Abstract
Linum album is a rich source of various phenolic compounds, especially lignans. Our previous studies revealed that phenylalanine (Phe), an aromatic amino acid, participates in lignans production. In this study, we tried to characterize how Phe as the precursor feeding affects metabolic fluxes in L. album cells. As expected, the results indicated that an increase in phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase activities (TAL) occurred at 250 μM. Their actions induced phenolic acids production, especially cinnamic acid and salicylic acid (SA). The low dosages of Phe stimulated hydrogen peroxide (H2O2) and nitric oxide (NO) generation, lipid peroxidation, and antioxidant enzymes activation, while the high dosages suppressed the mentioned physiological processes. A depletion in rhamnose/xylose, glucose, and mannose was observed at high concentrations of Phe compared with the control. These molecules altered amino acids profile through supplying carbon skeleton and cell required energy. Our observations also showed that amino acids contents were significantly influenced by Phe treatment. The reduction of endogenous Phe content in the treated cells may closely related to phenolics accumulation such as flavonoids and lignans, as the enhancement of catechin, myricetin, and kaempferol were observed in the treated cells. Likewise, phenylalanine increased lignans, except for lariciresionol (LARI), and the highest amounts of podophyllotoxin (PTOX), and 6-methoxy podophyllotoxin (6MPTOX) were detected at 1000 μM. In summary, metabolic and physiological changes can elucidate that Phe involves in the lignans production in L. album through a SA-dependent pathway.
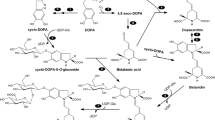
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Linum album Kotschy ex Bioss. is an important medicinal plant synthesizing lignans such as podophyllotoxin (PTOX). This plant geographically grows in Iran (Schmidt et al. 2010). PTOX, a valuable phenolic compound, exhibits antiviral and anticancer properties (Suzuki and Umezawa 2007). The phenolic compounds such as phenolic acids, flavonoids, and lignans are derived from a multistep pathway called the phenylpropanoid in which a variety of genes and enzymes are incorporated (Samari et al. 2020; 2022; Torabi et al. 2023). The phenolics are pivotal bioactive agents that compose of a phenyl ring accompanied by a side chain on C3 (Heldt and Piechulla 2011; Pascual et al. 2016). This pathway starts with deamination of phenylalanine (Phe) and tyrosine (Tyr) amino acids into cinnamic and p-coumaric acids through activation of phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase (TAL) enzymes (Barros et al. 2016; Sagharyan et al. 2020, 2023). Studies on the phenylpropanoid pathway also suggest that the pools of p-coumaric acid derived from Phe and Tyr direct toward independent pathways, which provide various end products (Simpson et al. 2021). One of the most important phenolic end products is salicylic acid (SA), a defense phytohormone, derived from the shikimate and the phenylpropanoid pathways in relation with isochorismate synthase and PAL enzymes, respectively (Dempsey and Klessig 2017; Koo et al. 2020). Likewise, the accumulation of SA can act as a signaling participant to mediate oxidative status in stress conditions (Koo et al. 2020; Chakraborty 2021).
Phe, a crucial amino acid in plant life processes, serve as a protein building block and a precursor of several bioactive agents that are necessary for plant reproduction, growth, development, and stress tolerance (Buchanan et al. 2000; Pascual et al. 2016; Rahmani Samani et al. 2019). Phe can be a source of nitrogen, carbon, hydrogen, and oxygen switching the primary metabolic dynamics to the specialized secondary metabolites production (Khataee et al. 2019; Meza et al. 2021, 2022). Furthermore, the metabolism of Phe can shift the fixed-carbon during the photosynthesis toward the provision of phenolic metabolites (Craven-Bartel et al. 2013).
One of the most important carbon sources in plants is soluble sugars that have critical roles in energy metabolism and signal transduction, as well as balancing the homeostasis of reactive oxygen species (ROS) (Couée et al. 2006; Sipari et al. 2020; Khataee et al. 2020). The soluble sugars can prevent the ROS production and oxidative stress through regulating the oxidative pentose phosphate pathway and NADPH production (Noctor and Mhamdi 2017). As signaling molecules, ROS molecules especially hydrogen peroxide (H2O2) are naturally generated by various reactions during plant aerobic metabolism (Mittler 2017; Waszczak et al. 2018). However, at a basal concentration, ROS are necessary for normal plant performance and development, while at the higher concentration leads to oxidative stress, cells damage and even cell death (Mittler 2017; Toghyani et al. 2020; Soltani et al., 2023; Esmaeili et al. 2023). In addition, nitric oxide (NO) is a dual-faceted molecule involved in physiological and biological processes within plants (Samari et al. 2020; Esmaeili et al. 2023; Sagharyan et al. 2023). Like ROS, NO behave differentially at a range of dosages as a signaling or a fatal factor (Fakhari et al. 2019; Samari et al. 2022; Sagharyan et al. 2023). Despite their importance, plants perfectly suppress the toxic effects of ROS and reactive nitrogen species (RNS) through amelioration of antioxidant enzymes such as catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) (Shahkarami et al., 2022; Sagharyan et al. 2023).
There are many studies declaring that Phe as a precursor feeding can induce phenolic compounds in different plant species such as Panax sikkimensis (Biswas et al. 2020), Buddleja cordata (Arano-Varela et al. 2020), and Triticum aestivum (Feduraev et al. 2020). It can be hypothesized that Phe may direct free sugars and amino acids content toward lignans accumulation by changes of oxidative status in L. album cells. Therefore, this study aims to determinate the inducing effects of Phe on the metabolic flux to the lignans accumulation in cells of L. album.
Materials and Methods
Plant Materials and Cell Culture
Callus was derived from the sterile leaves of L. album in solid-MS (Murashige and Skoog 1962) medium containing sucrose (3%), agar (0.8%), NAA (2 mg L−1) and Kinetin (0.4 mg L−1) as described by Yousefzadi et al. (2010). The pH of media was adjusted to 5.8. The friable callus was desegregated in 30 mL of liquid MS medium (3% sucrose, 2 mg L−1 of NAA and 0.4 mg L−1 of Kinetin). Cell culture of L. album (2 g) was moved to a rotary shaker at 110 × g at 27 °C in the darkened conditions. Subculture of cells was fulfilled at 14th intervals. The L-phenylalanine (Phe) was suspended in water and NaOH (1 M), and filter sterilized. After that, the growth curve was depicted based on dry weight to indicate the exponential phase of the cell growth in the Erlenmeyer flask. According to growth curve, 7-day old cells were exposed to different concentrations of Phe (250, 500, 750, and 1000 µM), near the log phase of the cell growth and survival. The cells were harvested after 5 days of treatment, weighed, rinsed in deionized water, frozen in liquid nitrogen and stored at − 80 °C for further analysis.
