Abstract
Polyamines, as regulatory compounds, contribute to plant growth, development, and defense responses. Despite attempts to elucidate polyamines effect on the secondary metabolites biosynthesis, their functional roles require further investigation. Here, the role of putrescine on the lignans production and different signaling and metabolic pathways modulation has been investigated in Linum album hairy roots. Our results revealed that putrescine affects the oxidative status of cells by increasing the hydrogen peroxide (H2O2) and malondialdehyde (MDA) levels, and activating superoxide dismutase (SOD), catalase (CAT) and peroxidases (POD) enzymes. Besides, nitric oxide (NO) content augmented during the first hours of the treatment with putrescine. Metabolic assays suggest that putrescine treatment shifts energy and metabolic flows, via changing the carbohydrates and amino acids biosynthesis, towards the phenolics production. Putrescine-induced activation of phenylalanine ammonia lyase (PAL) and tyrosine ammonia lyase (TAL) occurred, likely via the H2O2 and NO signaling pathways. The activation of the PAL and TAL enzymes ultimately led to an increase in phenolic acids, which either play a regulatory role or are precursors to other phenolic compounds such as flavonoids and lignans. The general assumption is that putrescine regulates the lignan biosynthesis by inducing signaling pathways, altering oxidative status, and modifying metabolite profiles in L. album hairy roots.
Key message
Putrescine treatment induces lignans accumulation accompanied by modulation of oxidative status and alteration of amino acids and carbohydrates contents in L. album hairy roots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is sufficient evidence that polyamines are involved in the various processes of plant growth and development (Liu et al. 2015; Chen et al. 2018; Pál et al. 2019). It has been determined that exogenous polyamines can accelerate the differentiation of flower bud in chrysanthemum (Xu 2015). Jiao et al. (2017) have indicated that appropriate amounts of polyamines in the culture medium significantly increase the efficiency of in vitro embryo rescue in seedless grapevine. Until now, numerous investigations have been carried out to identify the influence of polyamines on the various defense reactions in plants (Moschou and Roubelakis-Angelakis 2014; Liu et al. 2015; Gerlin et al. 2021). Darko et al. (2019) reported that polyamine metabolism can be significantly altered in response to stressful conditions in wheat seedlings (Darko et al. 2019). Furthermore, in transgenic tomatoes, overexpression of the genes involved in the polyamines biosynthesis led to greater resistance to biotic and abiotic stresses such as Fusarium oxysporum, Alternaria solani, salinity, drought, cold and high temperature (Hazarika and Rajam 2011). These results indicate that polyamines play a role in regulating the plant defense responses.
These nitrogenous compounds regulate the plant defense system directly thanks to their structural properties and/or indirectly through interaction with other metabolic and signaling pathways (Liu et al. 2015). Polyamine catabolism is one of the key events in the regulation of the plant defense system. The activation of enzymes that catalyze the degradation reactions of polyamines leads to the formation of hydrogen peroxide (H2O2) (Walters 2003). H2O2 and other reactive oxygen species (ROS) have critical roles in the plant cell fate, and act like a double-edged sword. Changes in their levels under different environmental conditions can cause oxidative damage to cellular membranes or initiate signaling pathways affecting proteins function and genes expression (Smirnoff and Arnaud 2019). Certain amounts of H2O2 induce the plant antioxidant machinery, including enzymatic and non-enzymatic antioxidants, to control cellular oxidative balance (Irato and Santovito 2021). Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) cooperate to maintain ROS hemostasis at a safe level (Rajput et al. 2021). Some reports suggest that polyamines can activate antioxidant enzymes in plants in response to stressful situations (Radhakrishnan and Lee 2013; Zhang et al. 2013). The H2O2 molecule can also regulate the biosynthesis of the secondary metabolites (Lv et al. 2019).
Secondary metabolites are small organic molecules with antioxidant function in plants whose medical, economic, industrial and agricultural importance is well-documented. Phenolic compounds including phenolic acids, flavonoids, anthocyanins, lignins, lignans, etc., are known as one of the largest groups of the secondary metabolites (França et al. 2001). Since understanding the regulation of the phenolic compounds biosynthesis is of considerable significance, a body of literature has appeared about which in recent decades (Hano et al. 2006; Baldi et al. 2010; Tahsili et al. 2014). According to some suggestions, polyamines can contribute to the induction of the secondary metabolite biosynthesis (Mustafavi et al. 2018). However, there is currently little information about their function in this process in medicinal plants. Lignans are a sub-group of phenolic compounds whose anticancer and antiviral properties have already been demonstrated. Accordingly, some types of cancer are currently being treated with lignans-derived medicines such as podophyllotoxin (PTOX) (Saleem et al. 2005). Linum album Kotschy ex Boiss., a species endemic to Iran, is one of the most valuable sources of lignans, including PTOX and 6-methoxypodophyllotoxin (6MPTOX) (Samari et al. 2022). Therefore, a lot of efforts have already been devoted to this species so as to stimulate lignans biosynthesis pathway and shed new light on the mechanisms of its regulation (Tahsili et al. 2014; Tashackori et al. 2016, 2018, 2021; Samari et al. 2022). Furthermore, Samari et al. (2020) showed that chitosan-induced lignans biosynthesis can be correlated with the polyamines changes in L. album hairy roots. We then tried to find out if and how polyamines can influence the lignans biosynthesis in L. album. For this, L. album hairy roots were exposed to putrescine, and changes in the lignans biosynthesis were examined. There is also a focus here to elucidate the effect of putrescine on upstream signaling and metabolic pathways including phenolic acids, amino acids, carbohydrates, H2O2, nitric oxide (NO) in the relation to the lignans biosynthesis.
Materials and methods
Hairy roots culture and treatment
The R1 hairy roots of Linum album have been developed with Agrobacterium rhizogenes, strain LBA9402, in previous studies (Chashmi et al. 2013). Hormone-free MS medium (Murashige and Skoog 1962) was used to cultivate the hairy roots. They were transferred to fresh medium every 3 weeks and kept on the incubator shaker at 100 rpm at 28 °C in the dark. To determine the optimum concentration of putrescine, different concentrations (0, 0.1, 0.25, 0.5, 0.75 and 1 mM) were added to 10-day-old hairy roots. A selected concentration of putrescine was then added to the culture medium for time course analysis at 0, 6, 12, 24, 48, 72, and 120 h after the treatment. In parallel, the control samples were treated with distilled water at the same time points. The experiment was performed in three repetitions for each group.
