Abstract
A field experiment was conducted to evaluate the combined or individual effects of biochar and nitrapyrin (a nitrification inhibitor) on N2O and NO emissions from a sandy loam soil cropped to maize. The study included nine treatments: addition of urea alone or combined with nitrapyrin to soils that had been amended with biochar at 0, 3, 6, and 12 t ha−1 in the preceding year, and a control without the addition of N fertilizer. Peaks in N2O and NO flux occurred simultaneously following fertilizer application and intense rainfall events, and the peak of NO flux was much higher than that of N2O following application of basal fertilizer. Mean emission ratios of NO/N2O ranged from 1.11 to 1.72, suggesting that N2O was primarily derived from nitrification. Cumulative N2O and NO emissions were 1.00 kg N2O-N ha−1 and 1.39 kg NO-N ha−1 in the N treatment, respectively, decreasing to 0.81–0.85 kg N2O-N ha−1 and 1.31–1.35 kg NO-N ha−1 in the biochar amended soils, respectively, while there was no significant difference among the treatments. NO emissions were significantly lower in the nitrapyrin treatments than in the N fertilization-alone treatments (P < 0.05), but there was no effect on N2O emissions. Neither biochar nor nitrapyrin amendment affected maize yield or N uptake. Overall, our results showed that biochar amendment in the preceding year had little effect on N2O and NO emissions in the following year, while the nitrapyrin decreased NO, but not N2O emissions, probably due to suppression of denitrification caused by the low soil moisture content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is an important contributor to global warming and is associated with the depletion of stratospheric ozone, whereas nitric oxide (NO) is a major precursor to atmospheric photo-oxidants that contribute to nitrogen (N) deposition and subsequent environmental acidification (Akiyama et al. 2010). Combined studies of NO and N2O emissions from agricultural ecosystems are uncommon due to the differing roles of the compounds in atmospheric dynamics. The use of N fertilizer and animal manure is considered to be a driver of N2O and NO emissions (Stehfest and Bouwman 2006) and agriculture accounts for almost 60 and 10% of global N2O and NO emissions, respectively (Ciais et al. 2013), derived from 2.8 and 1.6 Tg of N2O and NO emissions from fertilized agricultural fields, respectively (Bouwman et al. 2002). To satisfy the rising food demands of an increasing human population, especially in developing countries, greater inputs of N fertilizer not only reach levels of diminishing returns but also contribute to regional and global-scale environmental N-related issues (Chen et al. 2011).
The co-application of nitrification inhibitors with N fertilizer has been tested as an agronomic practice to decrease nitrate leaching and mitigate N2O and NO emissions (Di and Cameron 2006; Wu et al. 2017). Nitrification inhibitors can effectively reduce the oxidation rate of NH4+ to NO3−, thereby decreasing N loss from soils and increasing N uptake by crops (Di and Cameron 2006; Zaman et al. 2008). Nitrapyrin (2-chloro-6-(trichloromethyl) pyridine) is similar to other frequently used nitrification inhibitors, such as dicyandiamide (DCD) and 3,4-dimethylpyrazole phosphate (DMPP), and has been shown to inhibit the ammonia monooxygenase enzyme that catalyzes the first, rate-limiting step of nitrification (McCarty 1999; Abbasi and Adams 2000; Chen et al. 2010). Field measurements by Burzaco et al. (2013) and Martins et al. (2017) found that nitrapyrin reduced N2O emission by 49 and 24% with urea and urea-ammonium nitrate application during the maize growth season, respectively. The effectiveness of nitrification inhibitors on N2O emissions depends on environmental parameters, e.g., temperature, soil moisture content (Menéndez et al. 2012), soil available carbon (C) (Wu et al. 2017), the proportion of clays in soil (Shi et al. 2016), and the timing of fertilization (Parkin and Hatfield 2010). It is generally accepted that nitrification inhibitors have no direct effect on denitrification (Müller et al. 2002). However, some studies have shown that nitrification inhibitors affect N2O emissions more effectively under higher soil moisture levels by increasing the abundance of denitrifying genes (narG, nirK, and nosZ) (Barrena et al. 2017). Although the effects of nitrification inhibitor on N2O emission have been extensively studied, those investigating mitigation effects on NO emissions are poorly known (Wu et al. 2017), including key factors that influence effects of nitrapyrin on N2O and NO emissions from agricultural soils.
The addition of biochar to soil can be an effective mitigation technique for soil N2O emissions. Meta-analyses of laboratory and field studies by Cayuela et al. (2014) estimated reduction potentials of biochar amendment of soil on N2O emissions were 54 and 28%, respectively, where several mechanisms have been suggested. For example, biochar (1) improves soil aeration and immobilization of available N in the soil (van Zwieten et al. 2010b; Case et al. 2012), resulting in the suppression of denitrifier activities; (2) increases soil pH and the relative abundance of the bacterial N2O reductase nosZ gene that reduces N2O to N2 more efficiently (Harter et al. 2014); (3) increases adsorption of organic compounds (Kuzyakov et al. 2009) and microbial inhibiting compounds, such as ethylene (Spokas et al. 2010); and (4) increases adsorption of N2O, NO, and NH3 onto the biochar surface (Taghizadeh-Toosi et al. 2012; Cornelissen et al. 2013). However, effects of biochar on N2O emission may not be consistent among different types of biochar and soils (Ameloot et al. 2013; Cayuela et al. 2014); for example, Angst et al. (2014) did not observe effects on N2O emissions of biochar application to a sandy loam soil cultivated with ryegrass at rates of 5.7 and 18.8 t ha−1. To our knowledge, there have been few available studies on effects of biochar on NO emission (Nelissen et al. 2014; Obia et al. 2015). Data on biochar’s effects on NO emission are scarce, variable impacts on soil NO emission range from nearly no effect in a rice-wheat rotation field experiment (Xiang et al. 2015) to obvious reduction under incubation experiments (Nelissen et al. 2014; Obia et al. 2015), suggesting that biochar’s potential for reducing NO remains poorly regulated. The ingnored benefits of biochar applied on NO emission highlight the need for an improved understanding of the effect of biochar on the correlation bewteen N2O and NO emissions in agricultural soil.
