Abstract
Background and aims
Modern agriculture is driving the release of excessive amounts of reactive nitrogen (N) from the soils to the environment, thereby threatening ecological balances and functions. The amendment of soils with biochar has been suggested as a promising solution to regulate the soil N cycle and reduce N effluxes. However, a comprehensive and quantitative understanding of biochar impacts on soil N cycle remains elusive.
Methods
A meta-analysis was conducted to assess the influence of biochar on different variables involved in soil N cycle using data compiled across 208 peer-reviewed studies.
Results
On average, biochar beneficially increases symbiotic biological N2 fixation (63%), improves plant N uptake (11%), reduces soil N2O emissions (32%), and decreases soil N leaching (26%), but it poses a risk of increased soil NH3 volatilization (19%). Biochar-induced increase in soil NH3 volatilization commonly occurs in studies with soils of low buffering capacity (soil pH ≤ 5, organic carbon≤10 g kg−1, or clay texture), the application of high alkaline biochar (straw- or manure-derived biochar), or biochar at high application rate (>40 t ha−1). Besides, if the pyrolytic syngas is not purified, the biochar production process may be a potential source of N2O and NOx emissions which correspond to 2–4% and 3–24% of the feedstock-N, respectively.
Conclusions
This study suggests that to make biochar beneficial for decreasing soil N effluxes, clean advanced pyrolysis technique and adapted use of biochar are of great importance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since Fritz Haber discovered the industrial method to synthesize ammonia in 1908, the use of fertilizer nitrogen (N) has boosted food production and fed billions of people (Erisman et al. 2008). However, it also increases reactive N fluxes from the soils to the environment (NH3 volatilization, N2O emissions, and N leaching), which accelerates global warming, decreases stratospheric ozone, increases ecosystem eutrophication, and induces the formation of pollutant particulate matter in the atmosphere (Gruber and Galloway 2008). The current worldwide use of fertilizer N is about 100 Tg N per year (FAO 2014), and nearly two-fifths of N input are released to the air and water (Liu et al. 2010). It is estimated that N fertilizer use will be increased by two- to threefold by the second half of the twenty-first century, which will further raise the N pressures on the environment (Tilman et al. 2011). Thus, mitigation solutions to decrease soil N losses are in urgent need (Zhang 2017).
In recent years, biochar, a recalcitrant carbonaceous product derived from biomass pyrolysis under limited oxygen conditions (Sohi 2012), is attracting great attention as a potential tool to regulate the soil N cycle and reduce N effluxes. There are studies reporting that biochar could decrease soil NH3 volatilization via absorbing NH4+ by the surface negatively-charged functional groups (carboxyl and phenolic hydroxyl) (Taghizadeh-Toosi et al. 2012), mitigate soil N2O emissions through reducing N2O towards N2 (Cayuela et al. 2013), and decrease soil N leaching by elevating soil water holding capacity (Sun et al. 2017a). However, other studies observed negative effects of biochar, with increased soil NH3 volatilization due to raised soil alkalinity (Zhao et al. 2013b; Sun et al. 2014), accelerated soil N2O emissions through facilitated nitrification (Sánchez-García et al. 2014), and increased N leaching due to exacerbated soil structure (Singh et al. 2010; Mukherjee and Lal 2014).
The effect of biochar on soil N cycle depends on the interaction between biochar characteristics and soil properties (Clough et al. 2013). The match of the right biochar with the right soil will achieve benefits, whereas arbitrary application of biochar without considering biochar and soil properties may induce irreversible negative consequences (Mukherjee and Lal 2014). Previous research studies usually investigated the effect of biochar on soil N cycle from a single perspective. However, whether biochar holds promise in benefiting soil N cycle and how to optimize biochar application under different conditions require an integrated evaluation. There have been several studies synthesizing the results of soil N2O emissions and soil inorganic N in response to biochar application (Cayuela et al. 2014; Verhoeven et al. 2017; He et al. 2017; Nguyen et al. 2017). But currently, a comprehensive quantitative overview that covers the whole N cycle as affected by biochar is lacking. In addition, of special importance is the biochar production process. It may be a potential source of N2O and NOx emissions (Ren et al. 2013), which highly influences the effectiveness of biochar for decreasing soil N effluxes. However, this has received less attention and requires further evaluation.
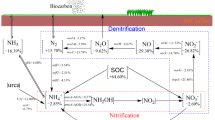
We therefore compiled results from peer-reviewed studies to evaluate the effects of biochar on soil N cycle, involving soil active N pools (dissolved organic N, inorganic N, and microbial biomass N), N transformations (mineralization, immobilization, and nitrification), N input (biological N2 fixation), and N outputs (plant N uptake, NH3 volatilization, N2O emissions, and N leaching) (Fig. 1). Moreover, the quantification of the potential N2O and NOx emissions derived from biochar production was also investigated. We aimed to (i) identify how and why the response of soil N cycle to biochar application varies across different biochar and soil properties; and (ii) explore whether biochar production process entails hidden risk of extra pollutant N emissions. The study is expected to develop constructive biochar management for decreasing soil N losses without incurring negative side effects.
Materials and methods
Data compilation
A literature search was performed through Web of Science, Google Scholar, Springer Link, Wiley Blackwell, and China Knowledge Resource Integrated (CNKI) databases using the keywords ‘biochar’, ‘black carbon’, ‘soil’, ‘nitrogen’. The resulting literature from this search was further screened to meet the following criteria: (i) the research was on soil N cycle in response to biochar addition; (ii) biochar was produced by pyrolyzing organic materials anaerobically (technology levels range from highly advanced facilities to simply equipped stoves); and (iii) control and biochar treatments were subjected to the same management (e.g. same tillage, watering, fertilization, or residue addition).
The resulting overall dataset consists of 208 papers published between 2003 and 2017, with 46 papers (796 observations) for soil active N pools, 23 papers (236 observations) for soil N transformations, 4 papers (25 observations) for biological N fixation, 35 papers (340 observations) for plant N uptake, 23 papers (99 observations) for soil NH3 volatilization, 70 papers (468 observations) for soil N2O emissions, and 36 papers (156 observations) for soil N leaching, respectively (Table S2). The dataset of soil N2O emissions was an update of the previous meta-analysis study by Cayuela et al. (2014) with 40 additional manuscripts. Experiments were performed under laboratory (n = 92, n represents the number of studies), greenhouse (n = 48) and field (n = 71) conditions. The biochar application pattern was either as a single dose (n = 199) or as multiple consecutive doses (n = 7). The experiment time scales ranged from 0 to 1 month (n = 37), 1–6 months (n = 96), 6–12 months (n = 25), to >12 months (n = 48). Biochar application rates expressed in weight percentage were transformed into t ha−1 based on a 10-cm application depth and a mean global agronomic soil bulk density of 1.3 g cm−3 (Batjes 2015) (e.g. 1% by weight corresponds to 13 t ha−1).