H2O2 and MDA contents determination
To determine the amount of H2O2, 0.2 g of fresh roots tissue was ground with 1.5 mL of 0.1% trichloroacetic acid (TCA) on the ice bath. The resulting mixtures were transferred to 2 mL micro tubes and centrifuged at 12,000 rpm for 15 min at 4 °C. 0.5 mL of the supernatant phase was added to 0.5 mL of 100 mM potassium phosphate buffer and 1 mL of 1 M potassium iodide (KI) in the dark. After 30 min, the absorbance of the samples was recorded at 390 nm using spectrophotometry method. The amount of H2O2 produced in the samples was calculated based on the standard curve, in µmol g−1 FW (Velikova et al. 2000).
To evaluate peroxidation of cell membranes, the amount of malondialdehyde (MDA) was determined. Fresh hairy roots tissue (0.2 g) was ground in 1.5 mL of 10% (w/v) TCA on the ice. The resulting mixture was centrifuged at 12,000 rpm for 15 min at 4 °C, and then 0.5 mL of the isolated liquid phase was mixed with 0.5 mL of 0.5% thiobarbituric acid (TBA) dissolved in 20% TCA solution. This mixture was put in a 95 °C water bath for 30 min. After reaching to ambient temperature, the absorbance of samples was read at 532 and 600 nm (Stewart and Bewley 1980).
Antioxidant enzymes activity assay
Total soluble proteins were extracted by homogenizing 200 mg of frozen tissue in 2 mL of ice-cold 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM dithiothreitol (DTT). The mixture was centrifuged at 12,000 rpm for 20 min at 4 °C, and then the supernatant was applied for the activity assay of enzymes. SOD activity was measured by determining the inhibition of nitro blue tetrazolium (NBT) photochemical reduction (Giannopolitis and Ries 1977). CAT activity was assayed by measuring the rate of H2O2 disappearance for 1 min at 240 nm (Cakmak and Marschner 1992). POD activity was determined by monitoring the variations in the absorbance of samples at 470 nm due to guaiacol oxidation in the presence of H2O2 (Pandolfini et al. 1992). Total protein concentration was determined according to the method of Bradford (1976) and bovine serum albumin (1 mg mL−1) was used as standard (Bradford 1976).
NO content measurement
The NO content was measured by determining nitrite concentration through grease reagent. Fresh hairy root tissue (0.2 g) was ground with 1.5 mL of 100 mM potassium phosphate buffer (pH 7), and centrifuged at 10,000 rpm for 15 min. 250 μL of liquid phase was added to 750 μL of extract buffer and 0.5 mL of grease reagent. The grease reagent contains 1% sulfanilamide and 0.1% N-naphthylethenyl amide in 5% (v/v) phosphoric acid solution. The resulting mixture was incubated at room temperature for 10 min. After the reaction time, the samples’ absorption was read at 540 nm with a spectrophotometer. NO amount was calculated based on the standard sodium nitrite curve and was reported in µmol g−1 fresh weight (Kaur et al. 2015).
Assessment of soluble carbohydrates
To determine soluble sugars contents, 0.2 g of fresh hairy root tissue was homogenized with 3 mL of 0.1 mM sodium phosphate buffer (pH 6.8). After centrifuging at 7,000 rpm for 10 min, the liquid phase was collected for carbohydrates analysis. 50 μL of extract and 450 μL of deionized water were transferred to test tubes, then, 2.5 mL of phenol solution was added to the samples. After 10 min, the light absorption of the samples was measured by spectrophotometer at 480, 485, and 490 nm for ascertaining the contents of rhamnose + xylose, glucose, and mannose, respectively. Finally, the amount of total carbohydrates in L. album hairy roots was ascertained based on the glucose standard curve (DuBois 1956).
Qualitative analysis of amino acids by HPLC
The fresh sample (0.2 g) was powdered and mixed with 2 mL of 80% (v/v) ethanol in water. The extract was centrifuged at 13,000 rpm for 5 min, and then the supernatant was dried at room temperature. The residue was resolved in 1 mL of H2O (Di Martino et al. 2003) and the amino acids were analyzed by HPLC (Agilent Technologies 1260 infinity, USA) using a FLD HP 1100 fluorimetric detector and precolumn derivatization with OPA. The separation was performed with a Zorbax Eclipse-AAA column (4.6 × 150 mm, 3.5-mm particle size; Agilent Technologies, USA). Mobile phase A was aqueous buffer (25 mM Na2HPO4/ NaH2PO, pH 7.2)/ tetrahydrofuran (95:5, v/v) and mobile phase B was aqueous buffer (25 mM Na2HPO4/ NaH2PO, pH 7.2)/ methanol/acetonitrile (50:35:15, v/v/v) (Biermann et al. 2013).
PAL and TAL enzymes activity
PAL enzyme activity was determined based on the cinnamic acid production (Beaudoin-Eagan and Thorpe 1985). The protein extract was incubated at 37 °C in 0.1 M potassium phosphate buffer (pH 8.8) containing 0.1 M L-phenylalanine for 1 h. Then, the cinnamic acid production was stopped by adding 50 μL of 6 M HCl. The mixture was extracted with 2 mL of ethyl acetate (3 times). The collected ethyl acetate phase was dried and the remainder was dissolved in 1 mL of 0.05 M NaOH. The solution’s absorbance was read at 290 nm using a spectrophotometer. The enzyme activity was expressed in μmol of cinnamic acid mg−1 protein min−1. TAL enzyme activity was measured according to Beaudoin-Eagan and Thorpe (1985) method as described for the PAL activity with L-tyrosine as substrate. The formation of p-coumaric acid was quantified as production by recording samples’ absorbance at 320 nm.
Total phenolics and flavonoids analysis
Total phenolics and flavonoids contents were determined based on Akkol et al. (2008). For phenolic acids measurement, 1 mL of methanolic extract was added to 5 mL of Folin–Ciocalteu reagent and 4 mL of 7.0% sodium carbonate solution. After 2 h, the samples’ absorbance was read at 765 nm. The total flavonoids also were determined by adding 1 mL of the methanolic extract to 1 mL of 20 mg mL−1 aluminum trichloride (AlCl3) in ethanol. The absorbance was measured after 40 min at 415 nm.
