Abstract
There are no reports indicating a prognostic difference based on normalization of left ventricular (LV) mechanical dyssynchrony after revascularization in patients with coronary artery disease (CAD). We retrospectively investigated 596 patients who underwent rest 201Tl and stress 99mTc-tetrofosmin electrocardiogram-gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging. All patients had significant stenosis with ≥ 75% narrowing of the coronary arterial diameter detected by coronary angiography performed after confirmation of ≥ 5% ischemia by the SPECT. Patients underwent revascularization and thereafter were re-evaluated by the SPECT during a chronic phase, and followed-up to confirm their prognosis for ≥ 1 year. The composite endpoint was the onset of major cardiac events (MCEs) consisting of cardiac death, non-fatal myocardial infarction (MI), unstable angina pectoris (UAP), and severe heart failure requiring hospitalization. The stress phase bandwidth (SPBW) was calculated by phase analysis with the Heart Risk View-F software and its normal upper limit was set to 38°. During the follow-up, 64 patients experienced MCEs: Cardiac death (n = 11), non-fatal MI (n = 5), UAP (n = 26), and severe heart failure (n = 22). The results of the multivariate analysis showed the ∆summed difference score %, ∆stress LV ejection fraction, and stress SPBW after revascularization to be independent predictors of MCEs. Additionally, the results of the multivariate logistic regression analysis showed the summed rest score%, summed difference score%, stress LV ejection fraction, and perfusion defects in the left circumflex artery region before revascularization to be independent predictors for normalized SPBW after revascularization. The prognosis of patients who normalized SPBW after revascularization was similar to that of patients with a normal SPBW before revascularization, while patients who did not normalize after revascularization had the worst prognosis. In conclusion, normalization of LV dyssynchrony after revascularization assessed with nuclear cardiology may help predict future MCEs and thus a useful indicator for predicting improved prognosis in patients with CAD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Left ventricular (LV) mechanical dyssynchrony, which is frequently observed in patients with cardiac dysfunction, is a very important prognostic factor in patients with cardiac disease. Recently, LV mechanical dyssynchrony indices were reportedly derived from a phase analysis with the electrocardiogram (ECG)-gated myocardial perfusion single-photon emission computed tomography (SPECT) [1,2,3]. The LV mechanical dyssynchrony indices derived from the phase analysis were found to show prognostic value in patients with non-ischemic cardiomyopathy [4,5,6]. However, few reports have described the prognostic value of LV mechanical dyssynchrony in patients with coronary artery disease (CAD) [7], and in particular, reports investigating Japanese patients with CAD are rare.
Therefore, we reported that the LV mechanical dyssynchrony index stress phase bandwidth (SPBW) was a predictor independent of the ischemic volume evaluated with SPECT in the previous study [8]. This index was shown to be useful in stratifying the risk of major cardiac events (MCEs) in patients with known or suspected stable CAD who had a preserved LV ejection fraction (LVEF) and were indicated for optimal medical therapy. We previously investigated the prediction and risk stratification of MCEs after treatment in Japanese CAD patients who underwent revascularization [9]. In this report, we concluded that patients with high SPBW before revascularization had a high MCE incidence, and suggested that SPBW assessed by ECG-gated SPECT was useful to predict future MCEs after revascularization. Our research results showed the prognostic value of SPBW in Japanese CAD patients [8, 9]. However, in the aspect of the prediction of MCEs in CAD patients undergoing revascularization, previous studies have suggested that ischemic reduction after revascularization evaluated with SPECT was an important prognostic predictor [10,11,12]. Similarly, improvement in LV mechanical dyssynchrony indices by revascularization is expected to improve prognosis, but no such study has been reported.
Fudim et al. examined the prognostic value of diastolic and systolic mechanical LV dyssynchrony among patients with CAD and reported that patients with normal LV mechanical dyssynchrony indices showed good long-term prognosis [13]. Therefore, we hypothesized that normalization of the SPBW after revascularization could be a predictive factor for prognosis in CAD patients who have undergone revascularization.
Given the above, we conducted a retrospective study to evaluate the aim as a hypothesis in patients with stable CAD, and to clarify the factors predicting normalization of SPBW after revascularization.
Method
Patient population
First, the study subjects were 596 patients who underwent rest 201Tl and stress 99mTc-tetrofosmin ECG-gated SPECT myocardial perfusion imaging (MPI) [8, 12, 14,15,16] at Nihon University Itabashi Hospital between April 2004 and November 2016. Second, after confirmation of ≥ 5% ischemia by the SPECT, a coronary angiography (CAG) was performed to detect the culprit stenotic lesion of the coronary artery. Significant stenosis was defined as ≥ 75% narrowing of the coronary arterial diameter according to the classification by the American Heart Association. Third, the patients underwent revascularization and, subsequently, were re-evaluated by the SPECT during a chronic phase.