Meta analyses of biochar impacts on soil N cycle
The meta-analysis was performed using the MetaWin 2.1 software (Sinauer Associates Inc., Sunderland, MA, USA) (Rosenberg et al. 2000). The biochar effect size was calculated as a natural logarithm-transformed response ratio (r):
where X c and X t represent the mean value of the control and biochar treatments, respectively (Hedges et al. 1999). The variance (v) of ln (r) was obtained as:
where S c and S t are the standard deviations in the control and biochar treatments, respectively; n c and n t are the number of replicates for the control and biochar treatments, respectively (Hedges et al. 1999). The weighting factor (w) for the effect size of each observation was the reciprocal of its variance (Adams, 1997):
The mean effect size (\( \overline{\ln (r)} \)) for all observations was estimated as:
where r i and w i are the value of r and w of the ith observation, respectively (Adams 1997).
Parameters potentially affecting the response of the soil N cycle to biochar addition were classified into the following categories: (i) soil pH (pH ≤ 5, 5 < pH ≤ 6.5, 6.5 < pH ≤ 7.5, and pH > 7.5); (ii) soil texture (sand, silt, loam, and clay) (based on USDA textural classification system; Shirazi and Boersma 1984); (iii) soil organic carbon (SOC ≤ 5, 5 < SOC ≤ 10, 10 < SOC ≤ 20, and SOC > 20 g kg−1), (iv) soil CEC (CEC ≤ 5, 5 < CEC ≤ 10, 10 < CEC ≤ 20, and CEC >20 cmol kg−1); (v) biochar feedstock species (wood, straw, and manure; “wood biochars” are those made from wood, bamboo or nutshell, “straw biochars” are those made from crop residues, leaves, grass, paper, or husks, “manure biochars” are those made from animal waste); (vi) biochar pyrolysis temperature (T) (T ≤ 350, 350 < T ≤ 500, and T > 500 °C); (vii) biochar pH (pH ≤ 7, 7 < pH ≤ 8, 8 < pH ≤ 9, 9 < pH ≤ 10, and pH > 10); and (viii) biochar amendment rate (R) (R ≤ 10, 10 < R ≤ 20, 20 < R ≤ 40, 40 < R ≤ 80, 80 < R ≤ 120, and R > 120 t ha−1).
Mean effect sizes and the 95% bootstrapped confidence intervals (CIs) based on 9999 iterations for each grouping categories were generated based on a random-effect model (Adams et al. 1997). The total heterogeneity of effect sizes among studies (QT) was partitioned into within-group (QW) and between-group (QB) heterogeneity. A QB larger than a critical value suggests a significant difference between subgroups (Table.S1) (Gurevitch and Hedge, 1993). If the 95% CI value around a mean effect size does not overlap zero, the response of a selected N cycling variable to biochar addition is considered significantly different from the control treatment. Means among different subgroups are significantly different from one another if their 95% CIs are non-overlapping. In this study, the mean effect size and 95% CI expressed in the natural log value of the response ratio were converted into a relative percentage change when results were presented in graphs. All statistically significant differences were identified at P < 0.05.
Quantification of the potential N2O and NOx emissions derived from biochar production
Pyrolysis of biomass produces syngas which usually goes to the downstream combustion for heat and power generation (Kwiatkowski et al. 2013). A fraction of the N in feedstock can be converted into N-containing compounds in syngas and interface with the downstream applications. The major N-containing compounds in syngas are NH3 and HCN, with lower concentrations of N2O and NOx (Leppälahti and Koljonen 1995; Ren and Zhao 2013). If the syngas is not purified, the contaminants NH3 and HCN in syngas can serve as precursors for N2O and NOx in the downstream burners, gas engines, or gas turbines (Hämäläinen and Aho 1996). To quantify the potential amount of N2O and NOx that may derive from biochar production without syngas purification, we summarized the conversion rates of feedstock-N to NH3 and HCN during the pyrolysis process from 6 studies with 211 observations (Table S3). In addition, the conversion rates of feedstock-N to N2O and NOx via pyrolysis were obtained from Sparrevik et al. (2013). Then, we used the reported conversion rates of reactive volatiles-N (NH3 and HCN) to N2O and NOx during the combustion process (Johansson et al. 1999; Hayhurst and Lawrence 1992; Adouane et al. 2002) to calculate the yield of N2O and NOx. The amount of N2O and NOx derived from NH3 and HCN oxidation, together with the original fraction of N2O and NOx in the syngas are considered as the total potential N2O and NOx emissions due to biochar production.
Results and discussion
Soil active N pools
On average across the documented data, biochar has no significant effect on soil dissolved organic N (DON) (6%, P = 0.412), but it significantly increases soil microbial biomass N (MBN) by 12% (P = 0.019). Soil NH4+ and NO3− are significantly decreased by biochar addition, with percentage changes of −6% (P = 0.045) and −12% (P < 0.001), respectively (Fig. 2).
Relative changes of soil active N pools in biochar-amended soils compared to unamended controls. Bars indicate 95% confidence intervals. Data in italics represent the number of observations. Soil active N pools include dissolved organic nitrogen (DON), microbial biomass nitrogen (MBN), ammonium (NH4+) and nitrate (NO3−)
The biochar-induced rise in soil microbial biomass N is in line with the increase of soil microbial biomass carbon (28%) as observed from a previous meta-analysis of 16 studies (Biederman and Harpole 2013). Biochar has been shown to stimulate the abundance of a variety of important soil microorganisms associated with soil N cycling (Ducey et al. 2013; Song et al. 2014; Prommer et al. 2014; Sánchez-García et al. 2014). The positive effect of biochar on soil microbial biomass may be related to its fine porous structure, high surface area, hydrophilicity, and mineral nutrients content, which render the biochar-soil system a more suitable habitat for microbial colonization and growth (Warnock et al. 2007; Steinbeiss et al. 2009).
Biochar itself contains little extractable inorganic N (Fig. S1), and the organic N contained in biochar (Fig. S2) is recalcitrant (Knicker 2010; Xie et al. 2013), therefore, biochar is not a source for available N. Upon biochar addition, soil available N is decreased, which may result from the promoted soil inorganic N assimilation (Fig. 3), increased plant N uptake (Fig. 5a), and raised soil NH3 volatilization loss (Fig. 5b).
