas S, Piatt J, Aiona M, Sussman M (2004) Spinal deformity after selective dorsal rhizotomy in ambulatory patients with cerebral palsy. J Pediatr Orthop 24:529–536
Spiegel D, Loder R, Alley K, Rowley S, Gutknecht S, Smith-Wright D, Dunn M (2004) Spinal deformity following selective dorsal rhizotomy. J Pediatr Orthop 24:30–36
Turi M, Kalen V (2000) The risk of spinal deformity after selective dorsal rhizotomy. J Pediatr Orthop 20:104–107
Yasuoka S, Peterson H, MacCarty C (1982) Incidence of spinal column deformity after multilevel laminectomy in children and adults. J Neurosurg 57:441–445
Meyer N, Flatley T, Dunn D (2003) Superiorly based laminoplasty in children: average 6.8 year follow up of 21 patients. J Spinal Disord Tech 16:156–162
Peter J, Hoffman E, Arens L, Peacock W (1990) Incidence of spinal deformity in children after multiple level laminectomy for selective posterior rhizotomy. Childs Nerv Syst 6:30–32
Park T, Liu J, Edwards C, Walter D, Dobbs M (2017) Functional outcomes of childhood selective dorsal rhizotomy 20-28 years later. Cureus 9:e1256
Funk J, Haberl H (2016) Monosegmental laminoplasty for selective dorsal rhizotomy—operative technique and influence on the development of scoliosis in ambulatory children with cerebral palsy. Childs Nerv Syst 32:819–825
Van Schie P, Schothorst M, Dallmeijer A, Vermeulen R, Van Ouwerkerk W, Strijers R, Becher J (2011) Short- and long-term effects of selective dorsal rhizotomy on gross motor function in ambulatory children with spastic diplegia. J Neurosurg 7:557–562
Nordmark E, Josenby A, Lagergren J, Andersson G, Stromblad L, Westbom L (2008) Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatr 8:54
Farmer J, Sabbagh A (2007) Selective dorsal rhizotomies in the treatment of spasticity related to cerebral palsy. Childs Nerv Syst 23:991–1002
Peter J, Hoffman E, Arens J (1993) Spondylolysis and spondylolisthesis after five-level lumbosacral laminectomy for selective posterior rhizotomy in cerebral palsy. Childs Nerv Syst 9:287–288
Sindou M, Georgoulis G (2015) Keyhole interlaminar dorsal rhizotomy for spastic diplegia in cerebral palsy. Acta Neurochir 157:1187–1196
Tedroff K, Lowing K, Jacobson DNO, Astrom E (2011) Does loss of spasticity matter? A 10-year follow up after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol 53:724–729
Ailon T, Beauchamp R, Miller S, Mortenson P, Kerr JM, Hengel AR, Steinbok P (2015) Long-term outcome after selective dorsal rhizotomy in children with spastic cerebral palsy. Childs Nerv Syst 31:415–423
Reamy B, Slakey J (2011) Adolescent idiopathic scoliosis: review and current concepts. Ann Fam Med 64:111–116
Tupman GS (1962) A study of bone growth in normal children and its relationship to skeletal maturation. J Bone Joint Surg (Br) 44-B:42–67
Konieczny M, Senyurt H, Krauspe R (2013) Epidemiology of adolescent idiopathic scoliosis. J Child Orthop 7:3–9
Tai C, Hsieh P, Chen P, Chen L, Chen W, Lai P (2008) Biomechanical comparison of lumbar spine instability between laminectomy and bilateral laminectomy for spinal stenosis syndrome—an experimental study in porcine model. BMC Musculoskelet Disord 9:84
Joaquim A, Cheng I, Patel A (2012) Postoperative spinal deformity after treatment of intracranal spinal lesions. Spine J 12:1067–1074
Park TS, Johnston J (2006) Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Neurosurg Focus 15;21(2):e7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wheelwright, M., Selvey, P.J., Steinbok, P. et al. Systematic review of spinal deformities following multi-level selective dorsal rhizotomy. Childs Nerv Syst 36, 1025–1035 (2020). https://doi.org/10.1007/s00381-019-04375-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04375-x