Between 1990 and 2014 in China, grain production and N fertilizer consumption increased by 36 and 46%, respectively, reaching 607 and 23.9 million t, respectively (China Agricultural Yearbook Editorial Committee 2015). It has been estimated that fertilizer-induced N2O emissions from croplands increased from 115.7 Gg N2O-N year−1 in the 1980s to 210.5 Gg N2O-N year−1 in the 1990s (Zou et al. 2010), while fertilizer-induced NO emissions from croplands in 2012 were estimated to have been 336.97 Gg NO-N (Huang and Li 2014). Qin et al. (2017) found that excessive N fertilization had the potential to increase N2O emissions due to reduction of N2O reductase activity in soils. There is an urgent need to develop effective techniques to improve N use efficiency (NUE) and minimize N loss emissions in agricultural ecosystems, due to the environmentally degrading effects of reactive N enrichment of the atmosphere, soil, and water. Therefore, the objective of this study was to evaluate the field scale effects of biochar and nitrapyrin on N2O and NO emissions, and how biochar’s effect varied when the nitrapyrin was applied. We tested the hypotheses that (i) biochar can decrease N2O and NO emissions and increase N uptake due to the relatively high N retaining capacity, and (ii) the combination of biochar and nitrapyrin further reduces N2O and NO emissions, especially at high rates of biochar application.
Materials and methods
Experimental site
The study site was located at the Fengqiu National Station for Agroecological Observation and Research, Henan Province, China (35°00′ N, 114°24′ E), where the traditional cropping system is wheat (Triticum aestivum L.) grown in winter and maize (Zea mays L.) cultivated in summer. The region has a semi-arid, sub-humid monsoon climate, with a mean annual temperature of 13.9 °C and precipitation of 615 mm. Soils are derived from alluvial sediments of the Yellow River and classified as calcaric Fluvisol (Shi et al. 2010), with a pH of 8.41 in soil-water suspension (1:2.5 v/v), bulk density of 1.41 g cm−3, and particle size distribution of 15.8% clay, 16.2% silt, and 68.0% sand, with 7.40 g kg−1 organic C, 0.85 g kg−1 total N, 1.06 mg kg−1 NO3−-N, and 8.23 mg kg−1 exchangeable NH4+-N.
Experimental design
The field experiment was established on 9 June 2014 when maize was drilled, with the addition of maize straw biochar at 0, 3, 6, and 12 t ha−1. The biochar, produced by the Sanli New Energy Company, was pyrolyzed at 450 °C in a vertical kiln constructed from refractory bricks, had a pH of 10.02, and comprised 38.3% ash, 450 g kg−1 total C, 14.8 g kg−1 total N, 2.82 mg kg−1 NO3−-N, and 4.48 mg kg−1 exchangeable NH4+-N.
In June 2015, nine treatments were arranged in a randomized complete block design with three replicates, where experimental plots measured 9 m2, except the control that was 18 m2. N fertilizer (urea) was applied to the biochar amended plots, either alone or in combination with a nitrification inhibitor (nitrapyrin). Treatments comprised N fertilizer only (N), N fertilizer + 3 t ha−1 biochar (NB3), N fertilizer + 6 t ha−1 biochar (NB6), N fertilizer + 12 t ha−1 biochar (NB12), N + nitrapyrin (NI), NB3 + nitrapyrin (NB3I), NB6 + nitripyrin (NB6I), NB12 + nitripyrin (NB12I), and a control without N fertilizer. Urea applied at 200 kg N ha−1 was added as basal and supplemental fertilizers on 9 June and 22 July 2015, respectively, at a ratio of 2:3, and calcium superphosphate and potassium sulfate were applied at 120 kg P2O5 ha−1 and 120 kg K2O ha−1, respectively, as a basal dressing. The application rate of nitrapyrin (2-chloro-6-(trichloromethyl) pyridine) was equivalent to 0.26% of the applied urea N (w/w), namely 525 g ha−1. Basal fertilizers were evenly broadcast onto the soil surface and immediately tilled into the surface (0–20 cm) soil. Unlike in local, conventional farming, irrigation was applied prior to tillage due to low soil moisture from low levels of precipitation. Maize was drilled on 9 June in rows 70 cm apart and was harvested on 25 September 2015, before it was dried at 60 °C to a constant weight for grain yield and aboveground biomass analysis.
Greenhouse gas flux measurement
Cylindrical polyvinyl chloride (PVC) plastic tubes (10 cm long, 10-cm outer diameter in the lower half, and 10-cm inner diameter in the upper half) were installed approximately 5 cm into the soil, and one maize plant per plot was subsequently established in the center of each plastic tube. Soil fluxes were measured using the closed chamber method, using a chamber that comprised two sections, which were joined using a hinge and airtight rubber seal, and was externally covered with plastic foam to minimize solar heating and temperature fluctuations (Ding et al. 2007). The stainless steel rectangular chamber base (70 cm × 30 cm × 10 cm), with a 5 cm groove around the upper edge, was inserted into the soil to a depth of 10 cm around the PVC tube in each plot, immediately after drilling. In order to exclude the plant from the gas sampling area and to avoid the need to raise the height of the chamber as the plant grew, a separate PVC pipe (35 cm long, 10 cm outer diameter) was inserted into the existing PVC tube, and upper end of the PVC pipe was sealed with a rubber seal. The stainless steel rectangular upper chamber (70 cm × 30 cm × 30 cm) with a 10-cm diameter central aperture (to accommodate the PVC pipe) was fitted to the base by inserting the flange of the upper chamber into the 5-cm groove. The chamber was equipped with two ports: A small, silicon-sealed vent for gas sampling and a second port for measuring chamber temperature and two battery-operated fans were placed in the upper chamber to ensure adequate gas mixing.
During the maize growth season, gas was sampled twice per week in the morning, between 09:00 and 12:00, to minimize the effects of diurnal variation in flux patterns. On each occasion, four samples of air were manually extracted, using a plastic syringe, from the chamber into pre-evacuated 20-ml glass vials fitted with butyl rubber stoppers at 0, 10, 20, and 30 min after chamber closure. The air temperature inside the chamber was simultaneously measured using a mercury thermometer. The gas samples were analyzed on an Agilent 7890D gas chromatograph equipped with an electron capture detector (ECD) for N2O, using high-purity Ar-CH4 carrier gases for the ECD.
NO fluxes were measured by the static chamber method. At the start and end of gas sampling, about 2 L of chamber gas was collected by a large syringe. The gas samples were stored in Teflon gas bags and immediately measured on a NOx analyzer (Model 42i, Thermo Fisher Scientific Inc.).
Weather and soil physicochemical measurements
Precipitation and air temperature were monitored at a local meteorological station. Soil temperature at a depth of 5 cm was measured using a geothermometer, and soil moisture was measured using a time domain reflectometry probe (TDR), expressed as water-filled pore space (WFPS) determined by the following equation:
where total soil porosity = 1 − (soil bulk density / 2.65), based on the assumption that particle density of the soil was 2.65 g cm−3.
