Abstract
Fall cultivation of field crops such as chickpea is prone to the risk of freezing stress. It is required to identify the mechanisms through which plants can tolerate low temperatures and provide conditions for fall cultivation of chickpea in the cold regions. To this, an experiment was carried out to evaluate the physiological, biochemical, and molecular alterations of chickpea genotypes (MCC797; cold-tolerant and MCC505; cold-sensitive) under freezing temperatures (− 3, − 6, − 9, and − 12 °C). Leaf malondialdehyde (MDA), hydrogen peroxide (H2O2), and electrolyte leakage (EL) were increased due to freezing stress in both genotypes, with a greater increase in the cold-sensitive genotype. The plant survival was decreased 20% at − 12 °C in the cold-sensitive genotype, while it remained constant (100%) in the cold-tolerant genotype. The cold-tolerant maximum efficiency of PSII and the PSII operating efficiency recovered faster (24 h after freezing stress; AFS) compare to the cold-sensitive genotype (48 h AFS) during the recovery period. Proline and enzymatic antioxidants activity, including ascorbate peroxidase, catalase (cat), peroxidase (pod), and superoxide dismutase, were increased more rapidly in the cold-tolerant genotype. The relative gene expression of cat, pod, and proline were more stimulated in the cold-tolerant genotype. The cat, pod, and proline were over-expressed on average by 4, 3, and 6 folds, and 16, 13, and 16 folds, in the cold-sensitive and cold-tolerant genotype, respectively, exposed to freezing temperatures. The greater gene expression and the higher antioxidant content of leaves led to lower lipid peroxidation (MDA and H2O2 content) in the cold-tolerant genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Legumes are the main protein source for humans and domestic animals and important agricultural ecosystem members (Calzadilla et al. 2016). Chickpea (Cicer arietinum L.) is the third important legume, after beans (Phaseolus vulgaris L.) and peas (Pisum sativum) in the world (Merga and Haji 2019), with an annual grain production of 17.2 million tons (FAOSTAT 2020). Chickpea cultivation has several advantages; chickpea can grow in marginal lands, fix the atmospheric nitrogen (Biabani et al. 2011), and have appropriate energy, protein, vitamins, minerals, and fiber (Wood and Grusak 2007).
Of the major problems of chickpea cultivation, late-season drought stress in spring cultivation is a severe yield-limiting factor (Gunes et al. 2008). A possible way to increase the chickpea yield is fall cultivation. In the fall cultivation, the plant can better use environmental conditions such as rainfall (Maqbool et al. 2017). Increasing the growing season duration in fall cultivation will also have a positive effect on increasing grain yield. Fall cultivation of chickpea in the Mediterranean region increased grain yield by ~ 70% compared with spring cultivation (Singh et al. 1997). Nevertheless, fall cultivation in areas such as highlands and cold regions is associated with freezing limitations.
Freezing stress is one of the most critical environmental stresses that adversely affect plant growth and survival, especially during the seedling stage (Zhu 2016). Plants respond to freezing stress through various mechanisms (Hu et al. 2017). They adapt to environmental conditions and have different freezing tolerance levels (Eremina et al. 2016). This difference between plants results from broad responses at the level of cell walls and membranes, organelles, and macromolecules, leading to different expression of related genes (De Filippi et al. 2007). Depending on the severity and duration, freezing stress leads to molecular, biochemical, physiological, and morphological responses in the stressed plants (Muzi et al. 2016). Loss of cellular proteins, metabolic disorders, changes in membrane stability and permeability, oxidative stress, and growth retardation are among the adverse effects of freezing stress on plants (Eremina et al. 2016).
Studies have evaluated the impact of chilling or freezing stresses on chickpea plants in the field (Srinivasan et al. 1998, 1999; Heidarvand et al. 2011; Habibpour et al. 2012). However, field screening is associated with such problems as unpredictable severity and irregularity of low-temperature frequency (Maqbool et al. 2010). Therefore, studying under a controlled condition provides more precious information on the freezing stress effects on plants.
One of the important biochemical changes under freezing stress conditions is the production of reactive oxygen species (ROS), e.g., superoxide, hydrogen peroxide (H2O2), and hydroxyl radical. Plant cells cope with these destructive compounds by setting up mechanisms to scavenge ROSs (Erdal et al. 2015). ROS’s scavenging system in plants includes enzymatic antioxidants, such as SOD, CAT, POD, and APX, and non-enzymatic antioxidants like ascorbate and proline (Zouari et al. 2016). Enhancement of enzymatic antioxidant activities has been correlated with an increased cold tolerance in chickpea (Cicer arietinum L.) (Kaur et al. 2009), cucumber (Cucumis sativus) (Han and Lee 2005), rice (Oryza sativa) (Morsy et al. 2007), and maize (Zea mays) (Hodges et al. 1997). Despite playing a role as a non-enzymatic antioxidant, proline increases the cell’s osmotic potential, membrane stability, and intracellular structures. Proline also adjusts the cell’s redox balance and induces its protection against oxidative stress (Liu et al. 2016).