Determination of Hydrogen Peroxide and Malondialdehyde Content
Estimation of hydrogen peroxide (H2O2) content was accomplished by Velikova et al. (2000) method with some modification. To analyze changes H2O2 content in response to Phe treatments, frozen-cells (0.2 g) were weighed and then blended by 2 mL of chilly trichloroacetic acid (TCA, 0.1% (w/v)). The mixture was poured into an amber tube and centrifuged at 12,000×g at 4 °C for 20 min. The extraction (0.5 mL) was added with 500 µL of phosphate buffer (100 mM; pH 7.0) and 1000 µL of potassium iodide (KI, 1 M) in the darkness at ambient temperature. The absorption of the test solution was assessed at 390 nm using a spectrophotometer (Cary 100-UV–vis, Agilent, USA) and the results were calculated based on the H2O2 standard curve (10–50 µmol mL−1) and expressed as µmol g−1 Fresh Weight (FW).
To measure cellular damage after treating with Phe, the malondialdehyde (MDA) content was quantified as an indicator for lipid peroxidation. Briefly, the frozen-cells (0.2 g) of L. album were powdered in the cold extraction buffer (TCA, 0.1% (w/v)). After that, each sample was centrifuged at 12,000×g at 4 °C for 20 min. Then, the supernatant was poured into glass vials containing TCA (0.1%) and 0.5% thiobarbituric acid (in TBA (20%)). The vials were subsequently placed in a benmari at 90 °C for 30 min. At the end of this period, the MDA content in the cells was evaluated at 532 and 600 nm via spectrophotometer (Cary 100-UV–vis, Agilent, USA) (Stewart and Bewley 1980). The concentration of MDA was computed as µmol g−1 FW.
Measurement of Nitric Oxide Content
To measure the nitric oxide (NO) content, the chilled-cells (0.2 g) were extracted with 1.5 mL of phosphate buffer (100 mM; pH 7.0). After centrifuging (12,000×g, 15 min, 4 °C), the test solution (500 µL) was dissolved with 500 µL of phosphate buffer (100 mM, pH 7.5), and Griess reagent (500 µL). After incubation for 30 min, the absorbance of test solution was analyzed at 540 nm via spectrophotometer (Cary 100-UV–vis, Agilent, USA) (Kaur et al. 2015). The content of NO was defined as µmol of sodium nitrite equivalents per g−1 FW.
Assessment of Antioxidant Enzymes Activity
For analysis of total protein, 200 mg of frozen cell powder was weighed into 2 mL of potassium phosphate buffer (50 mM; pH 7.0) containing polyvinylpyrrolidone (PVP, 1mM) and supernatant separated by centrifugation. Total protein concentration was tested using a Bradford (1976) procedure and calibrated using BSA (bovine serum) standard. Next, the possible role of antioxidant enzymes tethering in the treated cells was studied. For these experiments, CAT (EC 1.11.1.6) activity was tested by measuring the initiation levels of H2O2 disappearance at 240 nm for 1 min (Cakmak and Marschner 1992). The activity of POD (EC 1.11.1.7) assay was performed in accordance with the guaiacol oxidation by H2O2 at 470 nm (Pandolfini et al. (1992). SOD activity (EC 1.15.1.1) was quantified by the protocol of Giannopolitis and Ries (1977) as previously depicted, with minor modifications (Sagharyan et al. 2023). Each unit enzyme implies that the concentration of SOD can prevent nitroblue tetrazolium (NBT) photoreduction up to 50%.
Quantification of Soluble Sugar Content
The content of free soluble sugars was assessed with the colorimetric method involving the spectrophotometric analysis as described by DuBois et al. (1956) with slight modification. In brief, the frozen-sample (0.1 g FW) were added to 1.5 mL of 0.1 M phosphate buffer (pH 6.8). These extracts were purified by centrifugation (12,000×g, 20 min, 4 °C). After this, 500 µL of supernatant was transferred to the falcon tube supplemented with 5 mL of sulfuric acid. Then, 500 µL of phenol (5%) was mixed with the reaction mixture and allowed to stand for 5 min at ambient temperature. After incubation, the color absorption of the mixture was compared at 480 (rhamnose/xylose), 485 (glucose), and 490 (mannose) nm. The content of free sugars was calculated based on their standard curve and defined as mg g−1 FW.
Quantification of Amino Acids
The quantification of amino acid substances was processed with HPLC-FLD system model Agilent Technologies 1260 infinity, USA as previously described (Biermann et al. 2013). This device was operated by a Zorbax Eclipse-AAA column (4.6 × 150 mm, 3.5 µm particle size) with Na2HPO4/NaH2PO4 (25 mM, pH 7.2)/tetrahydrofuran (95:5, v/v) (Solvent A) Na2HPO4/NaH2PO4 (25mM, pH 7.2)/methanol/acetonitrile (50:35:15, v/v/v) (Solvent B) as mobile phase. To realize that, cell samples (0.2 g) was combined with 2 mL of ethanol 80% (v/v). After centrifugation (10,000×g, 15 min, 4 °C), the solvent was dried at ambient temperature. The dried-residue was added with deionized water (1 mL). Then, appropriate volume of the extraction (50 µL) was dissolved with 40 µL of orthophatalaldehyde (OPA) and buffer borate (80 µM). An equal volume (20 µL) was injected into the HPLC system. Fluorometric detection was performed by excitation wavelength (230 nm) and emission wavelength (435 nm). The column was utilized at 40 °C using 0.5 mL min−1 as the constant flow rate. The identification of individual amino acids was done according to comparison of retention times with standard substances.
Measurement of PAL and TAL Enzymes Activity
The activities of PAL (EC 4.3.1.24) and TAL (EC 4.3.1.23) enzymes was tested using the protocol of Beaudoin-Eagan and Thorpe (1985). Briefly, frozen cell (100 mg) was extracted with 1 mL of potassium phosphate buffer (50 mM; pH 7.0) containing PVP (1mM) and supernatant collected after centrifugation. This extract (100 µL) as a crude enzyme solution was added to 700 µL of potassium phosphate buffer (100 mM, pH 8.0) and 200 µL of L-phenylalanine and/or L-tyrosine solutions (100 mM), which were resolved in potassium phosphate buffer (100 mM, pH 8.0). The samples were heated in a water bath at a constant temperature (37 °C for PAL enzyme and/or 30 °C for TAL enzyme) for 1 h. To inactivate enzymes, 50 μL of HCl (6 N) was poured into the reaction vials. The mixture was purified 3 times through ethyl acetate (2 mL). The ethyl acetate extract was subject to dryness under air conditions and re-dissolved in 1 mL of sodium hydroxide (50 mM). The solution’s absorbance was measured at 290 nm (PAL) and 320 nm (TAL). The activities of PAL and TAL were defined based on μmol cinnamic and/or p-coumaric acids mg.
protein−1 min−1, respectively.