The patients were followed-up to confirm their prognoses for at least 1 year after the second SPECT procedure. The mean intervals between each procedure were as follows: the first SPECT and the CAG: 1.4 ± 3.0 months, the CAG and revascularization: 0.8 ± 3.7 months, revascularization and the second SPECT: 11.6 ± 9.9 months. The second SPECT procedure was performed 13.8 ± 11.1 months after the first SPECT procedure. Performing revascularization on all coronary vessels with ischemia evidenced by SPECT was defined as complete revascularization.
We excluded patients ≤ 20 years old, those with hypertrophic or dilated cardiomyopathy, those with serious valvular heart disease, those with an onset of acute coronary syndromes within 3 months, those with a non-sinus rhythm, those with left bundle branch block, those with pacemakers or implantable cardioverter-defibrillator implantation, and those with a history of cardiac resynchronization therapy.
Follow-up data were collected via medical records and retrospectively analyzed for 572 patients (96%). This study was approved by the institutional review board of Nihon University Itabashi Hospital.
ECG-gated SPECT MPI
The procedure of rest 201Tl and stress 99mTc-tetrofosmin ECG-gated SPECT MPI was performed according to a previously reported protocol [8, 12, 14,15,16]. All patients received an intravenous (i.v.) injection of 201Tl (111 MBq), and a 16-frame gated SPECT MPI was initiated 10 min after injection during rest. The i.v. injection of 99mTc-tetrofosmin (740 MBq) was then performed under stress induced by ergometer exercise in 23% of patients and by adenosine triphosphate in 77% of patients. Sixteen-frame gated SPECT MPI acquisition was initiated 30 min after exercise or 30–60 min after adenosine stress. The acquisition was performed first in a supine position and subsequently in a prone position. No attenuation or scatter correction was used. A 12-lead ECG was monitored continuously during the stress tests. The heart rate and blood pressure were recorded at baseline and every minute for at least 3 min after the stress test.
The projection data over 360° were obtained with 64 × 64 matrices and a circular orbit. A triple-detector SPECT MPI system equipped with low-energy high-resolution collimators was used (GCA9300A; Canon Medical Systems Corp., Tokyo, Japan). SPECT MPI scans were reconstructed from the data with a data processor (JETStream Workspace 3.0; Philips North America, Milpitas, CA, USA) combined with a Butterworth filter of 201Tl (order 5; cut-off frequency 0.42 cycles/cm), another of 99mTc (order 5; cut-off frequency 0.44 cycles/cm), and a ramp filter.
SPECT MPI interpretation
The SPECT MPI data were analyzed with the quantitative perfusion SPECT software program (Cedars-Sinai Medical Center, Los Angeles, CA, USA). The SPECT MPI scans were divided into 20 segments [14] on 3 short-axis slices (distal, mid, basal) and 1 vertical long-axis (mid) slice and the tracer uptake of each segment was visually scored using a 5-point scale (0: normal; 1: slight reduction in the uptake; 2: moderate reduction in the uptake; 3: severe reduction in the uptake; and 4: absence of the uptake). The sum of the scores of 20 segments in the stress and rest images provided the summed stress score (SSS) and summed rest score (SRS), respectively. The summed difference score (SDS) was calculated as the difference between the SSS and SRS. The respective summed scores were converted to a percentage of the total myocardium (visual % myocardium). The visual % myocardium was derived from the summed score divided by the maximum potential score (4 × 20) and multiplied by 100. When the SDS was 8, the visual ischemic % myocardium was 10% [17]. A difference between SDS% derived from the first and second SPECT (∆SDS%) was used for evaluation of improvement in ischemia. The visual semi-quantitative scoring was performed by two independent expert interpreters who were not provided with patient clinical information.
The determination of coronary arterial territories involved with perfusion defects on a polar map of SPECT MPI was based on the standard model recommended by the SPECT MPI guidelines of the American Society of Nuclear Cardiology [18].
The LV functional analysis with ECG-gated SPECT MPI
Sixteen-frame quantitative gated SPECT data were analyzed with the Heart Risk View-F software program (Nihon Medi-Physics, Tokyo, Japan) to calculate the LVEF (%), LV end-diastolic volume (LVEDV, mL), and LV end-systolic volume (LVESV, mL) [19]. LV mechanical dyssynchrony was evaluated with the phase histogram and phase map of the onset of myocardial contraction derived from the phase analysis of the Heart Risk View-F software program. The histogram analysis provided the standard deviation of the phase distribution (phase SD) and the 95% width of the histogram (phase bandwidth).