One of the ROS-induced injuries on the plants is the membrane lipid peroxidation, leading to the destruction of the cell membrane and cell contents leakage (Kaur et al. 2008). Hence, electrolyte leakage (EL) measurement is an appropriate index to identify the cold-tolerant genotypes. The chlorophyll fluorescence measurement is one of the other indices to distinguish between cold-tolerant and cold-sensitive genotypes. Zhou et al. (2018) evaluated 127 faba bean genotypes under cold stress. They observed that the maximum quantum efficiency of photosystem II (PSII) decreased under cold stress conditions, but the decrease was higher in cold-sensitive genotypes.
Freezing stress induces over- or down-expression of specific genes. Identifying the involved genes in freezing tolerance through evaluating their expression changes at low temperatures could provide a way to identify the mechanisms involved in freezing tolerance induced in the sensitive plants. Previous studies have evaluated the quantitative changes in some genes' expression in chickpea under freezing stress conditions. For instance, Karami-Moalem et al. (2018) reported that succinate dehydrogenase (CaSDH), alternative oxidase (CaAOX), and cytochrome c oxidase (CaCOX) were over-expressed in the cold-tolerant compared with the cold-sensitive chickpea genotypes. A high transcription level of CaCAT and CaSOD genes was also observed in chickpea under cold stress conditions (Kazemi-Shahandashti et al. 2014). However, studies that examined the expression of genes encoding enzymatic or non-enzymatic antioxidants in chickpea under freezing stress conditions are scarce.
Freezing and chilling stresses threaten chickpea fall cultivation. In cold and highland regions, freezing stress reduces plant survival. Therefore, it is necessary to identify freezing-tolerant mechanisms to achieve freezing-tolerant genotypes and provide conditions for fall cultivation of chickpea in such regions. This study was performed aiming to (i) Study the mechanisms involved in freezing stress tolerance of chickpea plants, (ii) compare cold-sensitive and cold-tolerant genotypes in terms of such mechanisms, and (iii) identify the indices related to freezing tolerance in chickpea plants.
Materials and Methods
Plant Material and Experimental Design
The experiment was performed in the Research center for plant science at the Ferdowsi University of Mashhad, Iran. A factorial experiment (4 × 2) based on a completely randomized design (CRD) with three replications was conducted to evaluate the effect of freezing temperatures (− 3, − 6, − 9, and − 12 °C) on Kabuli chickpea genotypes (MCCFootnote 1505; cold-sensitive and MCC797; cold-tolerant).
Growth Condition
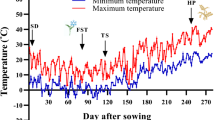
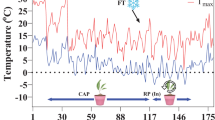
Seeds were first disinfected with 75% ethanol for one minute and then soaked in wet cotton wool in sterile plastic containers for 72 h to germinate. Ten germinated seeds of each genotype were then sown in a depth of 3–4 cm in each plastic pot (25 cm diameter), filled by a combination of field soil (2 mm sieved), leaf mold, and sand (one-third, v:v) in a glasshouse. The plants were grown under natural light at a temperature of 22/16 (± 2) °C (day/night), a photoperiod of 16 h, and relative humidity of 50 ± 5. The plants were kept in the glasshouse conditions up to the two-leaf stage (two weeks), and then cold-acclimated under a natural condition in a cold frame (to preserve the plants from sudden sub-zero temperatures) for four weeks. The outdoor temperature during this period is shown in Fig. 1. The cold frame door was kept open during the day to prevent high temperatures. When the temperature was approached zero °C, the cold frame door was being shut to prevent sub-zero temperatures. Plats were irrigated when needed. The last irrigation was done 24 h before the application of freezing treatments. The pots were then transferred to a thermogradient freezer.
The freezer temperature was 5 °C at the beginning of the experiment and decreased by 2 °C per h, where provides a condition for water redistribution to plant tissues and to prevent intracellular ice formation, which rarely happens naturally (Murray et al. 1988). To prevent ice nuclei formation in the seedlings, the plants were sprayed with an ice-nucleating active bacteria (INAB) suspension at − 2.5 to − 3 °C (Nezami et al. 2012). The pots were kept in each temperature treatment for one hour and then immediately transferred to a room with a temperature of 4 ± 1 °C and stored for 24 h to reduce the melting rate (Nezami et al. 2012). After which, the pots were moved to the glasshouse conditions (as mentioned above) for three weeks to recover.
Traits Measurement
Physiological Traits
Electrolyte Leakage (EL)
24 h after each freezing temperature, sampling for EL measurement was performed from the youngest fully developed leaves. 50 mg of leaf tissue was placed in vials containing 100 ml of double-distilled water and kept at the laboratory temperature for 24 h. Then, the electrical conductivity of the samples was measured with an electrical conductivity meter (Jenway Model 4510) and recorded as EC1. To measure the total amount of EL due to cell death, the samples were placed in an autoclave (120 °C and 1.5 bar) for 20 min and then placed in the laboratory temperature for 24 h; the electrical conductivity was recorded as EC2. The EL percentage was measured using Eq. 1 (Ghoulam et al. 2002).