Three replicate surface soil samples (0–20 cm) were taken weekly, at random from row and/or inter-row of each plot, using a 5-cm diameter auger. Nitrate and exchangeable NH4+ were extracted using 2 M KCl solution and quantified colorimetrically using a continuous-flow autoanalyzer (San++ System, Skalar Analytical BV, Breda, the Netherlands). Dissolved organic C (DOC) was determined using a TOC analyzer (vario TOC Cube, Elementar, Hanau, Germany), where fresh soil (equivalent to 10 g dry soil) was mixed with 50 ml of deionized water, shaken for 30 min, centrifuged for 15 min (7570×g), and filtered with a 0.45-μm polyethersulfone membrane filter. Soil pH was determined from soil-water suspensions (1:2.5 v/v), while soil organic C was measured using the wet oxidation-redox titration method and soil total N content was measured using an elemental analyzer (Vario Max CN, Elementar, Hanau, Germany). Particle size distribution was determined using a laser particle size analyzer (LS13320, Beckmann Coulter, Brea, USA).
Data analysis
Rates of N2O and NO increase in the chamber air were calculated using linear regression of gas concentration against time, using chamber air temperature and atmospheric pressure as follows:
where F is the flux in N2O and NO (μg N2O-N or NO-N m−2 h−1); ρ is the gas density at standard temperature and pressure; V is the volume of the chamber; S is the area of the chamber; dC/dt is the change in gas concentration with time; and T is the mean temperature inside the chamber during sampling. Cumulative N2O and NO emissions (E, N2O-N kg ha−1 or NO-N kg ha−1) were calculated using the following equation:
where f represents the flux in N2O or NO (μg N m−2 h−1); i is the ith measurement; (ti + 1 − t i ) is the number of days between two adjacent measurements; and 24 × 10−5 was used for unit conversion. N2O and NO direct emissions factor (EF, %) of N fertilizer applied to the soil, with adjustment to background levels, was calculated as follows:
where Efertilizer and Econtrol are cumulative N2O or NO emissions (kg N ha−1) in the N fertilizer treatments and the control, respectively, and applied N is the application rate of urea (200 kg N ha−1 for each treatment). Yield-scaled emissions (g N2O-N kg−1 grain or g NO-N kg−1 grain) were calculated as (after Venterea et al. 2011):
where cumulative emissions is cumulative N2O or NO emissions (kg N ha−1) in all the treatments.
Soil inorganic N intensity of NH4+ (NH4I), NO3− (NO3I), and NH4I plus NO3I (IONI) was calculated as the summation of daily exchangeable NH4+-N, NO3−-N, and (NO3− + NH4+)-N concentrations in the 0–20 cm layer soil over the same period as cumulative N2O emissions, using linear interpolation between sample dates (Zebarth et al. 2008).
All data were analyzed using the SPSS software package for Windows (Version 18.0, SPSS Inc.). Differences among treatments were tested using one-way ANOVA followed by least significant difference (LSD) test at P < 0.05. Interaction effects of biochar and nitrapyrin on N2O and NO emissions and grain yield were tested using two-way ANOVA, and Pearson correlation analysis was used to determine the relationship between N2O or NO flux and other factors.
Results
Yield and N uptake
Compared with the control, maize grain yield was higher in all the urea-amended treatments (P < 0.05, Table 1). The highest grain yield and aboveground biomass were 12,287 and 21,649 kg ha−1 in the NB3I treatment, respectively. N uptake in the measures of aboveground biomass was consistently greater in each of the urea-amended treatments (223–236 kg N ha−1) than the control (158 kg N ha−1) (P < 0.05), and there were no treatment effects among urea-amended treatments.
Environmental and soil variables
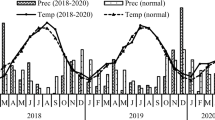
Average air temperature was 24.5 °C, with a range of 15.7–30.4 °C and soil temperature that varied from 16 to 34 °C (Fig. 1), and was correlated with air temperature in the treatments (R2 = 0.73–0.83, n = 31, P < 0.01). Total precipitation was 215.4 mm, and while soil moisture content ranged from 13 to 72% WFPS, there were no differences among the treatments. We recorded high soil WFPS values following irrigation events and/or heavy rainfall that were correlated with cumulative precipitation during the 3 days prior to the flux measurement (R2 = 0.25–0.34, n = 31, P < 0.05).
Across all treatments, average exchangeable NH4+ concentration ranged between 7.59 and 15.91 mg N kg−1. Soil exchangeable NH4+ concentration was higher in the NB3 treatment (48.83 mg N kg−1) than the control (5.17 mg N kg−1) after basal fertilization. After application of supplemental fertilizer, soil exchangeable NH4+ concentrations were higher in all the urea-amended treatments than the control, where peak concentration was 69% higher in the NB3I treatment than the NB3 treatment (P < 0.05, Fig. 2). Soil average NO3− concentrations ranged between 5.88 and 26.83 mg N kg−1 across all the treatments and were highest around 5 days after fertilizer application, before they gradually returned to background levels. After basal fertilization, the highest soil NO3− concentration was recorded in the NB3I treatment (29.25 mg N kg−1). Nitrapyrin lowered soil NO3− concentrations by 3–25%, compared with the application of urea alone after application of supplemental fertilizer (Fig. 2). Concentrations of both soil exchangeable NH4+ and NO3− showed small peaks when soil WFPS was > 65% after heavy rainfall events (24–25 June 2015). Mean DOC concentration in the urea-amended treatments ranged between 20.66 and 27.97 mg C kg−1 and was 29.88 mg C kg−1 in the control, and no apparent difference was found between urea-amended treatments (Fig. 2).
Mean level of soil NH4I was higher in the urea-amended treatments than in the control (P < 0.05, Table 2). Soil NH4I level was higher in the NB3I treatment than in the NB3 treatment, while in contrast, it was lower in the NB6I and NB12I treatments than in the NB6 and NB12 treatments, respectively (P < 0.05, Table 2). Mean level of soil NO3I in the NB12 treatment was higher than in the other urea-amended treatments (P < 0.05, Table 2).
N2O and NO fluxes
Mean N2O flux in the urea-amended treatments varied from 38.04 to 46.82 μg N2O-N m−2 h−1 and were higher than in the control (P < 0.05). Trends in N2O fluxes were generally similar among the treatments, with three distinct low peaks occurring after application of urea, heavy rainfall, and irrigation (Fig. 3). The first N2O flux peak was the day after application of basal fertilizer (10 June), when soil WFPS values ranged between 42 and 51% and soil temperature was > 28 °C; the highest peak was 153 μg N2O-N m−2 h−1 in the NB3I treatment. A second peak in N2O flux occurred on 26 June, when soil WFPS values increased to 67–72% following a heavy rainfall event (61.8 mm), where the highest peak, which was recorded in the N treatment, was 14% higher than the first peak. The third N2O flux peak occurred after supplemental fertilizer application (23 July) and subsequent irrigation that resulted in 56–66% soil WFPS; the highest peak (284 μg N2O-N m−2 h−1) occurred in the NI treatment. Compared with the N treatment, application of biochar increased the peaks in flux by 14–45%, whereas application of nitrapyrin to the biochar-amended soils reduced the peaks by 30–39% compared with the NI treatment. We found that N2O flux was correlated with soil WFPS in all the treatments and with soil exchangeable NH4+ concentration, except in the control (P < 0.05, Table 3). There was an exponential relationship between N2O fluxes and soil WPFS (Fig. 4).