In this study, the normal upper limit of phase bandwidth assessed with 99mTc-tetrofosmin was defined as 38° based on the mean + 2SDs of the normal value, which was reported in the Japanese Society of Nuclear Medicine working group normal database [20]. The LV mechanical dyssynchrony indices were estimated by two independent expert cardiologists who were not provided with patient clinical information.
Figure 1 shows representative phase histograms and phase map images in patients with no LV mechanical dyssynchrony (a) and severe LV mechanical dyssynchrony (b). The phase bandwidth and SD were 23.0° and 6.2 in patients without LV mechanical dyssynchrony, and 130.0° and 37.6, respectively, in patients with severe LV mechanical dyssynchrony.
Patient follow-up
The average follow-up period for all 572 analyzed patients was 29.5 ± 12.1 months after the second SPECT procedure. The primary endpoint was the onset of MCEs, which was a composite of cardiovascular death, non-fatal myocardial infarction (MI), unstable angina pectoris (UAP), and severe heart failure requiring hospitalization during follow-up.
Cardiac death was defined as death due to any cardiac cause, including fatal MI, heart failure, and sudden cardiac death. A diagnosis of UAP was provided for patients who required unscheduled hospitalization for the management of UAP occurring within 24 h of the most recent symptoms, and who had worsening ischemic discomfort, ischemic ECG changes without ST elevation, and negative troponins. A diagnosis of severe heart failure requiring hospitalization was provided for patients who required unscheduled hospitalization for the management of acute heart failure, and who had chest X-ray findings attributable to cardiac dysfunction (e.g. pulmonary edema) and respiratory distress. A patient who had insufficient data indicating the occurrence of MCEs was regarded as a non-event case. When a patient had several MCEs, only the first event was set as the follow-up endpoint.
Statistical analyses
All continuous variables were calculated as the means and standard deviations. Intergroup comparisons of continuous variables were performed with an independent t test. Intergroup comparisons of categorical variables were performed with the chi-square test. Cohen’s kappa (κ) was used to determine the inter-observer variability for the visual semi-quantitative scoring and LV mechanical dyssynchrony indices. A logistic regression model was used for univariate analyses to identify significant predictors for the abnormal SPBW (> 38°) and normalized SPBW after revascularization. A stepwise logistic regression model was employed for multivariate analyses with significant predictors as variables to determine independent predictors. A Cox proportional hazards model was used for univariate analyses to identify significant predictors of MCEs. A stepwise Cox proportional hazards model was performed for multivariate analyses with significant predictors as variables to determine independent predictors of MCEs. The Kaplan–Meier survival analysis was used to estimate the MCE-free survival rate in patients grouped according to normal or abnormal SPBW before and after revascularization. In the comparison of receiver operating characteristic (ROC) curves for detection of normalized SPBW after revascularization, DeLong’s test was used to compare the statistical difference in the area under the curve (AUC) of each parameter. Additionally, the optimal cut-off value of each parameter was calculated using the Youden index.
All data were analyzed using the MedCalc Statistical software program, version 19.7.1 (Mariakerke, Belgium). A P value of < 0.05 was considered statistically significant.
Results
Reproducibility of visual semi-quantitative scoring and LV mechanical dyssynchrony indices
Cohen’s kappa (κ) was 0.90 for the summed defect score in the visual semi-quantitative scoring and 0.96 for phase bandwidth in the LV mechanical dyssynchrony indices, indicating very good reproducibility.
Cardiac event rates and SPBW after revascularization with and without MCEs
During follow-up, 64 of 572 patients (11.2%) experienced MCEs consisting of cardiovascular death (11, 1.9%), non-fatal MI (5, 0.9%), UAP (26, 4.5%), and severe heart failure requiring hospitalization (22, 3.8%). The patients who developed MCEs had a significantly higher SPBW after revascularization than those without MCEs (61.0° ± 33.3° vs 41.3° ± 23.3°, P < 0.0001). From the comparison of each category, the SPBW after revascularization in patients who had cardiovascular death, non-fatal MI, and severe heart failure requiring hospitalization was significantly higher than in those without MCEs (Table 1).
Baseline characteristics of patients
Table 2 summarizes the baseline characteristics of the patients, divided into two groups according to normal or abnormal SPBW after revascularization. The proportion of male patients, patients with a history of MI or revascularization, and diabetes mellitus were significantly higher in the group with an abnormal SPBW after revascularization. Patients with an abnormal SPBW after revascularization had a significantly larger QRS width than those with a normal value (103.7 ± 18.6 vs. 97.2 ± 16.1 ms; P < 0.0001).