Measurement of Total Phenolics and Flavonoids
Folin-Ciocalteu assay was applied to estimate total phenolics content using the procedure of Akkol et al. (2008) with minor modifications. The reaction solution was composed of methanolic extract (500 µL), Folin-Ciocalteu reagent (2.5 mL) and sodium carbonate 7% (2 mL). The samples were carefully shaken and incubated at 25 ˚C for 2 h. Absorption was appraised with a spectrophotometer at 765 nm (Cary 100-UV–vis, Agilent, USA). The amount of total phenolic was quantified after drawing the gallic acid standard curve (10–100 µg mL−1; R2 = 0.993) in terms of μg g−1 FW. To evaluate total flavonoids, 500 µL of cell extracts was pooled aluminum trichloride dissolved in ethanol (20 mg mL−1) (Akkol et al. 2008). The absorbance was considered at 415 nm via spectrophotometer (Cary 100-UV–vis, Agilent, USA) after 40 min. The quercetin (10–100 µg mL−1; R2 = 0.998) was utilized as the standard for the scaling curve (μg g−1 FW).
Measurement of Phenolic Acids and Flavonoids by HPLC
The determination of phenolic acids was analyzed using the protocol of Owen et al. (2003). To perform HPLC analysis of phenolic acids present in the treated cells, frozen-cells (0.2 g) were mixed with addition of pure methanol, followed by centrifugation (12,000×g). The extraction was air-dried and resolved in acetonitrile (4 mL). To clear lipid components, the extract was prepared as a result of soaking 3 times with N-hexane (3 mL). Then, the acetonitrile solvent was collected and vaporized. The final sedimentary was solubilized with 0.5 mL of methanol. The measurement of phenolic acids was done in accordance with the method of HPLC device (Agilent Technologies 1260 infinity, USA) as previously described by Sagharyan et al. (2023). Isolation of phenolic acids was carried out with a C-18 column (Perfectsil Target ODS-3 (5 μm), 250 × 4.6 mm; MZ Analysentechnik, Mainz, Germany). The identification wavelengths of phenolic acids were arranged at 278–300 nm using a UV detector. The mobile solvent was consisted of aqueous acetic acid 2% (A) and methanol (B) (Zafari et al. 2016).
HPLC analysis of flavonoids content was conducted in correspondence with the procedure outlined by (Keinänen et al. 2001) with slight modification. To determine flavonoids content in the treated cells, the frozen-cells (0.2 g) were blended directly with 1.5 mL of methanol (40%, v/v) acidified with acetic acid (0.5%). Then, the samples were continuously shaken through a rotary shaker during the incubation time (4 h). Ultimately before injection, each sample were then centrifuged at 13,000×g for 10 min. For HPLC analysis, 30 µL of the filtered extract was injected. The mobile phase, composing of phosphoric acid (5%) in water (eluent A), and acetonitrile (eluent B), was pumped based on the eluent gradient as described by (Keinänen et al. 2001). The set wavelengths were 254, 280, 300, and 350 nm on UV-dual array detector. To detect individual flavonoids, the appropriate standards of flavonoids were injected. Sample peaks were analyzed based on the retention time of standards.
Chromatographic Analysis for Lignans
Lignans accumulation were detected as described method by Ahmadian Chashmi et al. (2013). So, 0.2 g dried cell of L. album was homogenized in methanol/water solvent at room temperature. Then, the suspensions were sonicated by ultrasonic bath for 30 min. The single tube test was centrifuged at 12,000×g, and the solvent was concentrated to dryness at ambient temperature. The residues were solubilized with ultrapure methanol (0.5 mL). 20 μL of extracts were applied to HPLC device. The HPLC system was equipped with a C18-ODS3 column (5μm; 250 × 4.6 mm). The mobile phase was ultrapure water and acetonitrile facilitated by a gradient program as previously designed by Ahmadian Chashmi et al. (2013).
Statistical Analysis
Each experimental unit was composed of three independent-biological replications. The normality distribution of data were tested using the Shapiro–Wilk test. Data were subjected to analysis of variance by the SPSS software (Version, 25) and Duncan’s multiple range test (α = 0.05). In the present study, the results were analyzed using statistical methods such as, Principal Component Analysis (PCA), Hierarchical Cluster Analysis (HCA), and Debiased Sparse Partial Correlation (DSPC), to better understand metabolites correlations, with the usage of MetaboAnalyst (https://www.metaboanalyst.ca). Also, Biorender online web (https://www.biorender.com) was used to design graphical abstract.
Results
The Determination of Growth Rate
L. album cells’ growth rate was considered as a sigmoid curve consisting of slow-growing, exponential, and linear phases (Fig. 1). Based on our results, the slow-growing phase was approximately 3–5 days after subculturing, and the exponential (fast-growing) phase was best captured 7–11 days after subculturing. In the present study, L. album cells were treated with different concentrations of Phe after 7 days of subculture.
The Induction Effects of Phe Applications on the Oxidative Status
In terms of oxidative status, exposure of the cell suspensions to different concentrations of Phe for 5 days, led to a variable increase in H2O2 as compared with control cultures (Fig. 2a). An increase in the level of H2O2 was detected at 250 μM of Phe, which was 1.11-fold higher than the control. On the other hand, NO formation was assayed to track its effect on soluble sugars and amino acids accumulations leading to increase phenolic metabolites in respect to Phe treatments. With 250 μM of Phe treatment, NO reached the highest level, which was 1.26-fold compared with the control (Fig. 2b), whereas under the higher levels of Phe treatments NO content was decreased in L. album cells after 5 days. As shown in Fig. 2c, MDA production in the cells induced with increasing H2O2 and NO content. The highest content of MDA (3.85 μmol g−1 FW) was found in cells of L. album exposed to 250 μM of Phe treatment. There was a decrease in MDA levels in cells treated with 1000 μM of Phe, where the content of MDA was 0.90-fold lower compared with the control (Fig. 2c).
The Effect of Phe Treatments on the Antioxidant Enzymes Activity
The inducing activities of the three important antioxidant mediator enzymes (i.e., CAT, POD, and SOD) were studied in the tested-cells to help understand the enzymatic antioxidant influences operative under the different concentrations of Phe. The activities of CAT and POD enzymes were increased at 250 μM of Phe (Fig. 3a, b). Also, a decrease in POD activity were observed at 750 and 1000 of Phe.
Moreover, changes in SOD activity were observed at different concentrations of Phe, where the activity of SOD was enhanced by 250 μM and 500 μM. Based on these results, the catabolism of Phe may have critical roles in altering oxidative status and antioxidant enzymes activities (Fig. 3c).