Survival Percentage
The number of plants after establishment (n) and after the recovery period (m) were counted and survival percentage (S) was calculated using Eq. 2.
Plant Dry Weight (DW)
Plant dry weight was measured (72 h at 75 °C) after the recovery period (3 weeks) and divided by the number of plants per pot.
Chlorophyll Fluorescence
Chlorophyll fluorescence parameters, including the light-adapted maximum efficiency of PSII photochemistry (Fvʹ/Fmʹ) and quantum efficiency of PSII electron transport (Fqʹ/Fmʹ), were measured on the adaxial surface of the youngest fully developed attached leaves by a portable OS1p Chlorophyll Fluorometer (OptiScience, Inc.) at 0, 12, 24, 48 and 72 h after freezing temperatures under actinic light.
Biochemical Traits
24 h after each freezing temperature, the youngest fully developed leaves were sampled for the biochemical and molecular assays. Proline, malondialdehyde (MDA), and H2O2 content were assayed by the methods described by Bates et al. (1973), Heath and Packer (1968), and Sergiev et al. (1997), respectively. The enzymatic antioxidant content, including APX, POD, CAT, and SOD were measured by the methods of Yamaguchi et al. (1995), Srinivas et al. (1999), Velikova et al. (2000), and Yu and Rengel (1999), respectively.
Gene Expression
The relative gene expression of cat, pod, proline, and actin11 (reference gene) was measured in this study. Total RNA was extracted by the Parstous kit (Parstous Biotechnology, Mashhad Iran) using 50 mg fresh weight of leaf tissues. The gel electrophoresis and nanodrop (Thermo Scientific, Nano Drop 2000 c) were used to determine the quality and quantity of extracted RNA. The first standard cDNA synthesis was performed using 5 μl of total RNA, 10 μl of buffer-mix, 2 μl of enzyme-mix, and 3 μl of DEPC-treated water. The cDNA synthesis was performed based on the protocol shown in Table 1 in the BIO-RAD instrument (T100, Thermal Cycler, USA). The real-time PCR was performed in Roche instrument (Lightcycler 96, Germany) using 10 μl of SYBR® GREEN master mix (Bio fact, Korea), 1 μl of the forward primer, 1 µl of reverse primer, 2 μl of cDNA, and 6 µl of DEPC-treated water. The protocol that was used for real-time PCR is shown in Table 2. Relative gene expression was computed using 2−ΔΔCT (Livak and Schmittgen 2001). Primers (Table 3) were designed using NCBI (https://www.ncbi.nlm.nih.gov/) to obtain 18–22 bp length, 59–61 °C melting temperature, and lowest CG content avoiding hairpins and complementarities between primers.
Statistical Analysis
For data analysis, ANOVA (analysis of variance) was performed using Proc GLM in SAS statistical package (v. 9.4). The least significant difference (LSD) was also used to mean comparison (p value ≤ 0.05). Correlation analysis was performed in the R environment (v. 4.0.2) using the corrplot package.
Results
Physiological Traits
Leaf EL
The effect of temperature, genotype, and their interaction was significant on the leaf EL (p ≤ 0.001). Leaf EL showed an increasing trend with decreasing temperature. Generally, leaf EL was greater in the cold-sensitive genotype at all temperatures. Although EL increased with a gentle slope to − 9 °C, it increased sharply to − 12 °C (Fig. 2a). The cold-sensitive genotype (MCC505) EL increased 10, 12, and, 124% at − 6, − 9, and − 12 °C, respectively, compare with − 3 °C, while in cold-tolerant (MCC797) genotype, EL increased by 17, 29 and, 138% at − 6, − 9 and − 12 °C, respectively.
Plant Survival (S)
The plant survival percentage was significantly affected by temperature, genotype, and temperature × genotype (p ≤ 0.001). The survival percentage changes in response to the temperature decline were different among the genotypes. The cold-tolerant genotype plant survival was constant at all temperatures (Fig. 2b). In contrast, while the plant survival of the cold-sensitive genotype remained constant up to − 6 °C, decreasing temperature to − 9 °C slightly decreased the plant survival (6% compared with − 3 °C). However, with decreasing temperature to − 12 °C, the cold-sensitive genotype survival sharply reduced (80% compared with − 3 °C).
Plant Dry Weight (DW)
Freezing temperature, genotype, and their interaction had a significant effect on DW (p ≤ 0.001). In both genotypes, DW showed a decreasing trend with the temperature decline; however, the cold-tolerant DW did not significantly decrease (Fig. 2c). Plant dry weight tended to decrease by 3, 4, and 8% when the temperature decline from − 3 °C to − 6, − 9 and, − 12 °C, respectively, in the cold-tolerant genotype. While the cold-sensitive DW was reduced by 14, 27, and 54% at − 6, − 9, and − 12 °C, respectively, compared with − 3 °C.