Qualitative analysis of phenolic compounds by HPLC
Dried roots (1 g) were powdered and mixed with methanol solution. The methanol extract was dried and the residue was dissolved in 50 mL of acetonitrile. The mixture was washed three times with 20 mL of n-Hexane. The subsequent acetonitrile solution was dried, and then re-suspended in 0.5 mL of methanol to analyze the individual phenolic acids by HPLC (Agilent Technologies 1260 infinity, USA) (Owen et al. 2003). The stationary phase was a C18 column (Perfectsil Target ODS-3 (5 μm), 250 × 4.6 mm; MZ Analysentechnik, Mainz, Germany). The eluent system consists of solvent A: 2% acetic acid in deionized water and B: methanol with a gradient system (Zafari et al. 2016). To determine lignans, the dried roots were extracted by sonication in 80% methanol (Yousefzadi et al. 2010). The extract obtained was dissolved in 0.5 mL of methanol for HPLC analysis. The stationary phase was a C18-ODS3, 5 μm (250 × 4.6 mm) column. The elution solvent was composed of acetonitrile and water with a gradient system based on Chashmi et al. (2013) (Chashmi et al. 2013).
Statistical analysis
All experiments were performed in three replicates. Data were processed using Graph Pad Prism 7 and IBM SPSS 26 software. Duncan’s multiple range test was used to present significant differences between the means. A p-value of less than 0.05 was used for significant differences. To clarify more detailed information on the correlation among metabolites, the hierarchical cluster analysis (HCA) was drawn by using the web-based metabolomics data processing software MetaboAnalyst (https://www.metaboanalyst.ca).
Results
Putrescine optimum concentration
To find optimum concentration of putrescine, the effect of various concentrations was studied on the hairy roots dry weight and lignans production. The results indicated that roots dry weight had no change under 0.1 and 0.25 mM putrescine, whereas higher concentrations resulted in substantial reduction in dry weight, changes in colour and thickness of the hairy roots (Fig. 1a and Online Resource 1). In addition, the HPLC data showed that lariciresinol production increases with increasing putrescine concentrations up to 0.5 mM, and reduces at higher concentrations. PTOX and 6MPTOX highest levels occurred in 0.25 mM putrescine, while increasing putrescine concentrations up to 1 mM significantly reduced their levels. The amount of pinoresinol remained constant up to 0.25 mM, and then decreased at higher putrescine concentrations, similar to other lignans (Fig. 1b). Based on these results, 0.25 mM putrescine was selected as the optimal concentration to investigate responses of L. album hairy roots over a period of time (Online Resource 2).
H2O2 and MDA contents
To investigate the effect of putrescine on the oxidative status of L. album hairy roots, the H2O2 and MDA levels were monitored. The results showed that the amount of intracellular H2O2 in the hairy roots of L. album increased significantly 6 and 12 h after putrescine treatment, but decreased thereafter and had no changes from 48 h of treatment (Fig. 2a). Similarly, MDA level which serves as a marker for the degree of cell membrane peroxidation due to oxidative stress, showed a significant increase up to 12 h after the treatment with putrescine, and then decreased to the level of the control samples (Fig. 2b).
Antioxidant enzymes activity
The effect of putrescine on the activity of antioxidant enzymes was investigated by measuring the activity of SOD, CAT and POD. According to Fig. 3a, SOD enzyme activity increased greatly 2.3, 2, 1.8 and 1.4-fold over the control samples at 6, 12, 24 and 48 h after the treatment, respectively. CAT activity in putrescine-affected L. album hairy roots increased significantly from 12 to 120 h, peaking at 48 h of treatment (2.5 times more than the control condition) (Fig. 3b). Putrescine also significantly enhanced POD enzyme activity in all-time points of the treatment period, while the maximum activity was observed at 24 and 48 h after the treatment (1.7-fold higher than the control) (Fig. 3c).
NO content
The changes in the NO content in the hairy roots when treated with putrescine have been shown in Fig. 4. The increase in the level of this molecule began 6 h after the treatment and reached a maximum level at 24 h by 1.3 times compared to the control. The NO content decreased to a constant level that was the same as the control sample level at 48 to 120 h.
Carbohydrates contents
The analysis of the sugars production showed that putrescine caused a significant increase in the contents of glucose, mannose and rhamnose + xylose 12 h after the treatment (1.3-fold) (Fig. 5). At 24 h of the treatment, mannose increased significantly, while at 120 h, the contents glucose and rhamnose + xylose increased in the hairy roots.
Free amino acids contents
Changes in individual free amino acids were measured in the hairy roots in response to putrescine (Table 1). HPLC data analysis revealed that amino acid glutamate (Glu) enhanced considerably from 24 h of the treatment, peaking at 72 h with a 1.5-fold increase over the control sample. Under the influence of putrescine, the amino acid phenylalanine (Phe) showed a significant increase from 6 to 48 h after the treatment, with the highest value at 12 and 24 h, and then decreased. The tyrosine (Tyr) and aspartate (Asp) contents showed an increasing trend until 24 and 48 h, respectively, and then their levels decreased. The amino acid arginine (Arg) showed an increasing trend in all-time points except 6 h of the treatment. A significant enhancement in methionine (Met) level occurred only 48 h after the addition of putrescine.
PAL and TAL enzymes activity
As Fig. 6 illustrates, putrescine treatment resulted in a slight increase in PAL enzyme activity after 6 h. It peaked after 48 h of the treatment. On the other hand, TAL enzyme activity showed an enhancement 12 and 24 h after putrescine treatment with a peak at 12 h.
Total phenolics and flavonoids contents
The results showed that putrescine caused a significant increase in total phenolics contents 6 h after the treatment compared to the control sample, and then remained constant (Fig. 7a). Also, the increase in total flavonoids contents started at 12 h after the treatment, peaking at 24 h. Their levels remained constant in the following hours of the treatment (Fig. 7b).
Phenolic acids contents
The results of the analysis of variance of phenolic acids at the concentration of 0.25 mM putrescine showed that the amount of cinnamic acid increased significantly at all-time points after the treatment. The maximum increase in cinnamic acid content was at 24 and 48 h after the treatment, which was 1.9 and 2.22 times higher than the control samples, respectively (Table 2). Putrescine also increased coumaric acid and salicylic acid (SA) contents 12 to 120 h after the treatment. The highest level of coumaric acid was about 1.7 times at 12 h after the treatment, and increased slightly in the following hours. Moreover, SA level peaked at 120 h after the treatment, which was 2.5 times higher than the level in the control sample. Eventually, ferulic acid and caffeic acid levels exhibited an enhancing trend in all-time points of putrescine treatment. As with SA, the greatest enhancement in caffeic acid was observed at 120 h after the treatment by 1.7 times, while the highest accumulation of ferulic acid occurred at 24 h (1.4 times).