The NO flux in the control treatment was consistently low, with an average of 8.20 μg NO-N m−2 h−1 (Fig. 3). Following application of basal fertilizer, the highest NO flux peak of 696 μg NO-N m−2 h−1 occurred in the NB6 treatment and this peak was 129% higher than in the NB6I treatment. No peaks in NO flux were identified following the heavy rainfall in June 2015. Following application of supplemental fertilizer, the highest NO flux peak was observed in the NB3 treatment and this peak was 34% higher than that in the NB3I treatment. NO flux was correlated with soil temperature and exchangeable NH4+ concentration (P < 0.05, Table 3).
Cumulative N2O and NO emissions
The lowest N2O emission was recorded in the control (0.31 kg N2O-N ha−1) and the highest in the N treatment (1.00 kg N2O-N ha−1) (Table 4). NO emissions recorded in all the urea-amended treatments were higher than the control (P < 0.05), and the highest emission was recorded in the N treatment (1.39 kg NO-N ha−1). While NO emissions were only reduced by 3–6% in plots that were treated with biochar alone (NB3, NB6, and NB12) compared with the N treatment, application of nitrapyrin significantly reduced NO emissions by 20–30% compared with the urea-amended-alone treatments. Cumulative NO emissions were higher than N2O emissions in the urea-amended treatments; however, an opposite pattern was observed in the control (P < 0.05). The ratios of NO/N2O emission ranged between 1.11 and 1.72 in the urea-amended treatments. There were no nitrapyrin × biochar interaction effects on N2O or NO emissions (Table S1).
Emission factors and yield-scaled emissions
The N2O emission factor of applied N was 0.34% in the N treatment, and the addition of biochar and nitrapyrin reduced this to between 0.22 and 0.28% (Table 4). The highest NO emission factor of applied N was 0.60% in the N treatment, and although there was no effect of the addition of biochar, application of nitrapyrin reduced NO emission factors by 23–34% compared with the urea-amended treatments (P < 0.05). The yield-scaled N2O emissions ranged from 0.064 to 0.085 g N2O-N kg−1 grain and were lower in the NB12I treatment than in the N treatment (P < 0.05). The highest yield-scaled NO emission was 0.118 g NO-N kg−1 grain in the N treatment, and the addition of nitrapyrin reduced this to 0.082–0.093 g NO-N kg−1 grain (P < 0.05, Table 4).
Discussion
Effect of nitrapyrin on N2O and NO emissions
We found that the N2O emission factor of applied N of 0.34% was much lower than the IPCC default value of 1.00% (IPCC 2006), and that measured in the neighboring field (1.06%) in a previous study (Cai et al. 2013). Nitrous oxide can increase due to denitrification (including nitrifier denitrification) following irrigation and/or intense rainfall (Wan et al. 2009; Zhou et al. 2016). In our study, irrigation was carried out prior to application of basal fertilizer to ensure germination under the low rainfall conditions. This practice reduced soil WPFS peak to c. 50%, due to the longer time available for evaporation, and contrasts with values of 65–85% that are typically recorded when irrigation is applied at the time of basal fertilizer application (Ding et al. 2007; Cai et al. 2013). Cai et al. (2013) suggested that irrigation prior to plowing minimized the formation of anaerobic conditions for denitrification, leading to a decrease in N2O emissions. Thus, management practices, such as tillage following appropriate irrigation to avoid excessive saturation of arable soils, may reduce conversion of applied N into N2O by denitrification.
We found that the estimated NO emission factor of 0.60% was within the previously reported range of 0.52–0.66% (Yan et al. 2003; Cui et al. 2012). The highest NO flux peaks occurred at 40–55% WFPS following the application of basal fertilizer, which was within the range of the optimal WFPS (40–60%) for NO emissions (Abalos et al. 2014), but much lower than the optimal WPFS (60–70%) for N2O emissions (Davidson et al. 2000). The relationship between N2O fluxes and soil WFPS indicated that optimal soil moisture for N2O emission in the experimental soils was 60–70% WFPS (Fig. 4). The ratio of NO/N2O emissions was > 1 in all the urea-amended treatments, suggesting that NO and N2O were primarily derived from nitrification (Anderson and Levine 1986) and confirmed previous studies that found nitrification was the dominant process (Ding et al. 2007; Cui et al. 2012). Thus, irrigation prior to plowing could contribute to increased NO emissions, creating a trade-off with N2O emission.
We found that nitrapyrin amendment significantly reduced NO emission by 20–30%, compared with the only N treatment, and this was slightly lower than the range of 35–84% reported previously (Baumgärtner and Conrad 1992; Akiyama et al. 2010). The use of nitrification inhibitors results in NH4+ retention by blocking the ammonia mono-oxygenase enzyme and preventing N2O and NO emissions through longer retention of N in the NH4+ form (Abbasi and Adams 2000; Wolt 2004). Using the 15N trace technique, Russow et al. (2009) found that NO2− was the main intermediate product for NO production under aerobic conditions and contributed 70% of the total emitted NO emissions. Nitrapyrin may effectively inhibit NO2− accumulation in alkaline soils (Shen et al. 2003) that would subsequently lead to the inhibition of conversion of NO2− to NO3− (Chen et al. 2010; Cui et al. 2013). In our study, lower soil NO3− intensity in the nitrapyrin-amended treatments, with the exception of the NB3I treatment, indicated that nitrapyrin effectively contributed to the inhibition of conversion of NH4+ to NO2− and NO3−. Fu et al. (2018) and Shi et al. (2016) have also confirmed negative responses of ammonia-oxidizing bacteria activity and nitrification to nitrification inhibitors in arable soils.