The inter-group comparison of the visual % myocardium, cardiac functions, angiographic findings, and MCE rates
Table 3 summarizes the visual % myocardium, cardiac functions, angiographic findings, and MCE rates in patients with normal or abnormal SPBW after revascularization. Patients with an abnormal SPBW after revascularization had significantly higher values for the SSS% and SRS% before and after revascularization, and SDS% after revascularization. The rest and stress LVEF before and after revascularization were significantly lower in patients with an abnormal SPBW after revascularization. The ΔSDS% and the difference between the stress LVEF derived from the first and second SPECT procedures (Δstress LVEF) were significantly higher in patients with a normal SPBW after revascularization. The proportion of patients with perfusion defects in the left circumflex artery (LCX) region was significantly higher in the group with an abnormal SPBW after revascularization than that with a normal value (52% vs. 28%). Regarding revascularization, there was no significant difference in the proportion of patients who underwent percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) between the groups. The proportion of patients who underwent complete revascularization was significantly higher in patients with a normal SPBW after revascularization. There was a significant difference in the MCE rates between patients with normal and abnormal SPBW after revascularization (6% vs. 14%; P < 0.0001). Among the overall MCEs, the incidence of cardiac death, non-fatal MI, or severe heart failure was significantly higher in patients with an abnormal SPBW after revascularization.
Predictors for MCEs
Table 4 summarizes the results of univariate and multivariate Cox proportional hazards regression analyses used to identify a predictor of MCEs. Univariate significant variables were a history of MI or revascularization, diabetes mellitus, eGFR, β-blockers, SSS% and SRS% before and after revascularization, SDS% after revascularization, ∆SDS%, ∆stress LVEF, rest and stress LVEF, LVEDV, LVESV, phase SD, and phase bandwidth before and after revascularization. Among those variables, ∆SDS%, ∆stress LVEF, and SPBW after revascularization were identified as multivariate independent predictors of MCEs.
Predictor of an abnormal SPBW after revascularization (> 38°)
Table 5 summarizes the results of a univariate and multivariate logistic regression analysis for evaluating predictors of an abnormal SPBW after revascularization (> 38°). The main significant predictors of an abnormal SPBW after revascularization were a history of MI or revascularization, diabetes mellitus, eGFR, SSS%, SRS%, ∆SDS%, rest and stress LVEF, LVEDV, and LVESV before and after revascularization, phase SD, and phase bandwidth before revascularization, ∆stress LVEF, perfusion defects in the LCX regions, CTO vessels, and complete revascularization. Among those variables, the multivariate analysis showed that the SSS% and stress LVEF after revascularization, ∆SDS%, and SBPW before revascularization were independent predictors of an abnormal SPBW after revascularization (> 38°).
Prediction of MCEs based on SPBW after revascularization
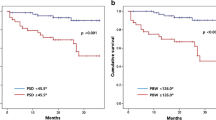
Figure 2 shows the Kaplan–Meier curves of the MCE-free survival in patients with a normal (≤ 38°) or abnormal (> 38°) SPBW after revascularization. The patients with an abnormal SPBW had a significantly worse prognosis than those with a normal SPBW.
Figure 3 shows the Kaplan–Meier curves of the MCE-free survival in patients with a normal SPBW before revascularization, normalized SPBW after revascularization, and abnormal SPBW before and after revascularization. The prognosis of patients who normalized SPBW after revascularization was as good as that of patients with a normal SPBW before revascularization, while patients who did not normalize after revascularization had the worst prognosis.
Predictor of the normalized SPBW after revascularization
After excluding patients with normal SPBW before revascularization, 328 patients with abnormal SPBW were examined using a univariate and multivariate logistic regression analysis for factors predicting SPBW normalization after revascularization. The result is shown in Table 6. The main significant predictors of the normalized SPBW after revascularization were a history of MI or revascularization, diabetes mellitus, eGFR, SSS%, SDS%, SRS%, rest and stress LVEF, LVEDV and LVESV, perfusion defects in the right coronary artery (RCA) or LCX region before revascularization, three-vessel CAD, CTO vessels, and complete revascularization. Among those variables, the multivariate analysis showed that the SRS%, SDS%, stress LVEF, and perfusion defects in the LCX region before revascularization were independent predictors of the normalized SPBW after revascularization.