Lignans contents
The results of lignans measurement showed that the amount of pinoresinol increased significantly at 6, 12, and 24 h of the treatment, and then remained constant. The greatest increase in pinoresinol content was observed at 12 h, which was almost three times higher than the amount of pinoresinol in the control sample (Fig. 8a). Lariciresinol content also showed a significant increase from 12 to 120 h after treatment with putrescine, peaking at 24 and 48 h with 1.7 and 2 times increase compared to their controls, respectively (Fig. 8b). In contrast, the increase in PTOX content started after 24 h of the treatment and lasted up to 120 h. The highest amount of PTOX was observed at 120 h of putrescine treatment (1.8-fold) (Fig. 8c). Putrescine also significantly increased the production of 6MPTOX 72 and 120 h after the treatment. Its level reached a peak at 120 h that was 1.4 times more than the control sample (Fig. 8d).
The effect of putrescine on the contents of a pinoresinol b lariciresinol c podophyllotoxin and d 6-metoxy podophyllotoxin in L. album hairy roots at the different time points. The values show the mean of the three replications ± standard deviation. Different letters indicate a significant difference at the 5% level
Data clustering
The correlations among the different signaling and metabolite pathways were determined according to Pearson correlation coefficient, by a hierarchical cluster analysis (HCA). The results illustrate 5 clusters displayed by double head arrows in Fig. 9. The different clusters reflect different response patterns of metabolites to putrescine.
HCA map was used for clustering of various metabolites and regulatory molecules based on Pearson correlations coefficient. Data is shown for three replicates for each variation at time points of elicitation. Colors in the heat map show the magnitude and direction of the correlations: the blue box means strong negative and the red box is the sign of strong positive correlation. There are 5 basic clusters that are indicated in the picture by double head arrows
Discussion
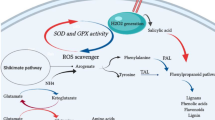
Although lignans biosynthesis has been widely discussed in recent decades, our information about the regulatory molecules governing this pathway is still limited and needs to be developed. Some studies have already shown that polyamines can increase the secondary metabolites production in plants (Mustafavi et al. 2018; Rakesh et al. 2021). For that reason, we made an effort to investigate the effect of putrescine on the biosynthesis of lignans, as well as the upstream signaling and metabolic pathways that participate in the lignans biosynthesis in hairy roots of L. album.
First, the use of different concentrations of putrescine showed that this polyamine is capable of stimulating the biosynthesis of lignans, and then optimum concentration of putrescine was selected, depending on the dry weight and lignans contents of the treated L. album hairy roots. The rapid rise in ROS, in particular H2O2, known as oxidative burst, is one of the first events occuring after the changes in plant conditions (Bhattacharjee 2005). Besides, the degradation of polyamines also leads to the production of H2O2 (Yoda et al. 2006). Because this molecule plays a dual role in cellular processes (a second messenger at low concentrations and a destructive agent at high concentrations), the plant antioxidant system must be ready to adjust the oxidative state of the cell. The activation of the antioxidant components, especially enzymes such as SOD, POD and CAT, is a significant factor in regulating the intracellular H2O2 level (Saxena et al. 2016). In our study, it was understood that putrescine increases H2O2 levels during the first 12 h of the treatment, resulting in mild membrane disruption and increased MDA production. Since the increase in SOD activity coincided with the onset of H2O2 production during the first hours of treatment, it can be concluded that the conversion of O2− to H2O2 is a cellular strategy to reduce the destructive effect of ROS in response to putrescine. The strong positive correlations between MDA and H2O2 as well as SOD and H2O2 can confirm these ideas. Subsequent increases in POD and CAT activity at the following time points indicated that each is respectively responsible for the removal of excessive amounts of putrescine-derived H2O2 in hairy roots of L. album. It appears that putrescine is able to control the destructive effects of high concentrations of H2O2 and reduce lipid peroxidation by inducing the activation of antioxidant enzymes (Mandal et al. 2013). It is reported that several studies have obtained similar results showing the role of putrescine in the activation of various antioxidant enzymes (Palma et al. 2016; Zhong et al. 2020). NO is another second messenger molecule involved in many plants developmental and defensive processes. Enzymatic oxidation of polyamines plays an important role in NO generation in plants (Yang et al. 2014). This molecule can influence the oxidative status of plant cells by inhibiting the activity of antioxidant enzymes such as CAT, and inducing the activity and gene expression of various biosynthetic enzymes such as PAL and TAL, leading to the production of antioxidant compounds (Begara-Morales et al. 2016; Samari et al. 2022; Khodamoradi et al. 2022). Our results revealed that putrescine increases No content in L. album hairy roots during the first 12 h of the treatment, and then reached to the level in the control sample after 48 h. Increase in CAT activity after this period demonstrates NO inhibitory effect on CAT activity in the early hours of treatment, followed by an increase in H2O2 level. Agurla et al. (2017) have reported that putrescine, spermine, and spermidine treatments activate amino oxidase and NADPH oxidase, which lead to an increase in H2O2 and NO contents (Agurla et al. 2018).
Carbohydrates metabolism is also related to the plant defense responses. Reprogramming their concentrations can regulate osmotic stress and induce the production of the secondary metabolites (Rojas et al. 2014). Several studies have provided evidence that polyamines affect carbohydrate metabolism in different plants (Palma et al. 2016; Luo et al. 2019). Our results showed that putrescine caused a significant increase in the levels of soluble carbohydrates glucose, mannose, rhamnose and xylose after 12 h of treatment in L. album hairy roots. Such carbohydrates accumulation during a 12 h treatment can be one of the mechanisms protecting plant cells from changes in the oxidative status. On the other hand, they can provide energy and materials needed to produce protective metabolites such as phenolic compounds, therefore, their concentrations reduction at subsequent time points in putrescine-treated samples can be justified. Accordingly, a former investigation indicated that increased sucrose level leads to increased lignan biosynthesis in L. album cell culture (Baldi et al. 2008).