Relative changes of soil N transformations in biochar-amended soils compared to unamended controls. Bars indicate 95% confidence intervals. Data in italics represent the number of observations. Soil N transformations include gross mineralization (MINgross), gross immobilization of NH4+-N to organic N (IMgross), net mineralization (MINnet), gross nitrification (NITgross), net nitrification (NITnet), and dissimilatory nitrate reduction to ammonium (DNRA)
Soil N transformations
The overall effects of biochar on soil N transformations suggest significant increases in gross immobilization (323%, P = 0.006), gross nitrification (67%, P < 0.001), net nitrification (92%, P = 0.004), and dissimilatory reduction of NO3− to NH4+ (72%, P < 0.001) (Fig. 3). A weak significant increase in soil gross N mineralization (50%, P = 0.075) following biochar application was also found. Soil net N mineralization shows no significant response to biochar addition (−23%, P = 0.108).
The trend of higher gross N mineralization in biochar-amended soils (Fig. 3) may be explained by the enhanced abundance of microorganisms that facilitate the degradation of soil organic N (Anderson et al. 2011; Nelissen et al. 2012). Meanwhile, the soil gross N immobilization is also stimulated by biochar addition (Fig. 3), which may be caused by the easily mineralizable aliphatic biochar components with a high C: N ratio (Deenik et al. 2010; Smith et al. 2010). Possibly due to the counterbalance between the increases of both soil gross N mineralization and gross N immobilization, soil net N mineralization is not significantly changed by biochar (Fig. 3). Even though biochar may not alter the size of soil organic N pool in the short term, the accelerated N turnover by biochar may facilitate the transfer of soil organic N from a recalcitrant pool to a more labile pool (Nelissen et al. 2012). Given that the stimulating effect of biochar on soil gross N immobilization may diminish along with the depletion of biochar labile carbon components (Deenik et al. 2010), biochar may be hypothesized to induce an increase in soil net N mineralization and subsequently a decrease in soil organic N pool in the long run. However, this requires further validation.
The overall increase of soil nitrification in biochar-amended soils is mainly attributed to two reasons: (1) biochar’s liming effect promotes the conversion of NH4+ to NH3 in soil solution, thereby supplying larger amount of available substrate NH3 for ammonia monooxygenase catalysis (Nelissen et al. 2012); (2) biochar usually raises the population of soil ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) (Fig. S5), which provides more basis for the biochemical reactions (Yao et al. 2011). The positive effects of biochar on soil net nitrification or the abundance of AOA and AOB seem to be more commonly observed in acidic soils than in neutral-to-alkaline soils (Fig. S5). This is likely because biochar addition to acidic soils could bring soil pH closer to the optimum condition (pH at 7.8) that favors nitrifier growth (Antoniou et al. 1990; Barnard et al. 2005). Soil net nitrification, together with the population size of nitrifiers shows little response to biochar addition in soils possessing high organic carbon (>20 g kg−1) (Fig. S5). It is likely that the organic carbon-rich soils generally support a high abundance in microorganisms, and already have a high level of nitrifier population and nitrification potential, thus being less sensitive to be mediated by biochar (Fig. S6; DeLuca et al. 2006; Kuroiwa et al. 2011).
Biological N2 fixation
In the presence of legumes, biochar amendment significantly increases symbiotic biological N2 fixation (BNF) by an average value of 63% (P < 0.001) (Fig. 4). Biochar application to acidic soils (pH ≤ 5) increases symbiotic BNF to a larger extent than at moderate acidic soils (5 < pH ≤ 6.5). Symbiotic BNF could be effectively increased by biochar at an addition rate ≤ 80 t ha−1, yet it shows no significant response when biochar is applied at >80 t ha−1 (Fig. 4). Because the current studies associated with biochar impact on symbiotic BNF are limited, the effect of other explanatory variables are not analyzed here, and the reliability of the results also requires further evaluation.
Relative changes of soil symbiotic biological N2 fixation (BNF) in biochar-amended soils compared to unamended soils. Responses are shown for different soil pH and biochar application rate. An overall mean is shown in the bottom panel. Bars indicate 95% confidence intervals. Data in italics represent the number of observations
Potential mechanisms for the positive response of symbiotic BNF in acidic soils to biochar are multiple: (1) increased soil pH may improve nodulation since the rhizobia prefers circum-neutral pH (Rondon et al. 2007); (2) enhanced availability of soil nutrients such as P, K, Mo, and B may benefit nitrogenase synthesis and function (Rondon et al. 2007; Tagoe et al. 2008; Mia et al. 2014); (3) reduced soil inorganic N (Fig. 2) may stimulate root nodulation and the rhizobia activity to supply fixed N to legumes (Kontopoulou et al. 2017). Very few studies have quantified the symbiotic BNF in neutral or alkaline soils under biochar amendment. However, it has been observed that the nodulation or nitrogenase activity (quantified by acetylene reduction) of the root system in these soils remains unresponsive, which may indicate a weak effect of biochar on symbiotic BNF from neutral or alkaline soils (Arif et al. 2015; Quillian et al. 2013).
Regarding biochar impacts on the non-symbiotic BNF by the free-living diazotrophs, there is a lack of study quantifying the amounts of N2 fixed using 15N tracing techniques. Some studies addressing soil nifH gene abundance indicate that biochar has the potential to stimulate the population of free-living N2-fixing bacteria and the subsequent non-symbiotic BNF under certain conditions (Ducey et al. 2013; Quilliam et al. 2013; Harter et al. 2014). However, the capacity of biochar to mediate the non-symbiotic BNF and the factors that control the heterogeneity require further investigation.
Plant N uptake
On average, biochar leads to an increase of 11% (P < 0.001) in plant N uptake, which is derived from an increase of 12% in plant biomass (P < 0.001) and a minor decrease of −2% (P = 0.014) in plant tissue N concentration (Figs. 5a and S7). Biochar generally increases plant biomass and N uptake in acidic soils (pH ≤ 6.5), but it shows little effect in neutral or alkaline soils. The increasing impacts of biochar on plant biomass and N uptake are usually maximized in soils with poor structure (rich in either sand or clay) or low CEC. Manure biochar induces a higher plant production and N uptake than wood biochar or straw biochar. The relationship of biochar application rate with the response of plant biomass or N uptake follows a convex curve, and over application of biochar (>80 t ha−1) will significantly inhibit plant biomass and N uptake (Figs. 5a and S7a).