The Effect of Phe Treatments on Soluble Sugars
Feeding of the cell suspensions with 250 μM and 500 μM of Phe for 5 days, leads to an enhancement in the concentration of rhamnose/xylose to 13.19 and 13.4 mg g−1 FW, respectively, in comparison to the control cultures (Fig. 4a). On the other hand, a depletion in rhamnose/xylose was noticed at 1000 μM of Phe. However, use of all the other three concentrations of the precursor feeding (250, 500, and 750 μM) were successful at increasing the glucose content, within 5 days of treatment (Fig. 4b). Maximum mannose content was measured when 500 μM of Phe was applied for 5 days (21.48 mg g−1) (Fig. 4c). While, the reduction of mannose content was found at 1000 μM of Phe after 5 days.
The Effect of Phe Treatments on Free Amino Acids
In the present study, we performed a comparative phytochemical analysis of the untreated and the treated cells of L. album to uncover the accumulations of amino acids, which can affect phenolic metabolites (Table 1). The HPLC analysis showed that aspartic acid (Asp) accumulation was affected by Phe treatments. Lower Phe concentration up to 750 μM did not reveal any significant difference in Asp accumulation. While the highest concentration of Asp was observed in 1000 μM of Phe that was up to 2.41-fold in comparison to the control. Under higher Phe concentrations (750 and 1000 μM), glutamic (Glu) accumulation was significantly decrease, compared with the control cells, the decrease in Glu accumulation was 26.17% in 750 μM of Phe followed by 1000 μM of Phe (18.85%). Also, the exogenous Phe application decreased the serine (Ser) content significantly compared with the control. The most significant depletion contributed by Phe was considered at 750 and 1000 μM concentration. Significant reduction of Phe at 1000 μM were observed in the treated cells, which was 6.13 μg g−1 FW. In contrast, significant accumulation of Tyr in the treated cells by 1000 μM of Phe treatment was found, which its peak was 2.5-fold more than the control. The content of leucine (Leu) and isoleucine (Ile) was changed by the application of Phe. An increase in Lue (15.67 μg g−1 FW) and Ile (17.74 μg g−1 FW) contents was realized at 500 μM of Phe treatment compared with the untreated control.
The Effect of Phe Treatments on PAL and TAL Enzymes Activities
The activities of PAL and TAL, two key enzymes associated with the phenylpropanoid pathway, was assayed at different concentrations of Phe treatments (Fig. 5a, b). As shown in Fig. 5a, PAL activity was enriched by Phe concentrations, where the highest activity of PAL was recorded at 250 and 500 μM of Phe after 5 days, which its peak was achieved 1.10- and 1.13-fold higher than its control, respectively. Also, the activity of PAL was decreased by 1000 μM of Phe after 5 days, where was 0.89-fold lower than the untreated cells.
Also, there was a significant enhance in TAL activity compared with the control (Fig. 5b). An increase in TAL activity was found at 500 1000 μM of Phe after 5 days, where its peak was 1.14-fold higher compared with the control. In addition, the activity of TAL was declined by 1000 μM of Phe after 5 days, which was 0.90-fold lower than the control.
The Effect of Phe Treatments on Phenolic Acids and Flavonoids Concentration
In order to investigate the metabolic changes on pathways upstream to lignans, we measured the content of total phenolics and phenolic acids (Table 2). Interestingly, maximum content of total phenolics was observed with the use of Phe (1000 μM). On the other hand, responses of phenolics to Phe feeding were shown different patterns in the accumulation of five phenolic acids. Here, an increase in cinnamic acid was found at 500, 750, and 1000 μM of Phe. The content of coumaric acid was achieved its peak at 750 μM of Phe, to extent of 3.28 μg g−1 FW. Use of Phe as precursor feeding, was also moderately successful at increasing caffeic acid content, only however at 500 μM of Phe (1.69 μg g−1 FW). With Phe feeding, an increase for ferulic acid occurred after the treatment period, which its highest content was considered at 250 μM of Phe (2.86 μg g−1 FW). Salicylic acid was significantly accumulated by Phe treatment, which its peak was detected at 1000 μM of Phe (12.78 μg g−1 FW).
In terms of total flavonoids accumulation, exposure of the cell suspension to different concentrations of Phe for 5 days, led to a variable increase in total flavonoids as compared with control cultures (Table 3). At both concentrations (250 and 500 μM of Phe) used, the content of total flavonoids was observed to be higher (715.33 and 708.66 μg g−1 FW, respectively). Also, there were significant differences in the accumulations of flavonoids such as myricetin, and kaempferol, catechin, among the L. album cells that were treated with different concentrations of Phe. Moreover, the content of myricetin in the treated cells that were treated with 250, 500, and 750 μM of Phe were induced by 5.51-, 6.45-, and 5.55-fold, respectively, compared with the untreated cells. The concentration of kaempferol was significantly higher in the 250 μM-treated plants that the other concentrations (Table 3). We thus presumed that the accumulations of flavonoids in the Phe-treated cells altered depending on the amount of applied Phe in the nutrition solution. Catechin accumulation in L. album cells were enhanced by Phe treatments. The highest content of catechin were observed at 500 and 750 μM of Phe, which was 2.92- and 3.11-fold as compared with the untreated cells, respectively (Table 2).
The Effect of Phe Concentrations on the Contents of Lignans
The contents of lignans significantly changed in different concentrations of Phe treatment (Fig. 6). As shown in Fig. 6a, there is no significant changes in the accumulation of lariciresinol (LARI) in the treated cells with Phe. Also, the accumulation of PTOX in the treated cells increased with Phe levels (Fig. 6b). The highest PTOX content (76.12 μg g−1 FW) was observed in cells exposed to 1000 μM of Phe, which was significantly higher by 37.75% than the control (Fig. 6b). Similar to PTOX content, the highest 6MPTOX content (0.673 μg g−1 FW) was also found at 1000 μM of Phe, which was 3.7-fold as that of the control (Fig. 6c).
Classification Metabolic Changes
According to our results, the first and second principal component (PCs) together illustrated 93.9% pf the variation within this data, and PC1 and PC3 clearly partitioned the samples based on metabolic changes exposed to Phe treatments (Fig. 7a). High diversity was detected in 1000 µM of Phe treatment suggesting that this concentration significantly influenced the total variance in this dataset. On the other hand, the results of HCA were shown that different metabolites received from the treated cells were categorized on the basis of a similarity in the clusters pattern through using Pearson correlation coefficient (Fig. 7b). 3 distinct cluster patterns were detected: 1- PTOX → SA; 2- H2O2 → MDA; 3- Coumaric acid → TAL. Consistent with this comprehensive information, Fig. 7b illustrated the possible correlation patterns of metabolic changes in Phe-treated L. album cells. As shown in Fig. 7b, 1000 µM of Phe can positively induce many phenolic compounds such as, SA, PTOX, and 6MPTOX.