Amino acids are another group of the primary metabolites involved in the induction of plant defense responses biochemically related to the polyamine metabolism (Vilas et al. 2018). Alterations in polyamines metabolism can influence the amino acid levels in plant cells (Majumdar et al. 2016). It has been elucidated that treatment with extracellular polyamines significantly increases the contents of various amino acids in rice grain (Xu et al. 2021). In addition, Moschou et al. (2012) pointed out that putrescine is positively related to the accumulation of the primary metabolites such as amino acids, glucose and sucrose (Moschou et al. 2012). Amino acids homeostasis is also related to the production of NO (Astier et al. 2018). The amino acids Glu and Asp -the main precursors of several amino acids- are used in the biosynthesis of Arg and Met, which are involved in the polyamines and NO biosynthesis (Winter et al. 2015). It was observed that putrescine increases the contents of amino acids Glu and Arg after 24 h of the treatment. It appears that when Glu increases, it leads to an increase in Arg level, possibly followed by the induction of polyamines and/or NO production. Therefore, the use of putrescine may increase the biosynthesis of endogenous polyamines in L. album hairy roots. On the other hand, due to a common precursor, there could be a possible competition between the formation of polyamines and NO, which increases the need of plants to produce more Arg. In addition, Asp level increased 12 h after treatment with putrescine, and then decreased after 48 h when the amino acid Met reached its maximum. The decreased in Asp level may be due to its consumption for Met production. HCA data shows a negative correlation between Asp content and Met accumulation. The amino acid Met is involved in the biosynthesis of polyamines and ethylene, leading to competition between these pathways and a reduction in Met content (Lasanajak et al. 2014). Amino acids are also known to be precursors for the production of the different secondary metabolites (Mur et al. 2017). The cyclic amino acids Phe and Tyr resulting from shikimate pathway play an important role in the biosynthesis of phenolic compounds (Santos-Sánchez et al. 2019). Previous studies have shown that chitosan and fungal treatments enhance the production of these amino acids in L. album hairy roots as a defensive strategy towards the production of phenolics, in particular PTOX and 6MPTOX (Tashackori et al. 2018; Samari et al. 2020). HPLC results showed that putrescine increased Phe and Tyr contents during 48 h after the treatment. Thereafter, their levels decreased, likely as a result of the consumption and negative feedback on the activity of key enzymes in the shikimate pathway (Tzin and Galili 2010).
Some studies have already demonstrated that polyamines are involved in inducing the phenylpropanoid biosynthesis by affecting the activity of PAL and TAL enzymes. These enzymes catalyse the key reactions of converting Phe and Tyr into cinnamic acid and coumaric acid, respectively, as entry points for the primary metabolites into phenylpropanoid pathway (Szalai et al. 2017). There are reports that spermine polyamine increases PAL enzyme activity and is involved in the production of phenolic acid (Orabi and Sadak 2015). Our results showed that PAL and TAL enzymes become activated at 6 h and 12 h of putrescine treatment in L. album hairy roots, respectively. This activation can be mediated through the putrescine degradation-induced H2O2 and NO signaling pathways. Accordingly, Fig. 9 shows that there is a positive correlation between this signaling molecules levels and the activity of PAL and TAL enzymes in the treated hairy roots of L. album. The increased activity of these enzymes is consistent with the increased levels of Phe and Tyr, as well as cinnamic and coumaric acids, respectively. Phenolic acids play an important role in the plant defense system as they contain an aromatic ring with side branches -OH and -OCH3 in their structure (Mishra et al. 2021). Furthermore, they are the precursors to more complex phenolic compounds such as lignans, lignins and flavonoids (Kumar and Goel 2019; Shahkarami et al. 2022). Phenolic acids analysis showed that putrescine stimulates the cinnamic acid and coumaric acid production 6 h and 12 h after treatment in L. album hairy roots. Furthermore, their downward trends in the last time points of the treatment can be attributed to their consumption as precursors of other phenolic compounds. Putrescine also significantly increased caffeic acid and ferulic acid in L. album hairy roots. Caffeic acid is converted into ferulic acid by the activity of caffeate–O–methyltransferase enzyme and is likely a precursor of lignans biosynthesis in the phenylpropanoid pathway (Guo et al. 2001). Yang et al. (2010) and Hao et al. (2012) reported that putrescine can stimulate rosmarinic acid and salvanoic acid production in the hairy root cultures of Nepeta cataria and Salvia miltiorrhiza, respectively (Yang et al. 2010; Hao et al. 2012). Salicylic acid (SA) is another important phenolic acid which increased in response to putrescine 12 h after the treatment, peaking at 120 h in L. album hairy roots. It is known as a phytohormone playing key roles in the many plant physiological processes. This regulatory molecule can interact with other signaling molecules such as NO and H2O2 to activate many defense responses and biosynthesis of secondary metabolites in plants (Samari et al. 2022). SA can also induce lignan production and genes expression involved in the biosynthesis of phenolic compounds (Yousefzadi et al. 2010). Our results showed a positive relationship between SA level and the content of various phenolic compounds. It has also been reported that polyamines can increase the flavonoids contents (Jahangir et al. 2011; Ahanger et al. 2019). Our results showed that putrescine causes a significant increase in total flavonoids from 12 to 120 h of the treatment. As potent antioxidants, flavonoids are branched from phenolic acids and their biosynthesis can affect the lignans accumulation, thanks to common precursor and competition (Serra et al. 2012).
Lignans measurement showed that putrescine increased pinoresinol content, reaching the maximum level 12 h after the treatment, while falling to control levels at 24 and 48 h. Likewise, the highest content of lariciresinol occurred 24 and 48 h after the treatment. These results suggest that putrescine presumably modifies pinoresinol and lariciresinol, consistent with pinoresinol role as a precursor of lariciresinol. On the other hand, lariciresinol decreased at 72 and 120 h after putrescine treatment, while PTOX and 6MPTOX contents significantly increased during these periods. These changes suggest the use of lariciresinol at 72 and 120 h to produce PTOX and 6MPTOX under putrescine treatment. It was also found that PTOX content increased faster and more than 6MPTOX in L. album hairy roots treated with putrescine. Therefore, treatment with putrescine has a stronger effect on PTOX production and accumulation in L. album hairy roots.
Conclusion
Polyamine putrescine can stimulate the lignans biosynthesis in L. album hairy roots. It can be suggested that putrescine by affecting the upstream metabolic and signaling pathways induces lignans biosynthesis. This polyamine probably directly and/or indirectly triggers H2O2 and NO signaling pathways, leading to changes in the oxidative status of L. album hairy root cells. Moreover, primary metabolites profile including carbohydrates and amino acids are modified in response to putrescine treatment. Reprogramming of signaling and metabolic pathways can direct information, materials, and energy toward inducing the production of phenolic compounds, especially lignans.