Ding et al. (2011) and Zhou et al. (2016) reported a 31–39% reduction of N2O emissions under DCD amendment of maize, compared with the application of N fertilizer alone. However, in our study, there was no reduction in N2O emissions following nitrapyrin amendment, supporting observations by Parkin and Hatfield (2010) in corn field, and was likely due to the reduction in denitrification activity in the experimental soil (Wan et al. 2009; Cai et al. 2013). We compiled data from the literature on N2O emissions under the application of N fertilizer with (N2ONi) and without (N2ON) nitrification inhibitors in the local region (North China Plain) for comparison with our data (Table 5) and analyzed the relationship between the response ratio of N2O emissions to nitrification inhibitor (RRNi = (N2ON − N2ONi) / N2ON) and soil WFPS. We found that efficacy of inhibitors on N2O emissions increased with soil WFPS (Fig. 5). Xue et al. (2012) found that DMPP (3,4-dimethylpyrazole phosphate) more efficiently reduced oxidation of NH4+ to NO3− at 40% water holding capacity (WHC) than at 60 and 80% WHC in an incubation experiment on brown soil, probably as a results of slower DMPP degradation in the drier conditions. In contrast, Balaine et al. (2015) observed the higher efficacy and longer half-time of DCD at wetter soil when targeted at urine-induced N2O emissions. The low efficacy of nitrapyrin can be explained by (1) biological process: microbial or plant uptake of the nitrification inhibitor (Marsden et al. 2016; Pal et al. 2016) and microbial degradation in warm conditions (Weiske et al. 2001); and (2) physicochemical process: solubility/leaching with rainfall or irrigation and sorption onto soil components (Shi et al. 2016; Marsden et al. 2016). We suggest that the small inhibitive effects of nitripyrin on N2O emission we found in this study were primarily driven by the low levels of soil moisture that reduced the denitrification process and N2O production and masked the potential effect of inhibitors. Further study is required to evaluate the effect of inhibitors on the relative contributions of nitrification and denitrification to N2O emissions at different levels of the soil moisture.
Correlation between the response ratio of N2O emissions to nitrification inhibitor (RRNi) and soil WFPS during the maize growth season. Squares and triangles represent the results from previous studies and the present study, respectively, as detailed in Table 6. Shaded sections indicate the 95% confidence intervals of the regression models
Combined effects of biochar and nitrapyrin on N2O and NO emissions
There are studies with conflicting results on the influence of biochar on N2O emissions, showing that inhibition (van Zwieten et al. 2010a; Zhang et al. 2012), stimulation (Saarnio et al. 2013), and no effect (Karhu et al. 2011) have been reported. Here, we found no effects of biochar on soil N2O or NO emissions during the maize growth season, unlike Niu et al. (2017) who reported significant reductions in emissions of N2O, and Nelissen et al. (2014) and Obia et al. (2015) who recorded reductions in NO emission under aerobic conditions and anaerobic conditions. It is known that biochar inhibits NO emissions due to an increase in N immobilization and non-electrostatic sorption of NH4+ and stimulation of ammonia volatilization (Nelissen et al. 2014).
The increased soil pH caused by biochar may alter the product stoichiometry of the denitrification process (reducing N2O/N2 ratio) by increasing the relative abundance of nosZ genes that encode for N2O reductase in acid soils (Harter et al. 2014; Obia et al. 2015). The soil acidification in our study (Table 6) due to the excessive use of fertilizer may lead to the higher N2O/N2 ratios during denitrification (Qu et al. 2014). Wang et al. (2018) demonstrated that the N2O emission increased by 21–119% under one unit decreasing of soil pH, based on a global meta-analysis of 1104 field experiments. As discussed above, N2O emission was mainly derived from nitrification in our study; thus, stimulation effect of soil acidification on N2O emission may be low. Baggs et al. (2010) reported that acidification of fertilized soil with H2SO4 lowered soil N2O emission, while liming of the acid soil with CaCO3 increased N2O emission with nitrification. The lower pH reduced the NH3 availability during nitrification (Blum et al. 2018). In this study, addition of biochar did not affect soil pH (Table 6), suggesting that there was absence of liming effect of biochar on N2O emission in the test alkaline soil, which was consistent with the finding of Liu et al. (2014). In addition, other mechanisms of biochar-mediated reductions in N2O emissions have been suggested: Firstly, the adsorption of inorganic N through abiotic or biotic processes by biochar decreases the availability of substrates for nitrifiers and denitrifiers (van Zwieten et al. 2010b; Kammann et al. 2012); secondly, reduction in the soil bulk density by biochar increases soil aeration and decreases soil WFPS, which in turn lowers the denitrification potential and N2O emissions (van Zwieten et al. 2010b; Harter et al. 2014). We found no significant difference in inorganic N and DOC concentrations between plots treated with urea alone and biochar, but soil bulk density reduced by biochar (Table 6). Thus, we suggest that the lack of effect of biochar on N2O emissions in this study may have been a result of a reduced denitrification process and N2O production in the low moisture experimental soils. A decrease in N immobilization and associated reduced effect on NO emission suppression caused by biochar in our study may have stemmed from the age of the biochar (degradation) and/or saturation with N.
Fuertes-Mendizábal et al. (2017) found that combined amendment with biochar and DMPP diminished the inhibition effect of the nitrification inhibitor on N2O emission at 40 and 80% WFPS, probably due to the adsorption of DMPP by the biochar (Shi et al. 2015). In comparison to DMPP, we speculate that the adsorption of the nitrapyrin might also occur after application of biochar. In the present study, no significant interactions of nitrapyrin and biochar on N2O and NO emissions were found mainly due to the small effect of the biochar. Continuous measurement in the deployed plots is required to evaluate the long-term effect of biochar on N2O and NO emission.
Impact of biochar and nitripyrin on grain yield and yield-scaled N2O emissions
Whereas we found that grain yield and N uptake increased in N fertilized treatments but were unaffected by the addition of biochar, Zhang et al. (2012) observed that biochar amendment at 20–40 t ha−1 increased maize yield by 12–18%. However, biochar has also been found to decrease crop yield (Rajkovich et al. 2012) or have no effect on maize growth (Nguyen et al. 2016). The N immobilization has been suggested as an important mechanism for decreasing availability of N, since biochar tends to have high C/N ratios (> 25) (van Zwieten et al. 2010b), but this may not have been applied in this study because mean levels of soil NO3I and IONI in the NB3 and NB12 treatments were shown to be high. It is likely that the high application rate of N fertilizer masked the negative effects of N immobilization in the subsequent year (Liu et al. 2014). Major et al. (2010) found a 3-year increase in maize yield following a single application of 8–20 t ha−1 of biochar to an infertile soil, and Yamato et al. (2006) similarly found an increase in maize yield following biochar amendment of an infertile soil, but not in fertile soils. Thus, we postulate that, under biochar amendment, an increase in crop yields may be expected in infertile, nutrient-poor, or acid soils (Major et al. 2010; Jeffery et al. 2011; Liu et al. 2013), but not in fertile or alkaline soil (Tammeorg et al. 2014; Hagemann et al. 2017).