Leaf Chlorophyll Fluorescence
The results showed that Fvʹ/Fmʹ of the genotypes was not similarly affected by different measurement times after freezing stress (Fig. 3a, b). Fvʹ/Fmʹ was significantly lower in − 9 and − 12 °C compare with − 3 and − 6 °C at all measurement times except for 72 h. (Fig. 3a, b). Furthermore, at all measurement times, Fvʹ/Fmʹ was higher in the cold-tolerant genotype. Fvʹ/Fmʹ was sharply reduced at − 12 °C in the cold-tolerant genotype; nevertheless, it recovered to the values close to − 3 °C 72 h AFS. Although Fvʹ/Fmʹ was slightly decreased at − 6 and − 9 °C compare to − 3 °C, it fully recovered to − 3 °C values 24 h AFS. The cold-sensitive Fvʹ/Fmʹ was dramatically reduced at − 9 and − 12 °C; however, it recovered to the values of − 3 and − 6 °C 72 h AFS.
Fqʹ/Fmʹ (also known as ΦPSII) trend of changes was almost the same as for Fvʹ/Fmʹ. Generally, Fqʹ/Fmʹ of the cold-sensitive was lower at all measurement times compared with the cold-tolerant genotype (Fig. 3c, d). The cold-tolerant genotype Fqʹ/Fmʹ was severely reduced only at − 12 °C; however, Fqʹ/Fmʹ increased with a sharp slope of 24 h AFS and recovered almost equal to − 9 and − 6 °C values 72 h AFS. Whereas, the cold-sensitive genotype Fqʹ/Fmʹ was significantly diminished at − 9 and − 12 °C; however, Fqʹ/Fmʹ recovered to the values of − 3 and − 6 °C 72 AFS.
Biochemical Traits
Leaf Malondialdehyde Content (MDA)
Temperature, genotype, and temperature × genotype significantly affected the leaf MDA content (p ≤ 0.001). The genotypes leaf MDA did not significantly differ at − 3 and − 6 °C; however, MDA was increased at − 9 °C in the cold-tolerant genotypes (Fig. 4a). A temperature decline from − 3 to − 9 °C significantly increased the leaf MDA content of the cold-tolerant genotype. The cold-tolerant MDA was increased by 10, 50, and 27% at − 6, − 9, and − 12 °C, respectively, compared with − 3 °C. In contrast, the leaf MDA increased by 8% and 1.25 times at − 9 and − 12 °C temperatures, respectively, in the cold-sensitive genotype.
Leaf Hydrogen Peroxide Content (H2O2)
Hydrogen peroxide was significantly affected by temperature, genotype, and their interaction (p ≤ 0.001). The genotypes H2O2 were increased with temperature decline to − 9 °C (Fig. 4b). Decreasing temperature from − 9 to − 12 °C significantly reduced the cold-tolerant genotype H2O2, while the cold-sensitive H2O2 was significantly enhanced at such temperature. The cold-sensitive genotype H2O2 increased 2, 2.1, and 2.4 times at − 6, − 9, and − 12 °C, respectively, compared to − 3 °C; however, it was increased 1.8, 2.3, and 1.3 times at − 6, − 9, and − 12 °C, respectively, in the cold-tolerant genotype.
Leaf Proline Content
Freezing temperature, genotype, and temperature × genotype significantly affected the leaf proline content (p ≤ 0.001). Leaf proline content showed an increasing trend by the temperature decline in both genotypes, with a higher increase in the cold-tolerant genotype. Leaf proline content of the cold-tolerant genotype was increased by 89% at − 6 °C, and 1.7 and 2.4 folds at − 9 and − 12 °C, respectively, compared with − 3 °C. The leaf proline content of the cold-sensitive genotype increased by 20, 32, and 109% at − 6, − 9, and − 12 °C, respectively. (Fig. 4c).
Leaf Enzymatic Antioxidant Content
The results of ANOVA indicated that genotype (p ≤ 0.05), temperature, and their interaction (p ≤ 0.001) had significant effects on APX. Besides, temperature, genotype, and their interaction significantly affected CAT, POD, and SOD (p ≤ 0.001). The temperature decline increased leaf APX content; however, the genotypes showed a different behavior (Fig. 5a). The cold-sensitive APX was higher than the cold-tolerant genotype at − 3 and − 6 °C; while, leaf APX was the same in both genotypes at − 9 °C. However, the cold-tolerant APX was higher than the cold-sensitive genotype at − 12 °C. The cold-sensitive genotype APX increased by 11, 18, and 53% at − 6, − 9, and − 12 °C, respectively, compared with − 3 °C. However, the cold-tolerant genotype APX increased by 17 and 61%, and 1.9 folds at − 6, − 9, and − 12 °C, respectively.