Detection of normalized SPBW after revascularization
ROC curve analysis was used to determine the relative strengths of the association between the predictor variables and normalized SPBW after revascularization (Fig. 4). Stress LVEF before revascularization had the highest AUC 0.764 (95% confidence interval [CI] 0.714–0.809) followed by SRS% before revascularization (AUC 0.743, 95% CI 0.692–0.790), perfusion defects in the LCX region before revascularization (AUC 0.596, 95% CI 0.541–0.650), and SDS% before revascularization (AUC 0.566, 95% CI 0.510–0.620). Using the Youden index, optimal cut-off values of each variable before revascularization were shown in Fig. 4.
ROC curves for detection of normalized SPBW after revascularization. LVEF left ventricular ejection fraction, revasc revascularization, SRS summed rest score, LCX left circumflex artery, SDS summed difference score, AUC area under the curve, CI confidence interval, Sens. sensitivity, Spec. specificity, ROC Receiver operating characteristic
Representative case images
Figure 5 shows two cases of representative images. Both patients showed abnormal SPBW before treatment. In Patient A, the SPBW normalized after revascularization, which resulted in a good prognosis; however, in Patient B, the SPBW did not normalize and the prognosis was poor.
CAG, polar map of SPECT MPI, and phase analysis in representative cases. Each picture shows a coronary angiographic image, polar map images, and phase histogram images from representative patients with normalized SPBW (A) and without normalized SPBW (B) after revascularization. SPBW stress phase bandwidth, CAG coronary angiography, SPECT single-photon emission computed tomography, MPI myocardial perfusion imaging
Patient A was a 65-year-old female with congestive heart failure. She underwent CAG, which revealed 99% stenosis in the proximal region of the LAD (left anterior descending artery), 90% stenosis in the mid-region of the LCX (a), and 50% stenosis in the mid-region of the RCA (b). The first SPECT demonstrated an MI with extensive ischemia in the region of the LAD and her SPBW was 105°(c). She underwent PCI for the stenosis in the LAD and LCX by drug-eluting stent (DES) implantation (red dotted line). The second SPECT showed a significant reduction of infarct and ischemic size, with the SPBW normalization (105° → 30°) (d). LVEF improved from 33 to 66% after revascularization, and she had a good prognosis without cardiac events.
Patient B was a 63-year-old male with congestive heart failure and a history of CABG: Left internal thoracic artery (LITA)-LAD, radial artery (RA)-RCA. CAG revealed 90% stenosis in the mid-region of the LAD (a). The mid-region of the LCX, LITA graft, and proximal region of the RCA all had chronic total occlusion (a–c). The RA graft was patent (d). The first SPECT demonstrated small ischemia in the LAD region and large infarction with peri-infarction ischemia in the LCX region, and his SPBW was 86° (e). He underwent PCI for the stenosis in the LAD by DES implantation (red dotted line). The LCX lesion was deferred due to the challenges of PCI requiring complex techniques and severely reduced tracer uptake in this lesion. The second SPECT showed no significant reduction of infarct and ischemic size, without SPBW normalization (86° → 94°) (f). LVEF showed no significant change from 26 to 28% after revascularization, and he experienced heart failure readmission after revascularization.
Discussion
Clinically useful and new findings
This is the first report to clarify that normalization of the SPBW can be a predictor of prognosis improvement and to determine the predictors of SPBW normalization after revascularization. As a result, patients with abnormal SPBW after revascularization had a significantly higher incidence of MCE after revascularization. Furthermore, the Kaplan–Meier analysis showed that the prognosis of patients in which the SPBW normalized after revascularization was similar to that of patients in which the SPBW was normal before revascularization. Additionally, patients in which the SPBW was abnormal before and after revascularization had the worst prognosis.
From the results of a multivariate logistic regression analysis (Table 6), the factors of lower SRS%, absence of perfusion defects in the LCX region, higher SDS%, and higher stress LVEF before revascularization were shown to be independent predictors that led to normalization of the SPBW after treatment. Therefore, the clinical course of high-risk patients with multiple abnormal predictors before revascularization should be monitored carefully in the chronic phase after treatment to reduce the onset of MCEs. Such precise management will surely benefit patients.
Mechanism of improvement of LV dyssynchrony index after revascularization
In this study, 57% of patients had an abnormal SPBW before treatment (328/572), which indicated that Japanese CAD patients targeted for revascularization had a high prevalence of LV mechanical dyssynchrony. From the results of our previous study of patients with known or suspected stable CAD with preserved LVEF [8], patients with a higher SPBW value had a poor prognosis. In these patients, revascularization is strongly recommended because an improvement of prognosis is expected. However, from the present study, only 36% of patients had a normalized SPBW after revascularization. The reasons for this result were that patients with a high SPBW before treatment, low stress LVEF after treatment, high SSS after treatment, and a small amount of ischemic reduction were indicated to have an abnormal SPBW after revascularization, which was suggested by the multivariate logistic regression analysis (Table 5). Therefore, not only improving ischemia but also the improvement of LVEF is particularly important for improving SPBW after revascularization. Sillanmäki et al. reported that LV mechanical dyssynchrony measured by SPECT was strongly associated with LV systolic dysfunction, and LVEF was the most powerful predictor of abnormal phase bandwidth [21].