According to DSPC resultants, different metabolites were determined by the nodes, while the correlations between them were deputed by lines (Fig. 8a). Data normalization was performed by the log or cubic root. In this network, some lignans were localized at the central positions in association with other metabolites. PTOX with 8 correlation edges, 6-MPTOX and LARI with 7 correlation edges are certain cases. PTOX and 6MPTOX were positively correlated to total phenolic and flavonoid compounds, as well as SA, while were negatively associated with Phe and Glu. Based on achieve information about the disarranging pathways and differential metabolites induced by Phe, the network of metabolic changes was developed. As shown in Fig. 8b, we found that the SA molecule can positively modulate PTOX and 6MPTOX accumulation in L. album cells exposed to Phe treatment. At the high levels of SA can act in the regulation of phenolics synthesis as a negative feedback mechanism.
Discussion
The ability of aromatic amino acids is well-known as primary metabolites; however, these compounds can be involved in the plant processes through providing nitrogenic groups for secondary metabolites pathways such as phenolic acids, flavonoids, coumarins, and alkaloids (Khataee et al. 2020; Feduraev et al. 2020; Torabi et al. 2023). Likewise, Phe is a crucial metabolic point that can link primary metabolism to the secondary metabolites (Manela et al. 2015; Meza et al. 2022). In this study, we tried to find how Phe as a precursor feeding might affect the accumulation of primary metabolites and phenolic compounds through the SA-dependent responses in the cells of L. album.
In plants, it has been found that changes in the various amino acids affect the nitrogenic compounds metabolism and/or their transport, leading to initiate a variety of phycological responses (Besnard et al. 2021). Phe can be converted to the simple phenolic blocks such as cinnamic and p-coumaric acids by the direct action of PAL/TAL enzymes (Barros and Dixon 2019; Torabi et al. 2023). Based on our results, the activity of PAL/TAL enzymes were increased in L. album cells in response to Phe treatment, accumulating phenolic acids. In our previous studies on L. album plant, it has been determined that increasing in PAL and TAL activities can play a key role in the synthesis and the storage of phenolic compounds, especially lignans (Samari et al. 2020; Tashackori et al. 2021; Sagharyan et al. 2023). Although, a decrease in PAL and TAL activities at high levels of Phe may depend on the accumulation of PTOX and 6MPTOX as the end products. This concept is agreement with the findings of Heldt and Piechulla (2011), who reported that the reduction in PAL and TAL activities may be modulated by the accumulation of the phenolic metabolites like cinnamic acid as a negative feedback mechanism.
Also, the exposure of experimental cells to Phe-enriched medium caused significant changes in phenolic acids contents. Among investigated phenolic acids, the SA level showed a notable increase in the cells elicited by different concentrations of Phe. It is known that SA is a phytohormone involved in the regulation of wide aspects of plant immune machinery, including enzymatic and non-enzymatic components (Wani et al. 2017; Samari et al. 2022). Probably, different concentrations of Phe improved the systemic acquired resistance (SAR) in L. album cells through SA-dependent and independent pathways. Accumulating of evidence shows that SA induces several up and down-stream molecules such as glycerol-3-phosphate (Chanda et al. 2011), ROS, and NO under stressful conditions (Wang et al. 2014; Samari et al. 2022). The generation of ROS, known as inevitable by-products of various metabolic pathways, is an obvious mechanism to trigger other important signaling networks (Zafari et al. 2017).
Our observations showed that the contents of H2O2 and MDA were increased at 250 µM of Phe, where the SA concentration did not change significantly compared to the control. On the other hand, the high levels of SA resulted in alleviation of MDA content in the treated cells with 1000 µM of Phe. Consistent with the role of SA in the modulation of ROS and MDA formation, Saleem et al. (2021) and Khan et al. (2022) have proposed that this molecule act as a dual-edged sword with both pro-oxidant (ROS generation) and antioxidant (ROS detoxification) activity versus adverse effectors. They have also suggested that at low contents, SA provokes ROS production and ROS-mediated signaling pathways at the initial stages of plant cell defense responses. SA appears to be a scavenger of hydroxyl radical and an iron-chelating compounds, which can diminish the toxic effect of oxidative stress in plants (Du et al. 2009; Popova et al. 2009; Wani et al. 2017).
In addition of SA, plants have deployed a flexible machinery of enzymes and metabolites that commonly modulate ROS catabolism in their production sites (Zafari et al. 2017; Sagharyan et al. 2023). In this study, we found that Phe changed the activity of antioxidant enzymes including CAT, POD, and SOD. According to our results, the activity of CAT, POD, and SOD enzymes were tightly associated with H2O2 and SA levels. The enhancement of POD activity in the treated cells could be related to its critical role in the biosynthesis of monolignols using H2O2 molecule. These findings are in line with the observations of Zafari et al. (2017), who proposed that an increase in POD activity can shift phenolics metabolism toward lignification in Prosopis farcta plants exposed to Pb stress.
NO molecule is another key regulator in plant signaling cascades by directly or indirectly activating several interplay participants such as Ca+2, Ca+2-protein binding, H2O2, mitogen-activated protein kinases (MAPKs) and effective stress hormones (Zheng et al. 2010; Sanz et al. 2015; Samari et al. 2022). The biological function of NO is dependent on its cellular levels that induce and/or inhibit nitro-oxidative stress (Asgher et al. 2017). Increasing evidence indicated that NO and SA either have a synergistic or antagonistic roles in the modulation of physiological events (Manjunatha et al. 2010). Our data was shown that Phe-induced NO level at 250 μM. These results can suggest that NO production is linked to SA level as an up-stream signal regulating NO content and NO-induced oxidative stress. This concept agreed with the findings of Lindermayr et al. (2006), who suggested that SA molecule can activate and/or suppress the biosynthesis of NO as a dose-dependent manner.
On the other hand, maintaining intracellular homeostasis is a complex biological function proceed by detoxification of ROS and RNS molecules, which requires high energy in plant cell (Mittler et al. 2004; Mittler 2017; Sagharyan et al. 2023). Phe treatment altered primary (e.g., soluble sugars and free amino acids) and secondary metabolites (e.g., flavonoids and lignans) accumulation in L. album cells. Soluble sugars participate in carbon storage, radical scavenging, and osmotic adjustment (Parida and Das 2005). The variations of soluble sugars probably depended on SA-mediated oxidative status in the treated cells. According to our results, a depletion of soluble sugars was found at the high concentrations of Phe, where the enhanced amount of SA was observed. In agreement with our assumption, previous studies have been explained that sugars as highly hydroxylated soluble molecules can reinforce stabilization of membranes and protection of proteins in cells under stress conditions (Pommerrenig et al. 2018; Toghyani et al. 2020). Furthermore, the simple carbohydrates as structural components can impact on the biosynthesis of amino acids by provision of the carbon skeleton and the cell energy flow (Pratelli and Pilot 2014; Hildebrandt et al. 2015).