The temperature declines also enhanced leaf CAT content, with a higher increase in the cold-tolerant genotype (Fig. 5b). The cold-sensitive genotype CAT increased by 59%, 1.5, and 3 folds, at − 6, − 9, and − 12 °C, respectively, while leaf CAT increased by 13%, 2.2, and 1.9 folds at − 6, − 9, and − 12 °C, respectively, in the cold-tolerant genotype compare with − 3 °C. Although leaf POD content was higher in the cold-sensitive genotype at − 3 °C, at the other freezing temperatures, POD was higher in the cold-tolerant genotype (Fig. 5c). Compared with − 3 °C, leaf POD content increased in the cold-sensitive genotype by 18, 25, 84%, respectively, at − 6, − 9, and − 12 °C. However, the cold-tolerant genotype POD increased by 1.1, 2.5, and 5.7 folds at − 6, − 9, and − 12 °C, respectively. Declining temperature from − 9 to − 12 °C increased leaf SOD content in the cold-tolerant genotype but decreased the SOD content in the cold-sensitive genotype (Fig. 5d). Leaf SOD content of the cold-tolerant genotype increased 25 and 39%, and 1.18 folds at − 6, − 9, and − 12 °C, respectively; however, the cold-sensitive genotype SOD increased 35 and 45% at − 6 and − 9 °C, respectively, and decreased by 22% at − 12 °C compared with − 3 °C.
Gene Expression
The expression of cat, pod, and proline genes was significantly affected by the temperature, genotype, and their interaction (p ≤ 0.001). The results showed that cat was over-expressed in the cold-tolerant genotype while the temperature declined (Fig. 6). Compared with − 3 °C, cat expression was enhanced by 2.5, 13.7, and 31.5 folds at − 6, − 9, and − 12 °C, respectively. However, the cold-sensitive genotype cat expression was decreased by 10% at − 6 °C and increased by 4.3 and 7.9 folds at − 9 and − 12 °C, respectively. The pod expression was not similarly affected in the two genotypes by declining temperature; the cold-sensitive genotype pod expression was decreased by 8% at − 6 °C; however, it was increased by 2.6 and 7.4 folds at − 9 and − 12 °C, respectively, compared with − 3 °C. The pod expression showed an increasing trend with decreasing temperature in the cold-tolerant genotype; the temperature decline increased the pod expression by 3, 14, and 21.3 folds at − 6, − 9, and − 12 °C, respectively (Fig. 6). The proline was also over-expressed in both genotypes by the temperature decline, with a lower increase in the cold-sensitive genotype (Fig. 6). The proline expression was stimulated by 50% and 4.7 folds, respectively, at − 6 °C in the cold-sensitive and -tolerant genotypes. The proline expression was also enhanced by 7.9 and 15.4 folds, respectively, at − 9 °C in the cold-sensitive and -tolerant genotype. The proline expression enhancement was 9.5 and 27.7 folds in the cold-sensitive and -tolerant genotype, respectively, at − 12 °C.
Discussion
Freezing stress imposes various molecular, biochemical, physiological, and morphological alterations in plants. Depending on their tolerance level, plants may respond differently to freezing temperatures. Different physiological and biochemical strategies, including remodeling of the plastidic and extraplastidic membrane lipid compositions, are used by plants under freezing conditions to prevent freezing-induced cell damage (Welti et al. 2002; Moellering et al. 2010; Chen and Thelen 2013). The results of the present study indicated that an increase in leaf EL due to a decline in temperature was accompanied by a decrease in plant DW in both genotypes; however, the cold-sensitive genotype showed higher sensitivity. On the other hand, the plant survival percentage decreased by the temperature decline only in the cold-sensitive genotype. Previous studies have also reported the adverse effect of cold stress on cell membrane integrity (Bertin et al. 1996; Kaur et al. 2008). Working on Trifolium hirtum also showed that leaf EL increased with declining temperature from − 6 to − 14 °C (Nunes and Smith 2003). In our study, a negative correlation was observed between leaf EL, plant survival, and DW (Fig. 7). These results revealed that an increased leaf EL resulting from the membrane deterioration induced by freezing temperatures led to a decrease in plant survival and DW.
At sub-zero temperatures, ice crystals form in the extracellular space, causing dehydration and plant cell damage (Thomashow 1999; Yamaguchi-Shinozaki and Shinozaki 2006). The plasma membrane has often been proposed as the primary site of freezing damage in plant cells (Steponkus 1984). Membrane fluidity is decreased by freezing stress, which may induce membrane damage and simultaneously, cell water loss and oxidation. As a result of the fluidity losses, the electron transport chain (ETC) is adversely affected, leading to an increased ROS production due to the uncoupling of the ETCs (Taïbi et al. 2018). In response to freezing temperatures, lower levels of lipids phosphatidic acid (PA) and diacylglycerol (DAG) and higher triacylglycerol (TAG) led to the plant tolerance enhancement to freezing stress by increasing the membrane integrity (Tan et al. 2018).