Additionally, the normalization of SPBW after revascularization was compared using a ROC analysis in patients with an abnormal SPBW before treatment in this study. As a result, stress LVEF before treatment had the highest prediction accuracy for normalization of SPBW after revascularization. This result was similar to the previous study [21]. Improvement of ischemia and improvement of LVEF by revascularization were independent factors, and it was considered that there were limits to the improvement of SPBW after revascularization in patients with a large infarct size before treatment and low LVEF. Therefore, to avoid MCEs after revascularization, and especially the onset of heart failure, it is important to strengthen and continue optimal medical treatment.
Association between revascularized coronary artery lesions and SPBW
In the present study, the results of the multivariate logistic regression analysis showed perfusion defects in the region of LCX before revascularization to be an independent predictor for a normalized SPBW after revascularization. Also, complete revascularization and CTO vessels were a significant univariate predictor for a normalized SPBW after revascularization.
In the multicenter CTO PCI registry, which examined PCI and procedural outcomes for CTO lesions, Christophoulos et al. reported that LCX was a common target vessel with incomplete revascularization. They assessed that LCX CTOs were technically difficult to revascularize due to anatomical features such as tortuosity, the steep angle of the LCX origin from LMT, and ipsilateral collateral [22], therefore, resulting in a lower rate of procedural success, less efficiency, and higher rates of complications compared with LAD or RCA CTOs. In the present study, 90% of revascularizations were performed by PCI, and many CTO patients were also included. Therefore, the LCX was considered to be an important vessel causing incomplete revascularization.
On the other hand, it has been reported in the Japanese CTO registry that PCI for LCX CTO lesions did not affect long-term cardiac mortality [23]. Our previous study has also shown that perfusion defects in the region of LCX was not a predictor of MCEs after revascularization, and was not associated with prognosis [9].
Regarding the correlation between coronary lesions and LV dyssynchrony, Ng et al. reported that the presence of proximal LCX stenosis might delay mechanical activation of the LV free wall and induce LV dyssynchrony, because the LCX normally supplies the LV lateral and posterior free walls that are finally activated by the cardiac conduction system [24].
LCX is also known to affect LV mechanical dyssynchrony and LVEF due to its wide perfusion area [25]. The previous study has shown that functional complete revascularization improved the prognosis of CAD patients [26]. Therefore, in patients with multivessel lesions including LCX with high SPBW, complete revascularization including LCX should be considered to improve cardiac function and prevent heart failure.
For the representative case B in which PCI was expected to be difficult due to a long LCX CTO lesion, it is important to perform complete revascularization by CABG as recommended by ESC/EACTS Guidelines [27]. This will not only lead to the significant reduction of infarct and ischemic size but also normalization of the SPBW. As a result, such a therapeutic strategy will lead to an improvement in the long-term outcome after revascularization and is expected to be extremely important for clinical management [28,29,30].
Limitations
This observational study has several limitations. First, the study design was a retrospective, single-center, and relatively small sample size investigation. In particular, the type of MCEs may bias due to the small sample size. Second, the study subjects included many patients (68%) with multi-vessel disease in whom perfusion defects existed in two or more coronary arterial stenoses. Therefore, direct comparison by SPBW estimated in each region of the coronary artery between LAD, RCA, and LCX was difficult. Third, there was also the potential for institutional bias in optimal treatment with medicine to prevent cardiovascular events, because this was an observational single center study.
Furthermore, 201Tl + 99mTc-tetrofosmin dual-isotope SPECT was utilized in the present study for improvement on throughput similar to the preceding studies [8, 12, 14,15,16]. The dual-isotope SPECT leads to higher radiation exposure than 1-day 99mTc-tetrofosmin low dose–high dose SPECT [31].
Finally, in this study, a direct comparison with the LV mechanical dyssynchrony index at rest and stress could not be performed due to the difference in tracers between the two conditions. However, in assessments of LV mechanical dyssynchrony, the low dose 99mTc has been reported to have a significantly higher phase SD than the high dose [31]. For this reason, the high dose tracer was used only at stress in the present study. The differences in protocols do not appear to affect the results of this study.
Conclusion
In conclusion, normalization of LV dyssynchrony after revascularization assessed with nuclear cardiology may help predict future MCEs and thus a useful indicator for predicting improved prognosis in patients with CAD.