Data availability
The data supporting the findings of this study are available from the corresponding authors, upon request.
Abbreviations
- 6MPTOX:
-
6-Methoxypodophyllotoxin
- CAT:
-
Catalase
- H2O2 :
-
Hydrogen peroxide
- LARI:
-
Lariciresinol
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- PAL:
-
Phenylalanine ammonia-lyase
- POD:
-
Peroxidase
- PTOX:
-
Podophyllotoxin
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SOD:
-
Superoxide dismutase
- TAL:
-
Tyrosine ammonia-lyase
References
Agurla S, Gayatri G, Raghavendra AS (2018) Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 255(1):153–162. https://doi.org/10.1007/s00709-017-1139-3
Ahanger MA, Qin C, Maodong Q, Dong XX, Ahmad P, Abd Allah EF, Zhang L (2019) Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol Biochem 144:1–13. https://doi.org/10.1016/j.plaphy.2019.09.021
Akkol EK, Göger F, Koşar M, Başer KHC (2008) Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem 108(3):942–949. https://doi.org/10.1016/j.foodchem.2007.11.071
Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69(14):3401–3411. https://doi.org/10.1093/jxb/erx420
Baldi A, Srivastava AK, Bisaria VS (2008) Effect of aeration on production of anticancer lignans by cell suspension cultures of Linum album. Appl Biochem Biotechnol 151(2):547–555. https://doi.org/10.1007/s12010-008-8230-3
Baldi A, Hussain W, Tailor Y (2010) Evaluation of in-vitro cultured cells of Withania somnifera for antioxidant activity. Curr Trends Biotechnol Pharm 4(1):589–595
Beaudoin-Eagan LD, Thorpe TA (1985) Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol 78(3):438–441. https://doi.org/10.1104/pp.78.3.438
Begara-Morales JC, Sánchez-Calvo B, Chaki M, Valderrama R, Mata-Pérez C, Padilla MN, Corpas FJ, Barroso JB (2016) Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Front Plant Sci 7:152. https://doi.org/10.3389/fpls.2016.00152
Bhattacharjee S (2005) Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transducation in plants. Curr Sci 89(7):1113–1121
Biermann M, Bardl B, Vollstädt S, Linnemann J, Knüpfer U, Seidel G, Horn U (2013) Simultaneous analysis of the non-canonical amino acids norleucine and norvaline in biopharmaceutical-related fermentation processes by a new ultra-high performance liquid chromatography approach. Amino Acids 44(4):1225–1231. https://doi.org/10.1007/s00726-013-1459-3
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98(4):1222–1227. https://doi.org/10.1104/pp.98.4.1222
Chashmi NA, Sharifi M, Yousefzadi M, Behmanesh M, Rezadoost H, Cardillo A, Palazon J (2013) Analysis of 6-methoxy podophyllotoxin and podophyllotoxin in hairy root cultures of Linum album Kotschy ex Boiss. Med Chem Res 22(2):745–752. https://doi.org/10.1007/s00044-012-0067-1
Chen D, Shao Q, Yin L, Younis A, Zheng B (2018) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci 9:1945. https://doi.org/10.3389/fpls.2018.01945
Darko E, Végh B, Khalil R, Marček T, Szalai G, Pál M, Janda T (2019) Metabolic responses of wheat seedlings to osmotic stress induced by various osmolytes under iso-osmotic conditions. PLoS ONE 14(12):e0226151. https://doi.org/10.1371/journal.pone.0226151
Di Martino C, Delfine S, Pizzuto R, Loreto F, Fuggi A (2003) Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol 158(3):455–463. https://doi.org/10.1046/j.1469-8137.2003.00770.x
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
França S, Roberto P, Marins M, Puga R, Rodrigues A, Pereira J (2001) Biosynthesis of secondary metabolites in sugarcane. Genet Mol Biol 24(1–4):243–250. https://doi.org/10.1590/S1415-47572001000100032
Gerlin L, Baroukh C, Genin S (2021) Polyamines: double agents in disease and plant immunity. Trends Plant Sci 26(10):1061–1071. https://doi.org/10.1016/j.tplants.2021.05.007
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59(2):309–314. https://doi.org/10.1104/pp.59.2.309
Guo D, Chen F, Inoue K, Blount JW, Dixon RA (2001) Downregulation of caffeic acid 3-O-methyltransferase and caffeoyl CoA 3-O-methyltransferase in transgenic alfalfa: impacts on lignin structure and implications for the biosynthesis of G and S lignin. Plant Cell 13(1):73–88. https://doi.org/10.1105/tpc.13.1.73
Hano C, Addi M, Bensaddek L, Crônier D, Baltora-Rosset S, Doussot J, Maury S, Mesnard F, Chabbert B, Hawkins S (2006) Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223(5):975–989. https://doi.org/10.1007/s00425-005-0156-1
Hao G, Ji H, Li Y, Shi R, Wang J, Feng L, Huang L (2012) Exogenous ABA and polyamines enhanced salvianolic acids contents in hairy root cultures of Salvia miltiorrhiza Bge. f. alba. Plant Omics 5(5):446–452. https://doi.org/10.3316/informit.776630483781125
Hazarika P, Rajam MV (2011) Biotic and abiotic stress tolerance in transgenic tomatoes by constitutive expression of S-adenosylmethionine decarboxylase gene. Physiol Mol Biol Plants 17(2):115–128. https://doi.org/10.1007/s12298-011-0053-y
Irato P, Santovito G (2021) Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 10(4):579. https://doi.org/10.3390/antiox10040579
Jahangir MM, Jiang T, Jiang Z, Amjad M, Ying T (2011) Effect of spermine on bioactive components and antioxidant properties of sliced button mushroom (Agaricus bisporus) during storage. Int J Agric Biol 13(5):771–775
Jiao Y, Li Z, Xu K, Al E (2017) Study on improving plantlet development and embryo germination rates in in vitro embryo rescue of seedless grapevine. N Z J Crop Horticult Sci 46:39–53. https://doi.org/10.1080/01140671.2017.1338301
Kaur G, Singh HP, Batish DR, Mahajan P, Kohli RK, Rishi V (2015) Exogenous nitric oxide (NO) interferes with lead (Pb)-induced toxicity by detoxifying reactive oxygen species in hydroponically grown wheat (Triticum aestivum) roots. PLoS ONE 10(9):e0138713. https://doi.org/10.1371/journal.pone.0138713