Relative changes of plant N uptake (a), soil NH3 volatilization (b), soil N2O emissions (c), and soil total inorganic N leaching (d) in biochar-amended soils compared to un-amended controls. Responses are shown in vertical order for different categories of soil pH, texture, organic C content (g kg−1), CEC (cmol kg−1), biochar feedstock type, pyrolysis temperature (°C), pH, and application rates (t ha−1). An overall mean is shown at the bottom of each panel. Bars indicate 95% confidence intervals. Data in italics at the right side of each panel represent the number of observations
The biochar-induced increases in plant productivity and N uptake are attributed to several mechanisms. First, biochar could increase soil pH towards optimum neutrality and reduce potential Al3+ toxicity from acidic soils (Jeffery et al. 2017). Second, biochar has the ability to alleviate soil tensile strength and enhance soil water-holding capacity, which may benefit root elongation and water uptake particularly in poorly-structured soils (Liu et al. 2017). Third, biochar has a large specific surface that contains a certain amount of negatively charged functional groups, and thus likely improves the nutrient retention of soils having low CEC (Liang et al. 2006). Finally, biochar is a source of available P, K, Ca and Mg, and can enhance soil fertility and plant nutrition (Silber et al. 2010). The greater beneficial effect of manure biochar than other type of biochar on plant growth and N uptake (Figs. 5a and S7) is likely due to the higher mineral nutrient content in manure biochar (Fig. S3). Biochar application rate has an optimum level for benefiting plant growth and N uptake (Figs. 5a and S7), above which phytotoxic effects may occur due to excessive soluble salts or ruined soil aggregate structure (Mukherjee and Lal 2014).
Soil NH3 volatilization
Biochar significantly enhances soil NH3 volatilization by 19% (P = 0.034) on average across different studies (Fig. 5b). Biochar stimulates soil NH3 volatilization to a larger extent from acidic soils (pH ≤ 5) than from moderately acidic soils (5 < pH ≤ 6.5), while it shows little effect on neutral or alkaline soils. Soil NH3 volatilization from clay textured soils are more prone to be increased by biochar than that from other types of soil. Biochar addition to soils with less than 10 g SOC kg−1 induces a significant increase in soil NH3 volatilization, while no significant response to biochar is observed in soils with SOC > 10 g kg−1. In terms of biochar species, the response of soil NH3 volatilization is bidirectional: manure biochar and straw biochar stimulate soil NH3 volatilization by an average of 43% and 27%, respectively, whereas wood biochar tends to decrease soil NH3 volatilization by an average of 30%. In general, biochar characterized by pH > 9, or being applied at the rate > 40 t ha−1 induces a significant increase in soil NH3 volatilization, however, biochar with pH lower than 9, or being applied at less than 40 t ha−1 shows no significant effect (Fig. 5b).
Soil NH4+ availability and soil pH are determinants of soil NH3 volatilization, with increasing alkalinity moving the equilibrium from NH4+ towards NH3 (Pan et al. 2016). When biochar is added into soils, on the one hand, the oxygen-containing functional groups on the biochar surface may adsorb NH4+ and decrease its availability (Kastner et al. 2009), while on the other hand, the alkaline minerals (e.g. carbonates, oxides, and hydroxides of Ca, Mg, Al, Mn, Zn, Fe) released from biochar will elevate soil pH and facilitate NH3 formation (Yang et al. 2015). Thus, the role of biochar in soil NH3 volatilization is a balance between its adsorption effect and liming effect.
Manure biochar or straw biochar has higher alkalinity, smaller surface area and less developed pore structure than wood biochar (Zhao et al. 2013a). Thus, for manure biochar or straw biochar, the liming effect likely plays the dominant role in stimulating soil NH3 volatilization, while for wood biochar, the adsorption effect may act as the main contributor to the decrease of soil NH3 volatilization.
The size of the risk for increased soil NH3 volatilization is likely related to the intrinsic soil NH4+ retention capacity and the extent of the change in soil pH. The volatilization of NH3 is less responsive to biochar addition in SOC-rich soils (Fig. 5b). This is probably because soils with high organic carbon have a greater ability to retain NH4+ on the surface of soil organic matter (SOM) through electrostatic attraction (Cameron et al. 2013), making NH4+ less available for de-protonation to NH3. The study also indicates a preference of biochar to increase NH3 volatilization from low pH soils (Fig. 5b), which could be explained by the phenomenon that biochar increases soil pH to a larger extent in acidic soils than in neutral-to-alkaline soils (Fig. S4). Soils high in clay content show a larger increase of NH3 volatilization in response to biochar addition (Fig. 5b). This is because in these soils, biochar may be more effective at increasing soil porosity, thus leading to a faster diffusion of NH3 to the atmosphere.
However, as biochar ages in soil, their negatively-charged functional groups may increase and their liming effect may fade (Yao et al. 2010). Therefore, the stimulating effect of biochar on NH3 volatilization is hypothesized to decrease as time goes on, verification of which requires further investigation. Although not identified in this study, production methods that result in oxidation of the biochar surface prior to application, such as steam pyrolysis or ozonolysis (Cha et al. 2016), are expected to lower the pH of the biochar and thus completely avoid stimulation of NH3 volatilization when the biochar is added to soil.
Soil N2O emissions
We found that biochar addition significantly decreases soil N2O emissions by an average of 32% (P < 0.001, Fig. 5c), which is lower than the previous estimate of 54% by Cayuela et al. (2014). The decreasing effect of biochar on soil N2O emissions is maximized in loam soils. The effect size is small and non-significant for soils with low organic carbon (≤5 g kg−1). Biochar made from manure or pyrolyzed at temperatures lower than 350 °C shows a weak and insignificant reducing impact on soil N2O emissions. Along with the increase of biochar addition rate, the magnitude of the reduction in soil N2O emissions increases, reaching the maximum when biochar addition rate is higher than 40 t ha−1 (Fig. 5c).
Soil N2O production is mainly a microbial process, with nitrifiers oxidizing NH4+ under aerobic conditions and denitrifiers reducing NO3− under anaerobic conditions (Cameron et al. 2013). Biochar effect on soil N2O flux may highly interact with soil N-transformation pathways as controlled by soil moisture (Fig. S8). In the presence of abundant inorganic N substrate (e.g., after N fertilization), if the soil is under relatively low moisture conditions [<80% water-filled pore space (WFPS)] where nitrification is the major pathway for N2O production (Bateman and Baggs 2005), biochar will likely increase soil N2O emissions (Fig. S8). This may be attributed to the function of biochar to facilitate soil nitrification, thus increasing the nitrification-derived N2O byproduct (Fig. 3). In comparison, if the soil is under high soil-moisture conditions (>80% WFPS) where denitrification dominates the N2O production pathway (Bateman and Baggs 2005), biochar will tend to decrease soil N2O emissions (Fig. S8). This may be a result of accelerated complete soil denitrification (i.e. increased conversion of N2O to N2), which could be induced by biochar in the following ways: (i) the “electronic conductor” of biochar matrix itself, together with the “electron shuttle” derived from biochar surface quinone-hydroquinone functional groups, might facilitate the transfer of electrons to soil denitrifying microorganisms (Cayuela et al. 2013; Sun et al. 2017b); (ii) biochar’s hydrophilic property and its combination with soil micro-aggregates (Lehmann et al. 2005) may protect soil microsites from exposure to oxygen, creating further reduced conditions favorable for N2O conversion towards N2; and (iii) biochar may increase the abundance of N2O-reducing bacteria in certain cases (Fig. S9), thus promoting the enzymatic activity of N2O reduction.