References
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, Iskandrian AE (2005) Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol 12:687–695
Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, Santana C (2007) The increasing role of quantification in clinical nuclear cardiology: the Emory approach. J Nucl Cardiol 14:420–432
Hämäläinen H, Corovai A, Laitinen J, Laitinen TM, Hedman M, Hedman A, Kivelä A, Laitinen TP (2021) Myocardial ischemia and previous infarction contribute to left ventricular dyssynchrony in patients with coronary artery disease. J Nucl Cardiol 28:3010–3020
Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, van der Wall EE, Schalij MJ, Garcia EV, Bax JJ (2007) Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med 48:1104–1111
Zafrir N, Bental T, Strasberg B, Solodky A, Mats I, Gutstein A, Kornowski R (2017) Yield of left ventricular dyssynchrony by gated SPECT MPI in patients with heart failure prior to implantable cardioverter-defibrillator or cardiac resynchronization therapy with a defibrillator: characteristics and prediction of cardiac outcome. J Nucl Cardiol 24:122–129
Goldberg AS, Alraies MC, Cerqueira MD, Jaber WA, Aljaroudi WA (2014) Prognostic value of left ventricular mechanical dyssynchrony by phase analysis in patients with non-ischemic cardiomyopathy with ejection fraction 35–50% and QRS < 150 ms. J Nucl Cardiol 21:57–66
Hess PL, Shaw LK, Fudim M, Iskandrian AE, Borges-Neto S (2017) The prognostic value of mechanical left ventricular dyssynchrony defined by phase analysis from gated single-photon emission computed tomography myocardial perfusion imaging among patients with coronary heart disease. J Nucl Cardiol 24:482–490
Hatta T, Yoda S, Hayase M, Monno K, Hori Y, Fujito H, Suzuki Y, Matsumoto N, Okumura Y (2020) Prognostic value of left ventricular dyssynchrony assessed with nuclear cardiology in patients with known or suspected stable coronary artery disease with preserved left ventricular ejection fraction. Int Heart J 61:685–694
Fujito H, Yoda S, Hatta T, Hori Y, Hayase M, Miyagawa M, Suzuki Y, Matsumoto N, Okumura Y (2021) Prognostic significance of left ventricular dyssynchrony assessed with nuclear cardiology for the prediction of major cardiac events after revascularization. Intern Med 60:3679–3692
Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE, Investigators COURAGE (2008) Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation 117:1283–1291
Nanasato M, Matsumoto N, Nakajima K, Chikamori T, Moroi M, Takehana K, Momose M, Nishina H, Kasai T, Yoda S, Kiso K, Yamamoto H, Nishimura S, Yamashina A, Kusuoka H, Hirayama A, Nishimura T (2018) Prognostic impact of reducing myocardial ischemia identified using ECG-gated myocardial perfusion SPECT in Japanese patients with coronary artery disease: J-ACCESS 4 study. Int J Cardiol 267:202–207
Hori Y, Yoda S, Nakanishi K, Tano A, Suzuki Y, Matsumoto N, Hirayama A (2015) Myocardial ischemic reduction evidenced by gated myocardial perfusion imaging after treatment results in good prognosis in patients with coronary artery disease. J Cardiol 65:278–284
Fudim M, Fathallah M, Shaw LK, Liu PR, James O, Samad Z, Piccini JP, Hess PL, Borges-Neto S (2019) The prognostic value of diastolic and systolic mechanical left ventricular dyssynchrony among patients with coronary heart disease. JACC Cardiovasc Imaging 12:1215–1226
Berman DS, Kiat H, Friedman JD, Wang FP, van Train K, Matzer L, Maddahi J, Germano G (1993) Separate acquisition rest thalium-201/stress technetium-99m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography: a clinical validation study. J Am Coll Cardiol 22:1455–1464
Hayase M, Yoda S, Hatta T, Hori Y, Monno K, Fujito H, Suzuki Y, Matsumoto N, Okumura Y (2020) Prognostic significance of the residual SYNTAX score and ischemic reduction detected with nuclear cardiology for prediction of major cardiac events after revascularization. Intern Med 59:1361–1371
Makita A, Matsumoto N, Suzuki Y, Hori Y, Kuronuma K, Yoda S, Kasama S, Iguchi N, Suzuki Y, Hirayama A (2016) Clinical feasibility of simultaneous acquisition rest (99m)Tc/stress (201)Tl dual-isotope myocardial perfusion single-photon emission computed tomography with semiconductor camera. Circ J 80:689–695
Berman DS, Abidov A, Kang X, Hayes SW, Friedman JD, Sciammarella MG, Cohen I, Gerlach J, Waechter PB, Germano G, Hachamovitch R (2004) Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion SPECT interpretation. J Nucl Cardiol 11:414–423