Khodamoradi S, Sagharyan M, Samari E, Sharifi M (2022) Changes in phenolic compounds production as a defensive mechanism against hydrogen sulfide pollution in Scrophularia striata. Plant Physiol Biochem 177:23–31. https://doi.org/10.1016/j.plaphy.2022.02.013
Kumar N, Goel N (2019) Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep 24:e00370. https://doi.org/10.1016/j.btre.2019.e00370
Lasanajak Y, Minocha R, Minocha SC, Goyal R, Fatima T, Handa AK, Mattoo AK (2014) Enhanced flux of substrates into polyamine biosynthesis but not ethylene in tomato fruit engineered with yeast S-adenosylmethionine decarboxylase gene. Amino Acids 46(3):729–742. https://doi.org/10.1007/s00726-013-1624-8
Liu JH, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci 6:827. https://doi.org/10.3389/fpls.2015.00827
Luo J, Wei B, Han J, Liao Y, Liu Y (2019) Spermidine increases the sucrose content in inferior grain of wheat and thereby promotes its grain filling. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01309
Lv F, Li S, Feng J, Liu P, Gao Z, Yang Y, Xu Y, Wei J (2019) Hydrogen peroxide burst triggers accumulation of jasmonates and salicylic acid inducing sesquiterpene biosynthesis in wounded Aquilaria sinesis. J Plant Physiol 234:167–175. https://doi.org/10.1016/j.jplph.2019.02.006
Majumdar R, Barchi B, Turlapati SA, Gagne M, Minocha R, Long S, Minocha SC (2016) Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: the pathway is regulated at the post-transcriptional level. Front Plant Sci 7:78. https://doi.org/10.3389/fpls.2016.00078
Mandal C, Ghosh N, Dey N, Adak M (2013) Physiological responses of Salvinia natans L. to aluminium stress and its interaction with putrescine. J Stress Physiol Biochem 9(4):163–179
Mishra P, Sohrab S, Mishra SK (2021) A review on the phytochemical and pharmacological properties of Hyptis suaveolens (L.) Poit. Future J Pharm Sci 7(1):1–11. https://doi.org/10.1186/s43094-021-00219-1
Moschou PN, Roubelakis-Angelakis KA (2014) Polyamines and programmed cell death. J Exp Bot 65(5):1285–1296. https://doi.org/10.1093/jxb/ert373
Moschou P, Wu J, Cona A, Tavladoraki P, Angelini R, Roubelakis-Angelakis K (2012) The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J Exp Bot 63(14):5003–5015. https://doi.org/10.1093/jxb/ers202
Mur LA, Simpson C, Kumari A, Gupta AK, Gupta KJ (2017) Moving nitrogen to the centre of plant defence against pathogens. Ann Bot 119(5):703–709. https://doi.org/10.1093/aob/mcw179
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Mustafavi SH, Naghdi Badi H, Sękara A, Mehrafarin A, Janda T, Ghorbanpour M, Rafiee H (2018) Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol Plant 40(6):1–19. https://doi.org/10.1007/s11738-018-2671-2
Orabi SA, Sadak MS (2015) Improvement of productivity and quality of two wheat cultivars by foliar application of spermine and paclobutrazol. Middle East J Agric Res 4(2):195–203
Owen R, Haubner R, Mier W, Giacosa A, Hull W, Spiegelhalder B, Bartsch H (2003) Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes. Food Chem Toxicol 41(5):703–717. https://doi.org/10.1016/s0278-6915(03)00011-5
Pál M, Ivanovska B, Oláh T, Tajti J, Szalai G, Khalil R, Vanková R, Dobrev P, Misheva SP, Janda T (2019) Role of polyamines in plant growth regulation of Rht wheat mutants. Plant Physiol Biochem 137:189–202. https://doi.org/10.1016/j.plaphy.2019.02.013
Palma F, Carvajal F, Jamilena M, Garrido D (2016) Putrescine treatment increases the antioxidant response and carbohydrate content in zucchini fruit stored at low temperature. Postharvest Biol Technol 118:68–70. https://doi.org/10.1016/j.postharvbio.2016.03.009
Pandolfini T, Gabbrielli R, Comparini C (1992) Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L. Plant Cell Environ 15(6):719–725. https://doi.org/10.1111/j.1365-3040.1992.tb01014.x
Radhakrishnan R, Lee IJ (2013) Ameliorative effects of spermine against osmotic stress through antioxidants and abscisic acid changes in soybean pods and seeds. Acta Physiol Plant 35(1):263–269. https://doi.org/10.1007/s11738-012-1072-1
Rajput VD, Singh RK, Verma KK, Sharma L, Quiroz-Figueroa FR, Meena M, Gour VS, Minkina T, Sushkova S, Mandzhieva S (2021) Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biol 10(4):267. https://doi.org/10.3390/biology10040267
Rakesh B, Sudheer W, Nagella P (2021) Role of polyamines in plant tissue culture: an overview. Plant Cell Tissue Organ Cult 145(3):487–506. https://doi.org/10.1007/s11240-021-02029-y
Rojas CM, Senthil-Kumar M, Tzin V, Mysore K (2014) Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front Plant Sci 5:17. https://doi.org/10.3389/fpls.2014.00017
Saleem M, Kim HJ, Ali SM, Lee YS (2005) An update on bioactive plant lignans. Nat Prod Rep 22(6):696–716. https://doi.org/10.1039/b514045p
Samari E, Sharifi M, Ghanati F, Fuss E, Ahmadian Chashmi N (2020) Chitosan-induced phenolics production is mediated by nitrogenous regulatory molecules: NO and PAs in Linum album hairy roots. Plant Cell Tissue Organ Cult 140(3):563–576. https://doi.org/10.1007/s11240-019-01753-w
Samari E, Chashmi NA, Ghanati F, Sajedi RH, Gust AA, Haghdoust F, Sharifi M, Fuss E (2022) Interactions between second messengers, SA and MAPK6 signaling pathways lead to chitosan-induced lignan production in Linum album cell culture. Ind Crops Prod 177:114525. https://doi.org/10.1016/j.indcrop.2022.114525
Santos-Sánchez NF, Salas-Coronado R, Hernández-Carlos B, Villanueva-Cañongo C (2019) Shikimic acid pathway in biosynthesis of phenolic compounds. Plant Physiol Aspects Phenolic Compd 1:1–15. https://doi.org/10.1093/oso/9780199860531.003.0009
Saxena I, Srikanth S, Chen Z (2016) Cross talk between H2O2 and interacting signal molecules under plant stress response. Front Plant Sci 7:570. https://doi.org/10.3389/fpls.2016.00570