The interactive effect of biochar with different soil N2O pathways indicates that the ability of biochar to decrease soil N2O emissions may be primarily contributed by the modified denitrification process. Higher reduction in soil N2O emissions by biochar occurs in fine textured loam soils (Fig. 5c). It is likely because loam soils have more capillary pores within aggregates than do sandy or clay soils, thereby holding soil water more tightly (Saxton et al. 1986). Anaerobic microsites favoring the denitrification process in these soils may be more easily formed, which could assist with biochar’s role in reducing soil N2O emissions by regulating the denitrification process. In soils with SOC lower than 5 g kg−1, biochar is less effective in decreasing soil N2O emissions (Fig. 5c). It may be that denitrification is less important in these soils due to the low amount of available carbon to support the heterotrophic process (Bouwman et al. 1993). In addition, biochar is more likely to increase soil nitrification in lower SOC soils (DeLuce et al. 2006), which might conversely promote nitrification-derived N2O production and thus cripple biochar’s overall function in reducing soil N2O emissions. Biochar made from manure or pyrolyzed at temperatures lower than 350 °C is less effective in reducing soil N2O emissions (Fig. 5c). This may be ascribed to their weaker aromatic structure and lower surface area which offer lower electric conduction capacity and less surface functionality for interacting with N2O turnover (Mandal et al. 2016).
Soil N leaching
Biochar on average significantly reduces the leaching of soil NH4+, NO3−, and total inorganic N by 22% (P = 0.009), 29% (P < 0.001), and 26% (P < 0.001), respectively (Figs. 5d and S10). In soils with lower organic carbon, biochar favors a larger decrease in soil total inorganic N leaching (Fig. 5d). Wood biochar leads to the highest decrease in soil total inorganic N leaching, which is slightly more efficient than straw biochar, while manure biochar has a non-significant effect on soil N-leaching. Biochar produced under lower pyrolysis temperature is more effective in reducing soil N leaching. Along with the increase of biochar addition rate, the extent of decrease in soil total inorganic N leaching increases (Fig. 5d).
On the one hand, biochar effects on soil N leaching are linked to its surface-charge properties. The presence of negatively charged carboxyl and phenolic hydroxyl groups on the biochar surface likely act as NH4+ retention sites (Gai et al. 2014). In addition, NO3− can also be attracted to these sites indirectly via electrostatic bridge-bonding with divalent cations such as Ca2+, Mg2+ or trivalent metals like Al3+ and Fe3+ (Gai et al. 2014). Therefore, biochar itself displays an adsorption capacity for both NH4+ and NO3− (Fig. S1). On the other hand, biochar may increase soil water holding capacity based on its large specific surface area and high porosity, thereby reducing soil water percolation and the N contained in it (Novak et al. 2012). Biochar addition in the range between 10 and 60 t ha−1 could decrease the volume of soil water leachate by 1.5–8.4% according to data compilation (Table S4).
Biochars produced under lower temperature conditions likely possess a larger fraction of charged functional groups (Ahmad et al. 2012), which may explain their more effective role in reducing soil N leaching (Fig. 5d). Soils with low organic carbon are usually characterized by low nutrient retention capacity (Parfitt et al. 1995; Kanthle et al. 2016), and therefore are relatively more responsive to biochar mediation for reducing soil N leaching (Fig. 5d). Soil water holding capacity has been shown to increase along with increasing biochar addition rate (Cao et al. 2014), which is consistent with the positive relationship observed between soil N leaching reduction and the amount of biochar application (Fig. 5d). Manure biochar shows less effect on soil N leaching (Fig. 5d), perhaps due to its low porosity, surface area, and number of functional groups (Zhao et al. 2013a).
Potential N2O and NOx emissions derived from biochar manufacture
Emissions of gaseous N pollutants (N2O and NOx) from the biochar production process is a key issue in the context of developing an effective biochar strategy. During the pyrolysis of biomass, a fraction of the feedstock-N is converted into the N components in syngas (Fig. 6). The major N components in syngas occur as N2 (22–47% of the feedstock-N, 95% CI, n = 21), NH3 (19–27% of the feedstock-N, 95% CI, n = 112), and HCN (7–12% of the feedstock-N, 95% CI, n = 78), with smaller amounts of N2O (1.2% of the feedstock-N, n = 1) and NOx (0.3% of the feedstock-N, n = 1) (Fig. 6). If the N contaminants in the pyrolytic syngas are not removed, the downstream applications of the syngas (e.g., combustion for heat or electricity) would lead to an oxidization of NH3 and HCN into N2, N2O, and NOx (Johansson et al. 1999). According to the conversion rates of the reactive volatiles-N (NH3, HCN) towards N2O (2–7%) and NOx (10–60%) via combustion (Johansson et al. 1999; Hayhurst and Lawrence 1992; Adouane et al. 2002), the total N2O and NOx released into the atmosphere after syngas combustion is on the order of 2–4% and 3–24% of the feedstock-N, respectively (Fig. 6).
Given the average available 2.5 t ha−1 yr.−1 crop residues derived from arable land for biochar production (Lal 2005) and the average N content of 0.83% for crop residues (Lu and Shi 1982), the N2O emissions due to biochar production in the absence of pyrolytic syngas purification would amount to 0.35–0.81 kg N2O-N ha−1 yr.−1, which can weaken or destroy the effectiveness of biochar in mitigating soil N2O emissions. Therefore, the removal of N-containing components from pyrolytic syngas before transferring to end users, either by trapping and subsequent conversion to harmless (i.e., N2) or useful (i.e., NH4+) forms of N, or by in-stack catalytic conversion to N2, is of great importance from a climate-change mitigation perspective (Bhandari et al. 2014). Previous studies indicate that a range of technologies exist to purify the raw syngas and can almost completely remove the unwanted contaminants (NH3, HCN, N2O, NOx) (Woolcock and Brown 2013). Therefore, developing advanced clean biochar production technology is feasible and essential.
Towards an effective and desirable biochar strategy
The rapid increase of global food demand depending on increased N inputs will further accelerate soil N losses (Tilman et al. 2011). Our meta-analysis reveals that the soil N cycle can be substantially altered by biochar amendment (Fig. 7). Can biochar be a strategy to alleviate soil N effluxes as well as benefit food production? This study suggests that biochar has the potential to achieve such goals, however, clean biochar production techniques and adapted use of biochar by considering biochar and soil properties are of great necessity.