Amino acids are a group of compatible solutes by which plant metabolic flux can be redirected toward the production of certain secondary metabolites (Yang et al. 2020; Sagharyan et al. 2023). In the present study, changes in amino caids contents such as Asp, Glu, Ser, Phe, Tyr, Lue, and Ile were found at different concentrations of Phe treatment. While a decrease occurred in Phe content in the treated cells, the content of Tyr increased. Unfortunately, there is no scientific information about the rating of affinity between PAL/TAL and the presence of Phe/Tyr in cells of L. album. A possible explanation may be that Phe can be contributed more that Tyr in the production of phenolic compounds in the L. album cells. In other words, the rate of Phe catabolism was probably higher than Tyr, thus leading to reduce of Phe pool. Similar observation was also made by Manela et al. (2015), whereby they reported that the catabolism of Tyr is slower than Phe in cells of Vitis vinifera.
For better understanding the induction mechanisms of Phe in regulating the defense responses, a comparative metabolic changes analysis was used to track its functional role in lignans accumulation in L. album cells. Likewise, SA molecule substantially induced in the presence of Phe treatment as a dose-dependent manner. However, excessive concentrations of Phe had a negative effect on PAL and PTAL enzyme activity due to overproduction of phenolic compounds.
Here, flavonoids accumulation was determined in L. album cells treated with Phe. These molecules are one of the main branches of the phenylpropanoid pathway, which have many biological functions in response to pathogens, herbivores, and environmental stresses (Fakhari et al. 2019; Abedi et al. 2023). The biosynthesis of flavonoids and lignans are directed by PAL activity, which are biochemically competed for primary substrates such as cinnamic acid (Abedi et al. 2023; Sagharyan et al. 2023). The enhancement of PTOX and 6MPTOX accumulation can be correlated to the decrease of central phenylpropanoid intermediates such as cinnamic, coumaric, caffeic, and ferulic acids, which are the main precursors to synthesize PTOX and 6MPTOX. The accumulation of lignans may maintain cellular redox condition, which can be regarded as an important adaptive response of cells to stresses. The biological action of lignans, known as antioxidant agents, is a mechanism for stress adjustment in L. album cells (Tashackori et al. 2021; Samari et al. 2022). It can be concluded that in treated cells of L. album, Phe as a precursor feeding redirected free sugars and amino acids to improve lignans accumulation through change of oxidative status as a SA-dependent pathway.
Conclusion
The present study analyzed the induction effects of phenylalanine as an aromatic amino acid on metabolic and physiological changes in L. album cells. It appears that Phe through induction of PAL/TAL enzymes modulates phenolic acids production such as SA. This phytohormone participates in formation of H2O2 and NO as a dose-dependent manner. The changes in oxidative status of the treated cells can result in the reprogramming of soluble sugars and free amino acids towards phenolics particularly lignans production. Based on our results, it can be proposed that phenylalanine provoked a salicylic acid-dependent regulatory mechanism, which positively modulated antioxidant enzymes activity and phenolics production including phenolic acids, flavonoids, and lignans in L. album cells. However, further studies are requisite to investigate the Phe-mediated regulatory factors like signaling molecules and miRNAs in the induction of lignans synthesis pathway in L. album cells.
Data Availability
All data related to this manuscript can be observed within this paper.
References
Abedi M, Karimi F, Saboora A, Razavi K (2023) NaCl-induced flavonoid biosynthesis and oxidative stress responses in suspension cells of Haplophyllum virgatum var. virgatum. Plant Cell Tissue Organ Cult. https://doi.org/10.1007/s11240-023-02455-0
Ahmadian Chashmi N, Sharifi M, Yousefzadi M, Behmanesh M, Rezadoost H, Cardillo A, Palazon J (2013) Analysis of 6-methoxy podophyllotoxin and podophyllotoxin in hairy root cultures of Linum album Kotschy ex Boiss. Med Chem Res 22:745–752. https://doi.org/10.1007/s00044-012-0067-1
Akkol EK, Goger F, Koşar M, Başer KHC (2008) Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem 108:942–949
Arano-Varela H, Cruz-Sosa F, Estrada-Zúiga ME, Fernández FJ (2020) Effects of phenylalanine and methyl jasmonate on verbascoside production in Buddleja cordata Kunth cell suspension cultures. S Afr J Bot 135:41–49. https://doi.org/10.1016/j.sajb.2020.08.005
Asgher M, Per TS, Masood A et al (2017) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pollut Res 24:2273–2285. https://doi.org/10.1007/s11356-016-7947-8
Barros J, Dixon RA (2019) Plant Phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci 25(1):66–79. https://doi.org/10.1016/j.tplants.2019.09.011
Barros J, Serrani-Yarce JC, Chen F, Baxter D, Venables BJ, Dixon RA (2016) Role of biofunctional ammonia-lyase in grass cell wall biosynthesis. Nat Plants 2(6):16050. https://doi.org/10.1038/nplants.2016.50
Beaudoin-Eagan LD, Thorpe TA (1985) Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol 78:438–441
Besnard J, Sonawala U, Maharjan B, Collakova E, Finlayson SA, Pilot G, McDowell J, Okumoto S (2021) Increased expression of umamit amino acid transporters results in activation of salicylic acid dependent stress response. Front Plant Sci 11:606386. https://doi.org/10.3389/fpls.2020.60638
Biermann M, Bardl B, Vollstdt S, Linnemann J, Knüpfer U, Seidel G, Horn U (2013) Simultaneous analysis of the non-canonical amino acids norleucine and norvaline in biopharmaceutical-related fermentation processes by a new ultra-high performance liquid chromatography approach. Amino Acids 44:1225–1231
Biswas T, Mathur A, Gupta V, Luqman S, Mathur AK (2020) Elicitation and phenylalanine precursor feeding based modulation of in vitro anthocyanin production, enzyme activity and gene expression in an Indian ginseng congener- Panax sikkimensis Ban. Ind Crops Prod 145:111986
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry & molecular biology of plants. American Society of Plant Physiologists, Rockville