Serra A, Macià A, Romero MP, Reguant J, Ortega N, Motilva MJ (2012) Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem 130(2):383–393. https://doi.org/10.1016/j.foodchem.2011.07.055
Shahkarami P, Ahmadian-Chashmi N, Samari E, Safaie N, Sharifi M (2022) Piriformospora indica induces phenylethanoid glycosides production and defense responses in Scrophularia striata cell culture. Plant Cell Tissue Organ Cult 149:381–395. https://doi.org/10.1007/s11240-021-02213-0
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221(3):1197–1214. https://doi.org/10.1111/nph.15488
Stewart RR, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65(2):245–248. https://doi.org/10.1104/pp.65.2.245
Szalai G, Janda K, Darkó É, Janda T, Peeva V, Pál M (2017) Comparative analysis of polyamine metabolism in wheat and maize plants. Plant Physiol Biochem 112:239–250. https://doi.org/10.1016/j.plaphy.2017.01.012
Tahsili J, Sharifi M, Safaie N, Esmaeilzadeh-Bahabadi S, Behmanesh M (2014) Induction of lignans and phenolic compounds in cell culture of Linum album by culture filtrate of Fusarium graminearum. J Plant Interact 9(1):412–417. https://doi.org/10.1080/17429145.2013.846419
Tashackori H, Sharifi M, Ahmadian Chashmi N, Safaie N, Behmanesh M (2016) Induced-differential changes on lignan and phenolic acid compounds in Linum album hairy roots by fungal extract of Piriformospora indica. Plant Cell Tissue Organ Cult 127(1):187–194. https://doi.org/10.1007/s11240-016-1041-2
Tashackori H, Sharifi M, Chashmi NA, Behmanesh M, Safaie N (2018) Piriformospora indica cell wall modulates gene expression and metabolite profile in Linum album hairy roots. Planta 248(5):1289–1306. https://doi.org/10.1007/s00425-018-2973-z
Tashackori H, Sharifi M, Ahmadian Chashmi N, Behmanesh M, Safaie N, Sagharyan M (2021) Physiological, biochemical, and molecular responses of Linum album to digested cell wall of Piriformospora indica. Physiol Mol Biol Plants 27(12):2695–2708. https://doi.org/10.1007/s12298-021-01106-y
Tzin V, Galili G (2010) New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol Plant 3(6):956–972. https://doi.org/10.1093/mp/ssq048
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vilas JM, Romero FM, Rossi FR, Marina M, Maiale SJ, Calzadilla PI, Pieckenstain FL, Ruiz OA, Gárriz A (2018) Modulation of plant and bacterial polyamine metabolism during the compatible interaction between tomato and Pseudomonas syringae. J Plant Physiol 231:281–290. https://doi.org/10.1016/j.jplph.2018.09.014
Walters D (2003) Resistance to plant pathogens: possible roles for free polyamines and polyamine catabolism. New Phyt 159(1):109–115. https://doi.org/10.1046/j.1469-8137.2003.00802.x
Winter G, Todd CD, Trovato M, Forlani G, Funck D (2015) Physiological implications of arginine metabolism in plants. Front Plant Sci 6:534. https://doi.org/10.3389/fpls.2015.00534
Xu L (2015) The effect of polyamineon flower bud differentiation and bud germination of chrysanthemum. Shandong Agric Univ 2:31–36
Xu Y, Jian C, Li K, Tian Y, Zhu K, Zhang W, Wang W, Wang Z, Yang J (2021) The role of polyamines in regulating amino acid biosynthesis in rice grains. Food Energy Secur 10(4):e306. https://doi.org/10.1002/fes3.306
Yang YK, Lee SY, Park WT, Park NI, Park SU (2010) Exogenous auxins and polyamines enhance growth and rosmarinic acid production in hairy root cultures of Nepeta cataria L. Plant Omics 3(6):190–193
Yang B, Wu J, Gao F, Wang J, Su G (2014) Polyamine-induced nitric oxide generation and its potential requirement for peroxide in suspension cells of soybean cotyledon node callus. Plant Physiol Biochem 79:41–47. https://doi.org/10.1016/j.plaphy.2014.02.025
Yoda H, Hiroi Y, Sano H (2006) Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol 142(1):193–206. https://doi.org/10.1104/pp.106.080515
Yousefzadi M, Sharifi M, Chashmi NA, Behmanesh M, Ghasempour A (2010) Optimization of podophyllotoxin extraction method from Linum album cell cultures. Pharm Biol 48(12):1421–1425. https://doi.org/10.3109/13880209.2010.489564
Zafari S, Sharifi M, Chashmi NA, Mur LA (2016) Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. Plant Physiol Biochem 99:11–20. https://doi.org/10.1016/j.plaphy.2015.12.004
Zhang G, Hu Q, Xu S, Gong Y (2013) Polyamines play a positive role in salt tolerant mechanisms by activating antioxidant enzymes in roots of vegetable soybean. Legum Res 36(3):234–240
Zhong M, Song R, Wang Y, Shu S, Sun J, Guo S (2020) TGase regulates salt stress tolerance through enhancing bound polyamines-mediated antioxidant enzymes activity in tomato. Environ Exp Bot 179:104191. https://doi.org/10.1016/j.envexpbot.2020.104191
Acknowledgements
The authors would like to thank Tarbiat Modares University for providing the laboratory facilities and financial support for this project. This research did not receive any specific grant from funding agencies in the public and not-for-profit sectors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to this research paper. MS performed the experiments and analyzed data and interpreted data. MSh designed and advised the study. RF supervised the study. NAC advised the study. ES wrote the final draft and revised it. SV analyzed data and wrote the first draft.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no competing interest.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Christophe Hano .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soltani, M., Samari, E., Vazirifar, S. et al. Putrescine induces lignans biosynthesis through changing the oxidative status and reprogramming amino acids and carbohydrates levels in Linum album hairy roots. Plant Cell Tiss Organ Cult 153, 387–402 (2023). https://doi.org/10.1007/s11240-023-02479-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02479-6