Our results showed that leaf MDA was positively correlated with leaf H2O2 content (Fig. 7). It is well-known that freezing temperatures primarily damage the thylakoid membranes and subsequently diminish the chlorophylls. Arslan et al. (2018) also observed a positive correlation between leaf chlorophyll content with PSII photochemical efficiency and a negative correlation with MDA under freezing temperatures. In the present study, leaf MDA and H2O2 content as indicators of lipid peroxidation were increased in both genotypes from − 3 to − 9 °C. However, the cold-sensitive genotype indicated a sharper increasing slope at temperatures below − 9 °C. Chen et al., (2000) also observed that MDA and H2O2 content were increased in maize (Zea mays L.) leaves due to chilling stress. Accumulation of ROS by low temperatures resulted in lipid peroxidation of the cell membrane, leading to the plant’s physiological dysfunction (Prasad et al. 1994). On the other hand, leaf MDA and H2O2 content were negatively correlated with plant survival and DW (Fig. 7). These findings may approve that a lower leaf MDA and H2O2 content leading to lower cell membrane damage, leaf EL, and ultimately, higher plant survival percentage and DW of the cold-tolerant genotype.
Chlorophyll fluorescence measurement is an appropriate and reproducible tool for evaluating plant performance under low-temperatures (Rizza et al. 2001; Ehlert and Hincha 2008). This method reveals the susceptibility levels of PSII and the damage to the photosynthetic ETC (Maxwell and Johnson 2000) and is a non-destructive diagnostic tool for evaluating the effect of freezing stress on plants and more effective and faster than destructive indices such as the EL (Christen et al. 2007; Su et al. 2015). Freezing stress decreased Fvʹ/Fmʹ and Fqʹ/Fmʹ of the genotypes differently. The cold-tolerant genotype Fvʹ/Fmʹ and Fqʹ/Fmʹ significantly decreased at − 12 °C. While, in the cold-sensitive genotype, those parameters were significantly diminished at − 9 °C. The genotypes were also different regarding the time of recovery onset. The cold-tolerant genotype recovery started earlier (24 h AFS) than the cold-sensitive genotype (48 h AFS). It has been observed that freezing temperatures significantly decreased Fv/Fm of wheat (Triticum aestivum L.) leaves (Si et al. 2017). Hasanfard et al., (2020) also reported that a temperature decline from − 12 °C disrupted the carbon exchange and PSII electron transport. They found that turnipweed (Rapistrum rugosum (L.) All.) Fvʹ/Fmʹ decreased by 28% with a temperature decline from − 12 °C during the first 24 h after compared with before the freezing stress. Taïbi et al. (2018) reported that cold-tolerant Pinus halepensis seedlings showed a higher stomatal conductance, transpiration, net photosynthetic rate, and Fv/Fm. In the present study, it seems that the cold-tolerant chickpea genotype could establish a balance between intercepting and converting light to dry matter during the active photosynthetic period under freezing temperatures.
Leaf proline content and enzymatic antioxidant (APX, CAT, POD, and SOD) were increased in both genotypes exposed to freezing temperatures; however, the increase was greater in the cold-tolerant genotype. A greater proline content led to a decrease in the cell internal water potential (Chen and Li 2002); resulting in a decrease in the freezing point and an increase in the cold resistance. Higher proline levels might be one reason for the greater freezing tolerance in the cold-tolerant genotype. Recent studies showed that proline had a distinct protective function in mitochondria and can enhance oxygen uptake by the alternative oxidase (AOX) through the catabolism cycle (Fang and Beattie 2003; Szabados and Savouré 2010); therefore, mitochondria may avoid ROS generation and supply cell survival (Rurek et al. 2015). Proline also can protect the complex II of the mitochondrial ETC (Hamilton and Heckathorn 2001).
Accumulation of ROS and cell death in plant leaves are triggered by freezing stress (Iba 2002; Chen et al. 2015). Evidence indicates that the plant's tolerance to freezing stress is tightly associated with the intracellular ROS level (Iba 2002); the accumulation of ROS can trigger irreversible oxidative damage and cause cell death (Apel and Hirt 2004). Tan et al. (2018) reported that freezing stress induces the accumulation of salicylic acid (SA), which contributes to freezing-induced ROS production and plant freezing sensitivity. They observed that SA content was higher under freezing temperatures and SA content was associated with the H2O2 content. In a plant cell, the production of antioxidants is activated by oxidative stress imposed by ROS accumulation to maintain cellular redox homeostasis (Foyer and Noctor 2011).
Our results also indicated that the greater enzymatic antioxidants content of the cold-tolerant genotype caused lower lipid peroxidation and ROS production. Hence, the membrane damage and leaf EL were reduced and led to greater plant survival and DW. These findings are consistent with those of Heidarvand and Maali-Amiri (2013) in chickpea (Cicer arietinum L.), Nazari et al., (2012) in black chickpea (Cicer arietinum L.), and Li et al., (2018) in tea (Camellia sinensis L.) plant. APX, CAT, and SOD increasing due to freezing stress in chickpea (Cicer arietinum L.) were previously reported by different studies (Fortunato et al. 2010; Gill and Tuteja 2010; Heidarvand and Maali-Amiri 2013). Studies suggested an association between freezing stress tolerance and an efficient antioxidant system (Kaur et al. 2009). Positive correlations were also observed between leaf SOD content with plant survival and DW (Fig. 7). Shulaev et al. (2008) reported that metabolic homeostasis was disrupted by chilling stress. Under low-temperature conditions, plants adjust different metabolic pathways to acquire stress tolerance, which antioxidant accumulation is one of the most important of those pathways.