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Chakraborty N (2021) Salicylic acid and nitric oxide cross-talks to improve innate immunity and plant vigor in tomato against Fusarium oxysporum stress. Plant Cell Rep 40:1415–1427. https://doi.org/10.1007/s00299-021-02729-x
Chanda B, Xia Y, Mandal MK, Yu K, Sekine K, Gao Q et al (2011) Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet 43:421–427. https://doi.org/10.1038/ng.798
Couée I, Sulmon C, Gouesbet G, Amrani AE (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot 57:449–459. https://doi.org/10.1093/jxb/erj027
Craven-Bartle B, Pascual MB, Cánovas FM, Ávila C (2013) A Myb transcription factor regulates genes of the phenylalanine pathway in maritime pine. Plant J 74:755–766. https://doi.org/10.1111/tpj.12158
Dempsey DA, Klessig DF (2017) How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans. BMC Biol 15:23
Du L, Ali GS, Simons KA, Hou J, Yang T, Reddy ASN, Poovaiah BW (2009) Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 457:1154–1159. https://doi.org/10.1038/nature07612
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Esmaeili S, Sharifi M, Ghanati F, Soltani BM, Samari E, Sagharyan M (2023) Exogenous melatonin induces phenolic compounds production in Linum album cells by altering nitric oxide and salicylic acid. Sci Rep 13:4158. https://doi.org/10.1038/s41598-023-30954-9
Fakhari S, Sharifi M, De Michele R, Ghanati F, Safaie N, Sadeghnejad E (2019) Hydrogen sulfide directs metabolic flux towards the lignan biosynthesis in Linum album hairy roots. Plant Physiol Biochem 135:359–371. https://doi.org/10.1016/j.plaphy.2018.12.015
Feduraev P, Skrypnik L, Riabova A, Pungin A, Tokupova E, Maslennikov P, Chupakhina G (2020) Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 9:476. https://doi.org/10.3390/plants9040476
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Heldt HW, Piechulla B (2011) Plant Biochemistry. Elsevier Academic Press, San Diego. https://doi.org/10.1016/B978-0-12-384986-1.00018-1
Hildebrandt TM, Nunes Nesi A, Araujo WL, Braun H (2015) Amino acid catabolism in plants. Mol Plant 8:1563–1579. https://doi.org/10.1016/j.molp.2015.09.005
Kaur G, Singh HP, Batish DR, Mahajan P, Kohli RK, Rishi V (2015) Exogenous nitric oxide (NO) interferes with lead (Pb)-induced toxicity by detoxifying reactive oxygen species in hydroponically grown wheat (Triticum aestivum) roots. PLoS ONE 10:e0138713. https://doi.org/10.1371/journal.pone.0138713
Keinänen M, Oldham NJ, Baldwin IT (2001) Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J Agric Food Chem 49:3553–3558. https://doi.org/10.1021/jf010200+
Khan MIR, Poor P, Janda T (2022) Salicylic Acid: A Versatile Signaling Molecule in Plants. J Plant Growth Regul 41:1887–1890. https://doi.org/10.1007/s00344-022-10692-4
Khataee E, Karimi F, Razavi K (2019) Chromium-induced alkaloid production in Catharanthus roseus (L.) G. Don in vitro cultured shoots and related gene expression patterns particularly for the novel gene GS. Acta Agric Slov 113(1):95–108
Khataee E, Karimi F, Razavi K (2020) Different carbon sources and their concentrations change alkaloid production and gene expression in Catharanthus roseus shoots in vitro. Funct Plant Biol 48(1):40–53. https://doi.org/10.1071/FP19254
Koo YM, Heo AY, Choi HW (2020) Salicylic acid as a safe plant protector and growth regulator. Plant Pathol J 36:1–10. https://doi.org/10.5423/PPJ.RW.12.2019.0295
Lindermayr C, Saalbach G, Bahnweg G, Durner J (2006) Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J Biol Chem 281:4285–4291. https://doi.org/10.1074/jbc.M511635200
Manela N, Oliva M, Ovadia R, Sikron-Persi N, Ayenew B, Fait A, Galili G, Perl A, Weiss D, Oren-Shamir M (2015) Phenylalanine and tyrosine levels are rate-limiting factors in production of health promoting metabolites in Vitis vinifera cv. Gamay Red cell suspension. Front Plant Sci 6:538. https://doi.org/10.3389/fpls.2015.00538
Manjunatha G, Lokesh V, Neelwarne B (2010) Nitric oxide in fruit ripening: trends and opportunities. Biotechnol Adv 28(4) 489–499. https://doi.org/10.1016/j.biotechadv.2010.03.001
Meza SR, Tobaruela EDC, Pascoal GB, Massaretto IL, Purgatto E (2021) Post-harvest treatment with methyl jasmonate impacts lipid metabolism in tomato pericarp (Solanum lycopersicum L. cv. Grape) at different ripening stages. Foods 10:877
Meza SLR, de Castro TE, Pascoal GB, Magalhães HCR, Massaretto IL, Purgatto E (2022) Induction of metabolic changes in amino acid, fatty acid, tocopherol, and phytosterol profiles by exogenous methyl jasmonate application in tomato fruits. Plants 11:366. https://doi.org/10.3390/plants11030366
Mittler R (2017) ROS are good. Trends in Plant Sci 22(1):11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Noctor G, Mhamdi A (2017) Climate change, CO2, and defense: the metabolic, redox, and signaling perspectives. Trends in Plant Sci 22(10):857–870. https://doi.org/10.1016/j.tplants.2017.07.007
Owen R, Haubner R, Mier W, Giacosa A, Hull W, Spiegelhalder B, Bartsch H (2003) Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brinedolive drupes. Food Chem Toxicol 41:703–717. https://doi.org/10.1016/S0278-6915(03)00011-5
Pandolfini T, Gabbrielli R, Comparini C (1992) Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L. Plant Cell Environ 15:719–725. https://doi.org/10.1111/j.1365-3040.1992.tb01014.x
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Pascual MB, El-Azaz J, de la Torre FN, Cañas RA, Avila C, Cánovas FM (2016) Biosynthesis and Metabolic Fate of Phenylalanine in Conifers. Front Plant Sci 7:1030. https://doi.org/10.3389/fpls.2016.01030
Pommerrenig B, Ludewig F, Cvetkovic J, Trentmann O, Klemens PAW, Neuhaus HE (2018) In concert: orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physiol 59(7):1290–1299
Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Krantev AP, Szalai G, Janda T (2009) Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem 47:224–231. https://doi.org/10.1016/j.plaphy.2008.11.007
Pratelli R, Pilot G (2014) Regulation of amino acid metabolic enzymes and transporters in plants. J Exp Bot 65:5535–5556. https://doi.org/10.1093/jxb/eru320
Rahmani Samani M, Ghasemi Pirbalouti A, Moattar F, Golparvar AR (2019) L-Phenylalanine and bio-fertilizers interaction effects on growth, yield and chemical compositions and content of essential oil from the sage (Salvia officinalis L.) leaves. Ind Crops Prod 137:1–8. https://doi.org/10.1016/j.indcrop.2019.05.019