We found that crop grain yield and N uptake did not increase in the NI treatment, compared with the N treatment. Chatterjee et al. (2016) found a similar phenomenon, where the combined addition of nitrapyrin with urea had no effect on corn yield or NUE; however, nitrapyrin combined with urea had a greater effect on yield in a vegetable system and paddy soil by increasing NUE (Ma et al. 2013; Li et al. 2014). We found that aboveground biomass and N uptake were lower in the NB6I and NB12I treatments than in the NI treatment (Table 1), and since the application of nitrification inhibitors may cause retention of NH4+ and induce higher NH3 emissions in alkaline soils (Kim et al. 2012), the supply of N at the rapid crop growth stage may have been reduced (Ding et al. 2015).
In our study, yield-scaled N2O emissions ranged from 0.031 to 0.085 g N2O-N kg−1 grain, which was within the range reported by Venterea et al. (2011), but lower than that found in the study of Ma et al. (2013). The lowest yield-scaled and lowest cumulative N2O emissions were recorded in the NB12I treatment, suggesting that this treatment may offer the optimal mitigation of N2O emissions.
Conclusions
We found that irrigation prior to plowing reduced the levels of soil moisture following application of basal fertilizer, compared with irrigation following fertilizer application, and this approach mitigated more effectively N2O emissions than NO emissions. Although biochar decreased soil bulk density, there was no effect on both N2O and NO emissions, whereas application of the nitrification inhibitor mitigated NO emissions, but not N2O emissions. We suggest that the potential effects of amendment with biochar in the previous year and addition of nitrapyrin on mitigating N2O emissions were probably offset by the low levels of moisture in the experimental soils that suppressed soil denitrification. Both biochar and nitrapyrin did not show any significant effect on maize yields and the N uptake in plant. Overall, we suggest that the NB12I treatment could be used as a “win-win” strategy with more agricultural income for farmers and less N2O emission for the environment in the North China Plain.
References
Abalos D, Sanchez-Martin L, Garcia-Torres L, van Groenigen JW, Vallejo A (2014) Management of irrigation frequency and nitrogen fertilization to mitigate GHG and NO emissions from drip-fertigated crops. Sci Total Environ 490:880−888
Abbasi MK, Adams WA (2000) Estimation of simultaneous nitrification and denitrification in grassland soil associated with urea-N using 15N and nitrification inhibitor. Biol Fertil Soils 31:38−44
Akiyama H, Yan XY, Yagi K (2010) Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta–analysis. Glob Change Biol 16:1837–1846
Ameloot N, Graber ER, Verheijen FGA, De Neve S (2013) Interactions between biochar stability and soil organisms: review and research needs. Eur J Soil Sci 64:379−390
Anderson IC, Levine JS (1986) Relative rates of nitric oxide and nitrous oxide production by nitrifiers, denitrifiers, and nitrate respirers. Appl Environ Microbiol 51:938–945
Angst TE, Six J, Reay DS, Sohi SP (2014) Impact of pine chip biochar on trace greenhouse gas emissions and soil nutrient dynamics in an annual ryegrass system in California. Agr Ecosyst Environ 191:17–26
Baggs EM, Smales CL, Bateman EJ (2010) Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol Fertil Soils 46:793–805
Balaine N, Clough TJ, Kelliher FM, van Koten C (2015) Increasing soil aeration reduces mitigation efficacy of dicyandiamide when targeted at ruminant urine-derived N2O emissions. New Zeal J Agr Res 58:441–453
Barrena I, Menéndez S, Correa-Galeote D, Vega-Mas I, Bedmar EJ, González-Murua C, Estavillo JM (2017) Soil water content modulates the effect of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 303:1–8
Baumgärtner M, Conrad R (1992) Effects of soil variables and season on the production and consumption of nitric oxide in oxic soils. Biol Fertil Soils 14:166–174
Blum JM, Su QX, Ma YJ, Valverde-Pérez B, Domingo-Félez C, Jensen MM, Smets BF (2018) The pH dependency of N-converting enzymatic processes, pathways and microbes: effect on net N2O production. Environ Microbiol 20:1623–1640
Bouwman AF, Boumans LJM, Batjes NH (2002) Modeling global annual N2O and NO emissions from fertilized fields. Glob Biogeochem Cycle 16(4):1080
Burzaco JP, Smith DR, Vyn TJ (2013) Nitrous oxide emissions in Midwest US maize production vary widely with band-injected N fertilizer rates, timing and nitrapyrin presence. Environ Res Lett 8:035031
Cai YJ, Ding WX, Luo JF (2013) Nitrous oxide emissions from Chinese maize–wheat rotation systems: a 3-year field measurement. Atmos Environ 65:112–122
Case SDC, McNamara NP, Reay DS, Whitaker J (2012) The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil-the role of soil aeration. Soil Biol Biochem 51:125–134
Cayuela ML, van Zwieten L, Singh BP, Jeffery S, Roig A, Sánchez-Monedero MA (2014) Biochar’s role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16
Chatterjee A, Cattanach N, Awale R, Thapa R (2016) Can we reduce rainfed maize (Zea mays L.) nitrogenous fertilizer application rate with addition of nitrapyrin? Commun Soil Sci Plant 47:527–532
Chen DL, Suter HC, Islam A, Edis R (2010) Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol Biochem 42:660–664
Chen XP, Cui ZL, Vitousek PM, Cassman KG, Matson PA, Bai JS, Meng QF, Hou P, Yue SC, Römheld V, Zhang FS (2011) Integrated soil-crop system management for food security. Proc Natl Acad Sci USA 108:6399–6404
China Agricultural Yearbook Editorial Committee (2015) China agricultural yearbook 2014. China Agricultural Publishing Press, Beijing, p 895
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S, Thornton P (2013) Carbon and other biogeochemical cycles, In: climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (eds stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM). Cambridge University press, Cambridge, UK and New York, NY. In: USA pp:465–570
Cornelissen G, Rutherford DW, Arp HPH, Dörsch P, Kelly CN, Rostad CE (2013) Sorption of pure N2O to biochars and other organic and inorganic materials under anhydrous conditions. Environ Sci Technol 47:7704–7712
Cui F, Yan GX, Zhou ZX, Zheng XH, Deng J (2012) Annual emissions of nitrous oxide and nitric oxide from a wheat-maize cropping system on a silt loam calcareous soil in the North China Plain. Soil Biol Biochem 48:10–19