The results showed that freezing stress increased cat, pod, and proline gene expression in both genotypes. However, their expressions were more significant in the cold-tolerant genotype. The results also showed a positive correlation between cat, pod, and proline gene expression and leaf CAT, POD, and proline content, respectively (Fig. 7). Few researchers studied plant gene expression alteration induced by freezing stress. For instance, CAT, APX, and GPX (glutathione peroxidase) expression were increased in the ginseng (Panax ginseng) plant under freezing stress (Devi et al. 2012). In chickpea, higher freezing tolerance was correlated with an increased Cu/Zn SOD gene expression (Arslan et al. 2018). Fan et al., (2014) also observed that SOD, POD, and APX genes were dramatically over-expressed in bermudagrass (Cynodon dactylon) under freezing stress conditions. Karami-Moalem et al., (2018) investigated the expression changes of AOX (alternative oxidase), CaCOX (cytochrome c oxidase), and CaSDH (succinate dehydrogenase) in chickpea (Cicer arietinum L.) under low temperatures and found that those genes were more over-expressed in the cold-tolerant genotype. Our results showed that gene expression, enzymatic antioxidant, and proline content were positively in line with each other (Fig. 8). In other words, similar mechanisms of freezing stress alleviation were simultaneously activated under freezing stress conditions in both genotypes. However, those mechanisms were more effective in the cold-tolerant genotype, where the cold-tolerant genotype could cope with freezing stress and remained higher survival and plant DW.
Conclusion
Due to the increasing cultivation area of autumn chickpea in Iran, screening the tolerant genotypes and recognizing the mechanisms upon which plants can tolerate the freezing temperatures, especially in cold regions, is of great importance. Laboratory- and controlled-condition-based studies provide repeatability situations to understand the plant responses to freezing stress. Our results indicated that the freezing stress hampered chickpea plants through ROS production, lipid peroxidation, EL, and ultimately decreasing plant DW and survival percentage. To cope with those damages, chickpea genotypes showed different responses in different levels, such as molecular (gene expression), biochemical (enzymatic and non-enzymatic antioxidant), and physiological (EL and chlorophyll fluorescence) modifications. However, the genotypes responded differently to freezing temperatures. The cold-tolerant genotype had a faster reaction to freezing stress; gene expression, antioxidant enzymes, and proline content increased faster than the cold-sensitive genotype. Lipid peroxidation (MDA and H2O2 content) showed a decreasing trend after the plant adaptation to freezing stress (below − 9 °C) in the cold-tolerant genotype. The cold-tolerant genotype showed a higher relative gene expression and a greater enzymatic and non-enzymatic compounds content than the cold-sensitive genotype. Fvʹ/Fmʹ and Fqʹ/Fmʹ were significantly decreased at − 12 and − 9 °C, respectively, in the cold-tolerant and cold-sensitive genotype. Furthermore, the cold-tolerant genotype Fvʹ/Fmʹ and Fqʹ/Fmʹ also recovered sooner (24 h AFS) than the cold-sensitive genotype (48 h AFS). Hence, the cold-tolerant genotype has advantages over the cold-sensitive genotype for chickpea fall cultivation.