Sagharyan M, Ganjeali A, Cheniyani M, Mousavi-Kouhi M (2020) Optimization of callus induction with enhancing production of phenolic compounds production and antioxidants activity in callus cultures of Nepeta binaloudensis Jamzad (Lamiaceae). Iran J Biotechnol 18(4):47–55. https://doi.org/10.30498/IJB.2020.2621
Sagharyan M, Sharifi M, Samari E (2023) Methyl jasmonate redirects the dynamics of carbohydrates and amino acids toward the lignans accumulations in Linum album cells. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2023.107677
Saleem M, Fariduddin Q, Castroverde CDM (2021) Salicylic acid: a key regulator of redox signaling and plant immunity. Plant Physiol Biochem 168:381–397. https://doi.org/10.1016/j.plaphy.2021.10.011
Samari E, Sharifi M, Ghanati F, Fuss E, Ahmadian Chashmi N (2020) Chitosan-induced phenolics production is mediated by nitrogenous regulatory molecules: NO and PAs in Linum album hairy roots. Plant Cell Tissue Organ Cult 140:63–576. https://doi.org/10.1007/s11240-019-01753-w
Samari E, Ahmadian Chashmi N, Ghanati F, Sajedi RH, Gust AA, Haghdoust F, Sharifi M, Fuss E (2022) Interactions between second messengers, SA and MAPK6 signaling pathways lead to chitosan-induced lignan production in Linum album cell culture. Ind Crop pro 177:114525. https://doi.org/10.1016/j.indcrop.2022.114525
Sanz L, Albertos P, Mateos I, Sánchez-Vicente I, Lechón T, Fernández-Marcos M, Lorenzo O (2015) Nitric oxide (NO) and phytohormones crosstalk during early plant development. J Exp Bot 66:2857–2868. https://doi.org/10.1093/jxb/erv213
Schmidt TJ, Hemmati S, Klaes M, Konuklugil B, Mohagheghzadeh A, Ionkova I, Fuss E, Alfermann AW (2010) Lignans in flowering aerial parts of Linum species—chemodiversity in the light of systematics and phylogeny. Phytochem 71:1714–1728
Shahkarami P, Ahmadian-Chashmi N, Samari E, Safaie N, Sharifi M (2022) Piriformospora indica induces phenylethanoid glycosides production and defense responses in Scrophularia striata cell culture. Plant Cell Tiss Organ Cult 149:381–395. https://doi.org/10.1007/s11240-021-02213-0
Simpson JP, Olson J, Dilkes B, Chapple C (2021) Identification of the tyrosine- and phenylalanine-derived soluble metabolomes of Sorghum. Front Plant Sci. https://doi.org/10.3389/fpls.2021.714164
Sipari N, Lihavainen J, Shapiguzov A, Kangasjärvi J, Keinänen M (2020) Primary metabolite responses to oxidative stress in early-senescing and paraquat resistant arabidopsis thaliana rcd1 (radical-induced cell death1). Front Plant Sci 11:194. https://doi.org/10.3389/fpls.2020.00194
Soltani M, Samari E, Vazirifar S, Ahmadian Chashmi N, Sharifi M, Fotovat R (2023) Putrescine induces lignans biosynthesis through changing the oxidative status and reprogramming amino acids and carbohydrates levels in Linum album hairy roots. Plant Cell Tiss Organ Cult 153(2):387–402. https://doi.org/10.1007/s11240-023-02479-6
Stewart RR, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–248
Suzuki S, Umezawa T (2007) Biosynthesis of lignans and norlignans. J Wood Sci 53:273–284
Tashackori H, Sharifi M, Ahmadian Chashmi N, Behmanesh M, Safaie N, Sagharyan M (2021) Physiological biochemical and molecular responses of Linum album to digested cell wall of Piriformospora indica. Physiol Mol Biol Plants 27(12):2695–2708. https://doi.org/10.1007/s12298-021-01106-y
Toghyani MA, Karimi F, Hosseini Tafreshi SA, Talei D (2020) Two distinct time dependent strategic mechanisms used by Chlorella vulgaris in response to gamma radiation. J Appl Phycol 32(3):1677–1695. https://doi.org/10.1007/s10811-020-02106-3
Torabi S, Karimi F, Razavi K (2023) Phenylpropanoid biosynthetic gene expression in cell suspension culture of Haplophyllum virgatum Spach. under chitin treatment. In Vitro Cell Dev Biol Plant 59(1):49–60. https://doi.org/10.1007/s11627-023-10327-7
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wang C, El-Shetehy M, Shine MB, Yu K, Navarre D, Wendehenne D et al (2014) Free radicals mediate systemic acquired resistance. Cell Rep 7:348–355. https://doi.org/10.1016/j.celrep.2014.03.032
Wani AB, Chadar H, Wani AH, Singh S, Upadhyay N (2017) Salicylic acid to decrease plant stress. Environ Chem Lett 15:101–123. https://doi.org/10.1007/s10311-016-0584-0
Waszczak C, Carmody M, Kangasjärvi J (2018) Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69:209–236. https://doi.org/10.1146/annurevarplant-042817-040322
Yang Q, Zhao D, Liu Q (2020) Connections between amino acid metabolisms in plants: lysine as an example. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00928
Yousefzadi M, Sharifi M, Chashmi NA, Behmanesh M, Ghasempour A (2010) Optimization of podophyllotoxin extraction method from Linum album cell cultures. Pharm Biol 48(12):1421–1425. https://doi.org/10.3109/13880209.2010.489564
Zafari S, Sharifi M, Ahmadian-Chashmi N, Mur LAJ (2016) Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds, and amino acids. Plant Physiol Biochem 99:11–20. https://doi.org/10.1016/j.plaphy.2015.12.004
Zafari S, Sharifi M, Mur LAJ, Chashmi NA (2017) Favouring NO over H2O2 production will increase Pb tolerance in Prosopis farcta via altered primary metabolism. Ecotox Environ Safe 142:293–302. https://doi.org/10.1016/j.ecoenv.2017.04.036
Zheng LP, Zhang B, Zou T, Chen ZH, Wang JW (2010) Nitric oxide interacts with reactive oxygen species to regulate oligosaccharide-induced artemisinin biosynthesis in Artemisia annua hairy roots. J Med Plant Res 4:758–766
Acknowledgements
This work is based upon research funded by Iran National Science Foundation (INSF) under project NO. 4020049. The authors also would like to thank Tarbiat Modares University for providing this project's laboratory facilities and financial support (Grant No. 87745).
Author information
Authors and Affiliations
Contributions
Authors contributed to this research paper. Mostafa Sagharyan: Investigation, Methodology, Data curation, Resources, Formal analysis, Software, Performing the experiments, Analyzing the data, and write the manuscript. Mohsen Sharifi: Design and Supervise the study, Project administration, Manuscript review, and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Vinay Kumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sagharyan, M., Sharifi, M. Metabolic and Physiological Changes Induced by Exogenous Phenylalanine in Linum album Cells. J Plant Growth Regul 43, 2785–2801 (2024). https://doi.org/10.1007/s00344-024-11307-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-024-11307-w