Summery of the average effects of biochar on soil N cycle. Data represents the percentage change of corresponding items induced by biochar amendment. MINgross, soil gross mineralization; MINnet, soil net mineralization; IMgross, soil gross immobilization of NH4+-N to organic N; NITgross, soil gross nitrification; NITnet, soil net nitrification; DNRA, soil dissimilatory nitrate reduction to ammonium
Biochar production techniques vary from low-efficient stoves without recycling pyrolytic syngas to highly-advanced facilities with application of syngas for power (Meyer et al. 2011). The low-efficient stoves with high emissions have been proved unsuitable for biochar production due to serious pollution (Liu et al. 2016). However, this study further warns that even for the highly-advanced facilities, if the reactive N-containing components in the pyrolytic syngas are not removed before the syngas goes to the downstream combustion, potential threats of extra N2O and NOx emissions still exist (Fig. 6). Therefore, syngas purification during biochar production is of primary importance for the effectiveness of biochar as a strategy to mitigate soil N emissions.
The choice of different biochar species could yield different benefits or risks (Fig. 5). This study indicates that manure biochar has a high potential for benefiting plant growth, but it has weak effects for decreasing soil N leaching and N2O emissions, and it poses a large risk for increasing soil NH3 volatilization (Fig. 5). Unlike manure biochar, wood biochar is more efficient in decreasing soil N losses (including N leaching, N2O emissions, as well as NH3 volatilization), but it is less beneficial for increasing crop production (Fig. 5). Straw biochar usually has properties and functions between that of wood biochar and manure biochar (Fig. 5). Therefore, if the aim is to achieve a higher benefit of food production instead of N retention, manure biochar could be primarily chosen. While if the goal is to realize higher N retention instead of food production, wood biochar could be considered as priority. In addition, a smart combination of different biochar species may be expected to integrally exert their respective advantages and cripple their weaknesses, which however requires further investigation.
The properties and functions of biochar can be mediated by pyrolysis temperature. Biochar produced under higher temperature tends to have a larger potential for mitigating soil N2O emissions, but a weaker effect for decreasing soil N leaching (Fig. 5). As such, a tradeoff between biochar functions should be taken into account for the design of targeted biochar.
Biochar application rate is an important parameter for influencing the advantages of biochar on soil N cycle (Fig. 5). In general, biochar addition rate tends to positively correlate with the extent of decreases in soil N2O emissions and N leaching, but it yields a convex curve for changes in plant production and plant N uptake (Fig. 5). Over application of biochar may induce negative effects such as reduced crop growth and accelerated soil NH3 volatilization (Fig. 5). In this study, it is recommended that the application rate of biochar better not exceed 40–80 t ha−1 (Fig. 5). The optimum biochar addition rate is generally within 10–40 t ha−1, which could achieve significant benefits for both crop production and N retention (Fig. 5).
To avoid negative side effects from biochar, soil properties should be carefully judged before biochar application (Fig. 5). For a range of weathered soils such as those characterized by soil pH less than 5, organic carbon less than 10 g kg−1, or clay texture, they may be previously considered as the adapted pools for biochar deployment due to a high agronomic benefit (Cranedroesch et al. 2013; Jeffery et al. 2017). However, this study indicates that these soils also suffer a risk of increased soil NH3 volatilization following biochar application (Fig. 5). To minimize or avoid such risk, the poorly-buffered soils may be suggested to use low alkaline biochar (such as wood biochar, or biochar that is activated, oxidized, or weathered), or high alkaline biochar at very low application rates.
For soils with neutral-to-alkaline pH, it was thought that biochar may be unsuitable to be applied due to the likelihood of its increasing soil pH towards more severe alkalinity and thus inhibiting crop growth (Mukherjee and Lal 2014). However, our study shows that a certain level of biochar application into neutral or alkaline soils shows neither negative nor positive effect on plant growth on average across different papers (Fig. S7). In addition, biochar in these soils is effective in decreasing soil N2O emissions and N leaching, and it has a low risk for stimulating soil NH3 volatilization (Fig. 5). Thus, neutral or alkaline soils are also expected to benefit from a proper biochar application in the view of mitigating soil N losses without endangering food production.
The biochar effects synthesized in the current paper are mainly derived from experiments characterized by single-dose designs and relatively short-term time scales (months to a few years). Biochar effects with respect to longer-term and repetitive additions require further evaluation with future more relevant experimental data.
Conclusions
Using a meta-analytical approach, this study reveals that the soil N cycle can be altered by biochar application, with a wide variation depending on certain characteristics of biochar and soil. Besides, clean advanced pyrolysis technique is of special importance, otherwise, pollutant N2O and NOx may be produced due to biochar production. Overall, this study provides a comprehensive insight into how different factors mediate the response of soil N cycle to biochar amendment, which is helpful towards the design of biochar projects for benefiting soil N cycle while minimizing undesirable side effects.