Cui PY, Fan FL, Yin C, Li ZJ, Song AL, Wan YF, Liang YC (2013) Urea-and nitrapyrin-affected N2O emission is coupled mainly with ammonia oxidizing bacteria growth in microcosms of three typical Chinese arable soils. Soil Biol Biochem 66:214−221
Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides: using two functions based on soil nitrogen availability and soil water content, the hole-in-the-pipe model characterizes a large fraction of the observed variation of nitric oxide and nitrous oxide emissions from soils. Bioscience 50:667–680
Di HJ, Cameron KC (2006) Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide. Biol Fertil Soils 42:472–480
Ding WX, Cai YJ, Cai ZC, Yagi K, Zheng XX (2007) Nitrous oxide emissions from an intensively cultivated maize–wheat rotation soil in the North China plain. Sci Total Environ 373:501–511
Ding WX, Yu HY, Cai ZC (2011) Impact of urease and nitrification inhibitors on nitrous oxide emissions from fluvo-aquic soil in the North China Plain. Biol Fertil Soils 47:91–99
Ding WX, Chen ZM, Yu HY, Luo JF, Yoo G, Xiang J, Zhang HJ, Yuan JJ (2015) Nitrous oxide emission and nitrogen use efficiency in response to nitrophosphate, N-(n-butyl) thiophosphoric triamide and dicyandiamide of a wheat cultivated soil under sub-humid monsoon conditions. Biogeosciences 12:803−815
Fu QL, Clark IM, Zhu J, Hu HQ, Hirsch PR (2018) The short-term effects of nitrification inhibitors on the abundance and expression of ammonia and nitrite oxidizers in a long-term field experiment comparing land management. Biol Fertil Soils 54:163–172
Fuertes-Mendizábal T, Huérfano X, Menéndez S, González-Murua C, Begoña González-Moro M, Ippolito J, Kamann C, Wrage-Mönnig N, Wrage-Mönnig N Borchard N, Cayuela ML, Spokas K, Sigua G, Novak J, Estavillo JM (2017) Biochar reduces efficiency of nitrification inhibitor 3,4-dymethylpyrazole phospate (DMPP) mitigating N2O emissions. EGU General Assembly Conference Abstracts p 5614
Hagemann N, Harter J, Kaldamukova R, Guzman-Bustamante I, Ruser R, Graeff S, Kappler A, Behrens S (2017) Does soil aging affect the N2O mitigation potential of biochar? A combined microcosm and field study. GCB Bioenergy 9:953–964
Harter J, Krause HM, Schuettler S, Ruser R, Fromme M, Scholten T, Kappler A, Behrens S (2014) Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J 8:660–674
Huang Y, Li DJ (2014) Soil nitric oxide emissions from terrestrial ecosystems in China: a synthesis of modeling and measurements. Sci Rep 4:7406
IPCC (2006) 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories, Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K. (eds.): Volume 4, Chapter 11, N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Application. IGES, Hayama, Japan
Jeffery S, Verheijen FGA, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187
Kammann C, Ratering S, Eckhard C, Mueller C (2012) Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J Environ Qual 41:1052–1066
Karhu K, Mattila T, Bergstrom I, Regina K (2011) Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—results from a short-term pilot field study. Agric Ecosyst Environ 140:309–313
Kim DG, Saggar S, Roudier P (2012) The effect of nitrification inhibitors on soil ammonia emissions in nitrogen managed soils: a meta-analysis. Nutr Cycl Agroecosys 93:51–64
Kuzyakov Y, Subbotina I, Chen HQ, Bogomolova I, Xu XL (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Li B, Fan CH, Xiong ZQ, Li QL, Zhang M (2014) The combined effects of nitrification inhibitor and biochar incorporation on yield-scaled N2O emissions from an intensively managed vegetable field in southeastern China. Biogeosci Discuss 11:15185–15214
Liu XY, Zhang AF, Ji CY, Joseph S, Bian RJ, Li LQ, Pan GX, Paz-Ferreiro J (2013) Biochar’s effect on crop productivity and the dependence on experimental conditions–a meta-analysis of literature data. Plant Soil 373:583–594
Liu XY, Ye YX, Liu YM, Zhang AF, Zhang XH, Li LQ, Pan GX, Kibue GW, Zheng JF, Zheng JW (2014) Sustainable biochar effects for low carbon crop production: a 5-crop season field experiment on a low fertility soil from Central China. Agric Syst 129:22–29
Ma YC, Sun LY, Zhang XX, Yang B, Wang JY, Yin B, Yan XY, Xiong ZQ (2013) Mitigation of nitrous oxide emissions from paddy soil under conventional and no-till practices using nitrification inhibitors during the winter wheat-growing season. Biol Fertil Soils 49:627–635
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333:117–128
Marsden KA, Marín-Martínez AJ, Vallejo A, Hill PW, Jones DL, Chadwick DR (2016) The mobility of nitrification inhibitors under simulated ruminant urine deposition and rainfall: a comparison between DCD and DMPP. Biol Fertil Soils 52:491–503
Martins MR, Sant’Anna SAC, Zaman M, Santos RC, Monteiro RC, Alves BJR, Jantalia CP, Boddey RM, Urquiaga S (2017) Strategies for the use of urease and nitrification inhibitors with urea: impact on N2O and NH3 emissions, fertilizer-15N recovery and maize yield in a tropical soil. Agric Ecosyst Environ 247:54–62
McCarty GW (1999) Modes of action of nitrification inhibitors. Biol Fertil Soils 29:1–9
Menéndez S, Barrena I, Setien I, González-Murua C, Estavillo JM (2012) Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biol Biochem 53:82–89
Müller C, Stevens RJ, Laughlin RJ, Azam F, Ottow JCG (2002) The nitrification inhibitor DMPP had no effect on denitrifying enzyme activity. Soil Biol Biochem 34:1825–1827
Nelissen V, Saha BK, Ruysschaert G, Boeckx P (2014) Effect of different biochar and fertilizer types on N2O and NO emissions. Soil Biol Biochem 70:244–255
Nguyen DH, Scheer C, Rowlings DW, Grace PR (2016) Rice husk biochar and crop residue amendment in subtropical cropping soils: effect on biomass production, nitrogen use efficiency and greenhouse gas emissions. Biol Fertil Soils 52:261–270
Niu YH, Chen ZM, Müller C, Zaman M, Kim DG, Yu HY, Ding WX (2017) Yield-scaled N2O emissions were effectively reduced by biochar amendment of sandy loam soil under maize-wheat rotation in the North China plain. Atmos Environ 170:58–70
Obia A, Cornelissen G, Mulder J, Dörsch P (2015) Effect of soil pH increase by biochar on NO, N2O and N2 production during denitrification in acid soils. PLoS One 10:e0138781
Pal P, McMillan AMS, Saggar S (2016) Pathways of dicyandiamide uptake in pasture plants: a laboratory study. Biol Fertil Soils 52:539–546