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol 9:240–245
Nakae I, Hayashi H, Matsumoto T, Mitsunami K, Horie M (2014) Clinical usefulness of a novel program “Heart Function View” for evaluating cardiac function from gated myocardial perfusion SPECT. Ann Nucl Med 28:812–823
Nakajima K, Okuda K, Matsuo S, Kiso K, Kinuya S, Garcia EV (2017) Comparison of phase dyssynchrony analysis using gated myocardial perfusion imaging with four software programs: based on the Japanese Society of Nuclear Medicine working group normal database. J Nucl Cardiol 24:611–621
Sillanmäki S, Gimelli A, Ahmad S, Samir S, Laitinen T, Soman P (2021) Mechanisms of left ventricular dyssynchrony: a multinational SPECT study of patients with bundle branch block. J Nucl Cardiol 28:1140–1150
Christopoulos G, Karmpaliotis D, Wyman MR, Alaswad K, McCabe J, Lombardi WL, Grantham JA, Marso SP, Kotsia AP, Rangan BV, Garcia SA, Lembo N, Kandzari D, Lee J, Kalynych A, Carlson H, Thompson CA, Banerjee S, Brilakis ES (2014) Percutaneous intervention of circumflex chronic total occlusions is associated with worse procedural outcomes: insights from a multicentre US registry. Can J Cardiol 30:1588–1594
Mitomo S, Naganuma T, Jabbour RJ, Sato K, Takebayashi H, Kobayashi T, Obata JE, Sakamoto K, Tsujita K, Kugiyama K, Ogawa H, Nakamura S (2017) Impact of target vessel on long-term cardiac mortality after successful chronic total occlusion percutaneous coronary intervention: Insights from a Japanese multicenter registry. Int J Cardiol 245:77–82
Ng AC, da Tran T, Allman C, Vidaic J, Leung DY (2010) Prognostic implications of left ventricular dyssynchrony early after non-ST elevation myocardial infarction without congestive heart failure. Eur Heart J 31:298–308
Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW (2005) The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 1:219–227
Choi KH, Lee JM, Koo BK, Nam CW, Shin ES, Doh JH, Rhee TM, Hwang D, Park J, Zhang J, Kim KJ, Hu X, Wang J, Ye F, Chen S, Yang J, Chen J, Tanaka N, Yokoi H, Matsuo H, Takashima H, Shiono Y, Akasaka T (2018) Prognostic implication of functional incomplete revascularization and residual functional SYNTAX score in patients with coronary artery disease. JACC Cardiovasc Interv 11:237–245
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, ESC Scientific Document Group (2019) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 55:4–90
Sun LY, Gaudino M, Chen RJ, Bader Eddeen A, Ruel M (2020) Long-term outcomes in patients with severely reduced left ventricular ejection fraction undergoing percutaneous coronary intervention vs coronary artery bypass grafting. JAMA Cardiol 5:631–641
Marino M, Leonardi S, Crimi G, Ferrario M, Musumeci G, Tarantini G, Lettieri C, Bettari L, Maddalena L, De Luca L, Varbella F, De Servi S (2020) Lack of implementation of guidelines recommendations for coronary revascularization in stable patients with complex disease is associated with high rates of incomplete revascularization. Heart Vessels 35:30–37
Allahwala UK, Kiat H, Ekmejian A, Mughal N, Bassin L, Ward M, Weaver JC, Bhindi R (2021) Both surgical and percutaneous revascularization improve prognosis in patients with a coronary chronic total occlusion (CTO) irrespective of collateral robustness. Heart Vessels 36:1653–1660
Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, Grunwald MA, Levy D, Lytle BW, O’Rourke RA, Schafer WP, Williams SV, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Russell RO, Ryan TJ, Smith SC Jr (1999) ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 33:2092–2197
AlJaroudi W, Jaber WA, Cerqueira MD (2012) Effect of tracer dose on left ventricular mechanical dyssynchrony indices by phase analysis of gated single photon emission computed tomography myocardial perfusion imaging. J Nucl Cardiol 19:63–72
Acknowledgements
We appreciate Miss Yukiko Inoue for her assistance with this study.
Funding
This research received no grants from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujito, H., Yoda, S., Hatta, T. et al. Prognostic value of the normalization of left ventricular mechanical dyssynchrony after revascularization in patients with coronary artery disease. Heart Vessels 37, 1395–1410 (2022). https://doi.org/10.1007/s00380-022-02045-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-022-02045-8