References
Adams DC, Gurevitch J, Rosenberg MS (1997) Resampling tests for meta-analysis of ecological data. Ecology 78:1277–1283
Adouane B, Hoppesteyn P, de Jong W, van der Wel M, Hein KR, Spliethoff H (2002) Gas turbine combustor for biomass derived LCV gas, a first approach towards fuel-NOx modelling and experimental validation. Appl Therm Eng 22:959–970
Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, Yang JE, Ok YS (2012) Effects of pyrolysis temperature on soybean Stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol 118:536–544
Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR (2011) Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 54:309–320
Antoniou P, Hamilton J, Koopman B, Jain R, Holloway B, Lyberatos G, Svoronos SA (1990) Effect of temperature and pH on the effective maximum specific growth rate of nitrifying bacteria. Water Res 24:97–101
Arif M, Jalal F, Jan MT, Muhammad D, Quilliam RS (2015) Incorporation of biochar and legumes into the summer gap: improving productivity of cereal-based cropping systems in Pakistan. Agroecol Sust Food 39:391–398
Barnard R, Leadley PW, Hungate BA (2005) Global change, nitrification, and denitrification: a review. Global Biogeochem Cy 19:GB1007
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fert Soils 41:379–388
Batjes NH (2015) World soil property estimates for broad-scale modelling (WISE30sec). Report 2015/01, ISRIC-World Soil Information, Wageningen (with data set, available at www.isric.org)
Bhandari PN, Kumar A, Huhnke RL (2014) Simultaneous removal of toluene (model tar), NH3, and H2S, from biomass-generated producer gas using biochar-based and mixed-metal oxide catalysts. Energy Fuel 28:1918–1925
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214
Bouwman AF, Fung I, Matthews E, John J (1993) Global analysis of the potential for N2O production in natural soils. Global Biogeochem Cy 7:557–597
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann Appl Biol 162:145–173
Cao CT, Farrell C, Kristiansen PE, Rayner JP (2014) Biochar makes green roof substrates lighter and improves water supply to plants. Ecol Eng 71:368–374
Cayuela ML, Sánchez-Monedero MA, Roig A, Hanley K, Enders A, Lehmann J (2013) Biochar and denitrification in soils: when, how much and why does biochar reduce N2O emissions? Sci Rep 3:1732
Cayuela ML, Zwieten LV, Singh BP, Jeffery S, Roig A, Sánchez-Monedero MA (2014) Biochar's role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16
Clough TJ, Condron LM, Kammann C, Müller C (2013) A review of biochar and soil nitrogen dynamics. Agronomy 3:275–293
Cranedroesch A, Abiven S, Jeffery S, Torn MS (2013) Heterogeneous global crop yield response to biochar: a meta-regression analysis. Environ Res Lett 8:925–932
Deenik JL, McClellan T, Uehara G, Antal MJ, Campbell S (2010) Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci Soc Am J 74:1259–1270
DeLuca TH, MacKenzie MD, Gundale MJ, Holben WE (2006) Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70:448–453
Ducey TF, Ippolito JA, Cantrell KB, Novak JM, Lentz RD (2013) Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl Soil Ecol 65:65–72
Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W (2008) How a century of ammonia synthesis changed the world. Nat Geosci 1:636–639
Food and Agricultural Organization of the United Nations (2014) Statistics: Fertilizers input. (http://www.fao.org/faostat/en/#data/RF)
Gai X, Wang H, Liu J, Zhai L, Liu S, Ren T, Liu H (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS One 9:e113888
Gruber N, Galloway JN (2008) An earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Gurevitch J, Hedges LV (1993) Meta-analysis: combining the results of independent experiments. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, New York, USA, pp 378–389
Harter J, Krause HM, Schuettler S, Ruser R, Fromme M, Scholten T, Behrens S (2014) Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. The ISME journal 8:660–674
Hayhurst AN, Lawrence AD (1992) Emissions of nitrous oxide from combustion sources. Prog Energ Combust 18:529–552
Hämäläinen JP, Aho MJ (1996) Conversion of fuel nitrogen through HCN and NH3 to nitrogen oxides at elevated pressure. Fuel 75:1377–1386
He Y, Zhou X, Jiang L, Li M, Du Z, Zhou G, Wallace H (2017) Effects of biochar application on soil greenhouse gas fluxes: a meta-analysis. GCB Bioenergy 9:743–755
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Jeffery S, Abalos D, Prodana M, Bastos A, van Groenigen JW, Hungate B, Verheijen F (2017) Biochar boosts tropical but not temperate crop yields. Environ Res Lett 12:053001
Cha JS, Park SH, Jung SC, Ryu C, Jeon JK, Shin MC, Park YK (2016) Production and utilization of biochar: a review. J Ind Eng Chem 40:1–15
Johansson EM, Järås SG (1999) Circumventing fuel-NOx formation in catalytic combustion of gasified biomass. Catal Today 47:359–367
Kanthle AK, Lenka NK, Lenka S, Tedia K (2016) Biochar impact on nitrate leaching as influenced by native soil organic carbon in an Inceptisol of Central India. Soil Till Res 157:65–72
Kastner JR, Miller J, Das KC (2009) Pyrolysis conditions and ozone oxidation effects on ammonia adsorption in biomass generated chars. J Hazard Mater 164:1420–1427
Knicker H (2010) “Black nitrogen”–an important fraction in determining the recalcitrance of charcoal. Org Geochem 41:947–950
Kontopoulou CK, Liasis E, Iannetta PPM, Tampakaki A, Savvas D (2017) Impact of rhizobial inoculation and reduced N supply on biomass production and biological N2 fixation in common bean grown hydroponically. J Sci Food Agric 97:4353–4361
Kuroiwa M, Koba K, Isobe K, Tateno R, Nakanishi A, Inagaki Y, Yoh M (2011) Gross nitrification rates in four Japanese forest soils: heterotrophic versus autotrophic and the regulation factors for the nitrification. J Forest Res 16:363–373
Kwiatkowski K, Dudyński M, Bajer K (2013) Combustion of low-calorific waste biomass syngas. Flow Turbul Combust 91:749–772
Lal R (2005) World crop residues production and implications of its use as a biofuel. Environ Int 31:575–584
Lehmann J, Liang B, Solomon D, Lerotic M, Luizão F, Kinyangi J, Schafer T, Wirick S, Jacobsen C (2005) Near-edge X-ray absorption fine structure (NEXAFS) spectroscopy for mapping nano-scale distribution of organic carbon forms in soils: application to black carbon particles. Global Biogeochem Cy 19: GB1013
Leppälahti J, Koljonen T (1995) Nitrogen evolution from coal, peat and wood during gasification: literature review. Fuel Process Technol 43:1–45
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'neill B, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Liu J, You L, Amini M, Obersteiner M, Herrero M, Zehnder AJ, Yang H (2010) A high-resolution assessment on global nitrogen flows in cropland. Proc Natl Acad Sci U S A 107:8035–8040
Liu Q, Liu BJ, Ambus P, Zhang Y, Hansen V, Lin Z, Shen D, Liu G, Bei Q, Zhu J, Wang X, Ma J, Lin X, Yu Y, Zhu C, Xie Z (2016) Carbon footprint of rice production under biochar amendment-a case study in a Chinese rice cropping system. GCB Bioenergy 8:148–159
Liu Q, Liu B, Zhang Y, Lin Z, Zhu T, Sun R, Wang X, Ma J, Bei Q, Liu G, Lin X, Xie Z (2017) Can biochar alleviate soil compaction stress on wheat growth and mitigate soil N2O emissions? Soil Biol Biochem 104:8–17