Parkin TB, Hatfield JL (2010) Influence of nitrapyrin on N2O losses from soil receiving fall-applied anhydrous ammonia. Agric Ecosyst Environ 136:81–86
Qin SP, Ding KR, Clough TJ, Hu CS, Luo JF (2017) Temporal in situ dynamics of N2O reductase activity as affected by nitrogen fertilization and implications for the N2O/(N2O + N2) product ratio and N2O mitigation. Biol Fertil Soils 53:723–727
Qu Z, Wang JG, Almøy T, Bakken LR (2014) Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils. Glob Change Biol 20:1685–1698
Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR, Lehmann J (2012) Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol Fertil Soils 48:271–284
Russow R, Stange CF, Neue HU (2009) Role of nitrite and nitric oxide in the processes of nitrification and denitrification in soil: results from 15N tracer experiments. Soil Biol Biochem 41:785–795
Saarnio S, Heimonen K, Kettunen R (2013) Biochar addition indirectly affects N2O emissions via soil moisture and plant N uptake. Soil Biol Biochem 58:99–106
Shen QR, Ran W, Cao ZH (2003) Mechanisms of nitrite accumulation occurring in soil nitrification. Chemosphere 50:747–753
Shi XZ, Yang GX, Yu DS, Xu SX, Warner ED, Petersen GW, Sun WX, Zhao YC, Easterling WE, Wang HJ (2010) A WebGIS system for relating genetic soil classification of China to soil taxonomy. Comput Geosci 36:768–775
Shi YF, Zhang LL, Zhao MQ (2015) Effect of biochar application on the efficacy of the nitrification inhibitor dicyandiamide in soils. Bioresources 10:1330–1345
Shi XZ, Hu HW, He JZ, Chen DL, Suter HC (2016) Effects of 3,4-dimethylpyrazole phosphate (DMPP) on nitrification and the abundance and community composition of soil ammonia oxidizers in three land uses. Biol Fertil Soils 52:927–939
Spokas KA, Baker JM, Reicosky DC (2010) Ethylene: potential key for biochar amendment impacts. Plant Soil 333:443–452
Stehfest E, Bouwman L (2006) N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosys 74:207–228
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) A wood based low-temperature biochar captures NH3-N generated from ruminant urine-N, retaining its bioavailability. Plant Soil 353:73–84
Tammeorg P, Simojoki A, Mäkelä P, Stoddard FL, Alakukku L, Helenius J (2014) Biochar application to a fertile sandy clay loam in boreal conditions: effects on soil properties and yield formation of wheat, turnip rape and faba bean. Plant Soil 374:89–107
Venterea RT, Maharjan B, Dolan MS (2011) Fertilizer source and tillage effects on yield-scaled nitrous oxide emissions in a corn cropping system. J Environ Qual 40:1521–1531
Wan YJ, Ju XT, Ingwersen J, Schwarz U, Stange CF, Zhang FS, Streck T (2009) Gross nitrogen transformations and related nitrous oxide emissions in an intensively used calcareous soil. Soil Sci Soc Am J 73:102–112
Wang YJ, Guo JH, Vogt RD, Mulder J, Wang JG, Zhang XS (2018) Soil pH as the chief modifier for regional nitrous oxide emissions: new evidence and implications for global estimates and mitigation. Glob Change Biol 24:e617–e626
Weiske A, Benckiser G, Herbert T, Ottow J (2001) Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol Fertil Soils 34:109–117
Wolt JD (2004) A meta-evaluation of nitrapyrin agronomic and environmental effectiveness with emphasis on corn production in the Midwestern USA. Nutr Cycl Agroecosys 69:23–41
Wu D, Cárdenas LM, Calvet S, Brüggemann N, Loick N, Liu SR, Bol R (2017) The effect of nitrification inhibitor on N2O, NO and N2 emissions under different soil moisture levels in a permanent grassland soil. Soil Biol Biochem 113:153–160
Xiang J, Liu DY, Ding WX, Yuan JJ, Lin YX (2015) Effects of biochar on nitrous oxide and nitric oxide emissions from paddy field during the wheat growth season. J Clean Prod 104:52–58
Xue Y, Wu ZJ, Zhang LL, Gong P, Dong XX, Nie YX (2012) Inhibitory effect of DMPP on soil nitrification as affected by soil moisture content, pH and organic matter. Chin J App Ecol 23:2663–2669
Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52:489–495
Yan XY, Akimoto H, Ohara T (2003) Estimation of nitrous oxide, nitric oxide and ammonia emissions from croplands in East, Southeast and South Asia. Glob Change Biol 9:1080–1096
Zaman M, Nguyen ML, Blennerhassett JD, Quin BF (2008) Reducing NH3, N2O and NO3 −–N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol Fertil Soils 44:693–705
Zebarth BJ, Rochette P, Burton DL (2008) N2O emissions from spring barley production as influenced by fertilizer nitrogen rate. Can J Soil Sci 88:197–205
Zhang AF, Liu YM, Pan GX, Hussain Q, Li LQ, Zheng JW, Zhang XH (2012) Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 351:263–275
Zhao ZC, Han X, Shi YF, Wu WL, Meng FQ (2016) Effect of nitrification and urease inhibitor on carbon sequestration and greenhouse gas emissions in winter wheat and summer maize rotation system in North China. T Chin Society Agr Eng 32:254–262
Zhou YZ, Zhang YY, Tian D, Mu YJ (2016) Impact of dicyandiamide on emissions of nitrous oxide, nitric oxide and ammonia from agricultural field in the North China Plain. J Environ Sci 40:20–27
Zhu YC, Li YE, Qin XB, Duan ZY, Wan YF, Zhou WP, Wang B, He JN (2016) Effects of controlled release fertilizer and nitrification inhibitor additions on nitrous oxide emissions from spring maize field in Northern China. J Agro-Environ Sci 35:1421–1428
Zou JW, Lu YY, Huang Y (2010) Estimates of synthetic fertilizer N-induced direct nitrous oxide emission from Chinese croplands during 1980–2000. Environ Pollut 158:631–635
van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010a) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
van Zwieten L, Kimber S, Morris S, Downie A, Berger E, Rust J, Scheer C (2010b) Influence of biochars on flux of N2O and CO2 from ferrosol. Aust J Soil Res 48:555–568
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0200304, 2016YFD0200309), National Natural Science Foundation of China (41471207, 41501274), IAEA Coordinated Research Project (18627/R0), and the Frontier Project from the Institute of Soil Science, Chinese Academy of Sciences (ISSASIP1607).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 20.5 kb)
Rights and permissions
About this article
Cite this article
Niu, Y., Luo, J., Liu, D. et al. Effect of biochar and nitrapyrin on nitrous oxide and nitric oxide emissions from a sandy loam soil cropped to maize. Biol Fertil Soils 54, 645–658 (2018). https://doi.org/10.1007/s00374-018-1289-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-1289-2