Lu RK, Shi TJ (1982) Handbook of agricultural chemistry. Science Press, Beijing, China (In Chinese)
Mandal S, Sarkar B, Bolan N, Novak J, Ok YS, van Zwieten L, Singh BP, Kirkham MB, Choppala G, Spokas K, Naidu R (2016) Designing advanced biochar products for maximizing greenhouse gas mitigation potential. Crit Revt Env Sci Tec 46:1367–1401
Meyer S, Glaser B, Quicker P (2011) Technical, economical, and climate-related aspects of biochar production technologies: a literature review. Environ Sci Technol 45:9473–9483
Mia S, Van Groenigen JW, Van de Voorde TFJ, Oram NJ, Bezemer TM, Mommer L, Jeffery S (2014) Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric Ecosyst Environ 191:83–91
Mukherjee A, Lal R (2014) The biochar dilemma. Soil Res 52:217–230
Nelissen V, Rütting T, Huygens D, Staelens J, Ruysschaert G, Boeckx P (2012) Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol Biochem 55:20–27
Nguyen TTN, Xu CY, Tahmasbian I, Che R, Xu Z, Zhou X, Bai SH (2017) Effects of biochar on soil available inorganic nitrogen: a review and meta-analysis. Geoderma 288:79–96
Novak JM, Busscher WJ, Watts DW (2012) Biochars impact on soil-moisture storage in an Ultisol and two Aridisols. Soil Sci 177:310–320
Pan B, Lam SK, Mosier A, Luo Y, Chen D (2016) Ammonia volatilization from synthetic fertilizers and its mitigation strategies: a global synthesis. Agric Ecosyst Environ 232:283–289
Parfitt RL, Giltrap DJ, Whitton JS (1995) Contribution of organic matter and clay minerals to the cation exchange capacity of soils. Commun Soil Sci Plan 26:1343–1355
Prommer J, Wanek W, Hofhansl F, Trojan D, Offre P, Urich T, Hood-Nowotny RC (2014) Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS One 9:e86388
Quilliam RS, DeLuca TH, Jones DL (2013) Biochar application reduces nodulation but increases nitrogenase activity in clover. Plant Soil 366:83–92
Ren Q, Zhao C (2013) NOx and N2O precursors from biomass pyrolysis: role of cellulose, hemicellulose and lignin. Environ Sci Technol 47(15):8955–8961
Rondon MA, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fert Soils 43:699–708
Rosenberg MS, Adams DC, Gurevitch J (2000) MetaWin: statistical software for meta-analysis. Version 2. Sunderland, Massachusetts. Sinauer Associates
Saxton KE, Rawls W, Romberger JS, Papendick RI (1986) Estimating generalized soil-water characteristics from texture. Soil Sci Soc Am J 50:1031–1036
Sánchez-García M, Roig A, Sánchez-Monedero MA, Cayuela ML (2014) Biochar increases soil N2O emissions produced by nitrification-mediated pathways. Front Environ Sci 2:25
Shirazi MA, Boersma L (1984) A unifying quantitative analysis of soil texture. Soil Sci Soc Am J 48:142–147
Silber A, Levkovitch I, Graber ER (2010) pH-dependent mineral release and surface properties of corn straw biochar: agronomic implications. Environ Sci Technol 44:9318–9323
Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A (2010) Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J Environ Qual 39:1224–1235
Smith JL, Collins HP, Bailey VL (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42:2345–2347
Sohi SP (2012) Carbon storage with benefits. Science 338:1034–1035
Song Y, Zhang X, Ma B, Chang SX, Gong J (2014) Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol Fert Soils 50:321–332
Sparrevik M, Field JL, Martinsen V, Breedveld GD, Cornelissen G (2013) Life cycle assessment to evaluate the environmental impact of biochar implementation in conservation agriculture in Zambia. Environ Sci Technol 47:1206–1215
Sun L, Li L, Chen Z, Wang J, Xiong Z (2014) Combined effects of nitrogen deposition and biochar application on emissions of N2O, CO2 and NH3 from agricultural and forest soils. Soil Sci Plant Nutr 60:254–265
Sun H, Min J, Zhang H, Feng Y et al (2017a) Biochar application mode influences nitrogen leaching and NH3 volatilization losses in a rice paddy soil irrigated with N-rich wastewater. Environ Technol:1–7
Sun T, Levin BDA, Guzman JJL, Enders A, Muller DA, Angenent LT, Lehmann J (2017b) Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat Commun 8:14873
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) A wood based low-temperature biochar captures NH3-N generated from ruminant urine-N, retaining its bioavailability. Plant Soil 353:73–84
Tagoe SO, Horiuchi T, Matsui T (2008) Effects of carbonized and dried chicken manures on the growth, yield, and N content of soybean. Plant Soil 306:211–220
Tilman D, Balzer C, Hill J, Befort BL (2011) Global food demand and the sustainable intensification of agriculture. P Natl Acad Sci USA 108: 20260, 20264
Verhoeven E, Pereira E, Decock C, Suddick E, Angst T, Six J (2017) Toward a better assessment of biochar-nitrous oxide mitigation potential at the field scale. J Environ Qual 46:237–246
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil----concepts and mechanisms. Plant Soil 300:9–20
Woolcock PJ, Brown RC (2013) A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 52:54–84
Xie Z, Xu Y, Liu G, Liu Q, Zhu J, Tu C, Hu S (2013) Impact of biochar application on nitrogen nutrition of rice, greenhouse-gas emissions and soil organic carbon dynamics in two paddy soils of China. Plant Soil 370:527–540
Yang F, Cao X, Gao B, Zhao L, Li F (2015) Short-term effects of rice straw biochar on sorption, emission, and transformation of soil NH4 +-N. Environ Sci Pollut R 22:9184–9192
Yao FX, Arbestain MC, Virgel S, Blanco F, Arostegui J, Maciá-Agulló JA, Macías F (2010) Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor. Chemosphere 80:724–732
Yao H, Gao Y, Nicol GW, Campbell CD, Prosser JI, Zhang L, Singh BK (2011) Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl Environ Microb 77:4618–4625
Zhang X (2017) Biogeochemistry: a plan for efficient use of nitrogen fertilizers. Nature 543:322–323
Zhao L, Cao X, Mašek O, Zimmerman A (2013a) Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J Hazard Mater 256:1–9
Zhao X, Yan X, Wang S, Xing G, Zhou Y (2013b) Effects of the addition of rice-straw-based biochar on leaching and retention of fertilizer N in highly fertilized cropland soils. Soil Sci Plant Nutr 59:771–782
Acknowledgements
We gratefully acknowledge support for this research from the Natural Science Foundation of China (grant no. NFSC-41171191), Special Project on Agricultural Science and Technology (201503137), Special Project on the Basis of National Science and Technology of China: National Survey of Biological Nitrogen Fixation Resources in Paddies of China (2015FY110700),the Danish Agency for Science, Technology and Innovation for financial support to Sino-Danish cooperation on biochar as a tool to mitigate climate change (No 1370-00036B), the Science and Technology Supporting Project of China (2013BAD11B01), and the Science and Technology Supporting Project of Jiangsu Province (BE2013451), and Blue Moon Fund USA.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Erik J. Joner.
Electronic supplementary material
ESM 1
(DOCX 325 kb)
Rights and permissions
About this article
Cite this article
Liu, Q., Zhang, Y., Liu, B. et al. How does biochar influence soil N cycle? A meta-analysis. Plant Soil 426, 211–225 (2018). https://doi.org/10.1007/s11104-018-3619-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3619-4