Notes
Mashhad Chickpea Collection.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- EL:
-
Electrolyte leakage
- F v ʹ/F m ʹ :
-
Maximum efficiency of PSII
- F q ʹ/F m ʹ :
-
PSII operating efficiency (ΦPSII)
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- cat :
-
Catalase gene
- pod :
-
Peroxidase gene
- proline :
-
Proline gene
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arslan Ö, Eyidoğan F, Ekmekçi Y (2018) Freezing tolerance of chickpea: biochemical and molecular changes at vegetative stage. Biol Plant 62:140–148
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bertin P, Bouharmont J, Kinet J (1996) Somaclonal variation and improvement in chilling tolerance in rice: changes in chilling-induced electrolyte leakage. Plant Breed 115:268–272
Biabani A, Carpenter-Boggs L, Coyne CJ et al (2011) Nitrogen fixation potential in global chickpea mini-core collection. Biol Fertil Soils 47:679–685. https://doi.org/10.1007/s00374-011-0574-0
Calzadilla PI, Maiale SJ, Ruiz OA, Escaray FJ (2016) Transcriptome response mediated by cold stress in Lotus japonicus. Front Plant Sci 7:374
Chen WP, Li PH (2002) Membrane stabilization by abscisic acid under cold aids proline in alleviating chilling injury in maize (Zea mays L.) cultured cells. Plant Cell Environ 25:955–962
Chen M, Thelen JJ (2013) Acyl-lipid desaturase2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell 25:1430–1444
Chen WP, Li PH, Chen THH (2000) Glycinebetaine increases chilling tolerance and reduces chilling-induced lipid peroxidation in Zea mays L. Plant Cell Environ 23:609–618
Chen Q-F, Xu L, Tan W-J et al (2015) Disruption of the Arabidopsis defense regulator genes SAG101, EDS1, and PAD4 confers enhanced freezing tolerance. Mol Plant 8:1536–1549
Christen D, Schönmann S, Jermini M et al (2007) Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ Exp Bot 60:504–514
De Filippi L, Fournier M, Cameroni E et al (2007) Membrane stress is coupled to a rapid translational control of gene expression in chlorpromazine-treated cells. Curr Genet 52:171–185
Devi BSR, Kim YJ, Selvi SK et al (2012) Influence of potassium nitrate on antioxidant level and secondary metabolite genes under cold stress in panax ginseng. Russ J Plant Physiol 59:318–325
Ehlert B, Hincha DK (2008) Chlorophyll fluorescence imaging accurately quantifies freezing damage and cold acclimation responses in Arabidopsis leaves. Plant Methods 4:12
Erdal S, Genisel M, Turk H et al (2015) Modulation of alternative oxidase to enhance tolerance against cold stress of chickpea by chemical treatments. J Plant Physiol 175:95–101
Eremina M, Rozhon W, Poppenberger B (2016) Hormonal control of cold stress responses in plants. Cell Mol life Sci 73:797–810
Fan J, Ren J, Zhu W et al (2014) Antioxidant responses and gene expression in bermudagrass under cold stress. J Am Soc Hortic Sci 139:699–705
Fang J, Beattie DS (2003) Alternative oxidase present in procyclic Trypanosoma brucei may act to lower the mitochondrial production of superoxide. Arch Biochem Biophys 414:294–302
FAOSTAT (2020) Food and Agriculture Organization Corporate Statistical Database. http://www.fao.org/faostat/en/#data/QC. Accessed Nov 2020
Fortunato AS, Lidon FC, Batista-Santos P et al (2010) Biochemical and molecular characterization of the antioxidative system of Coffea sp. under cold conditions in genotypes with contrasting tolerance. J Plant Physiol 167:333–342
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Ghoulam C, Foursy A, Fares K (2002) Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 47:39–50
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gunes A, Inal A, Adak MS et al (2008) Effect of drought stress implemented at pre- or post-anthesis stage on some physiological parameters as screening criteria in chickpea cultivars. Russ J Plant Physiol 55:59–67. https://doi.org/10.1134/S102144370801007X
Habibpour F, Zeinali H, Amiri RM, Nazari M (2012) Genotypic variability and physio-biochemical characteristics of Iranian black chickpea to cold stress. Rom Agric Res 29:121–130
Hamilton EW, Heckathorn SA (2001) Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiol 126:1266–1274
Han HS, Lee KD (2005) Phosphate and potassium solubilizing bacteria effect on mineral uptake, soil availability and growth of eggplant. Res J Agric Biol Sci 1:176–180
Hasanfard A, Rastgoo M, Darbandi EI et al (2020) Regeneration capacity after exposure to freezing in wild oat (Avena ludoviciana Durieu.) and turnipweed (Rapistrum rugosum (L.) All.) in comparison with winter wheat. Environ Exp Bot 181:104271
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Heidarvand L, Maali-Amiri R (2013) Physio-biochemical and proteome analysis of chickpea in early phases of cold stress. J Plant Physiol 170:459–469
Heidarvand L, Amiri RM, Naghavi MR et al (2011) Physiological and morphological characteristics of chickpea accessions under low temperature stress. Russ J Plant Physiol 58:157–163
Hodges DM, Andrews CJ, Johnson DA, Hamilton RI (1997) Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J Exp Bot 48:1105–1113
Hu Y, Jiang Y, Han X et al (2017) Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot 68:1361–1369
Iba K (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53:225–245
Karami-Moalem S, Maali-Amiri R, Kazemi-Shahandashti S-S (2018) Effect of cold stress on oxidative damage and mitochondrial respiratory properties in chickpea. Plant Physiol Biochem 122:31–39
Kaur G, Kumar S, Nayyar H, Upadhyaya HD (2008) Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): effects on quantitative and qualitative components of seeds. J Agron Crop Sci 194:457–464
Kaur S, Gupta AK, Kaur N et al (2009) Antioxidative enzymes and sucrose synthase contribute to cold stress tolerance in chickpea. J Agron Crop Sci 195:393–397
Kazemi-Shahandashti S-S, Maali-Amiri R, Zeinali H et al (2014) Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J Plant Physiol 171:1106–1116
Li X, Wei J-P, Scott ER et al (2018) Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 23:165
Liu Z, Li L, Luo Z et al (2016) Effect of brassinolide on energy status and proline metabolism in postharvest bamboo shoot during chilling stress. Postharvest Biol Technol 111:240–246
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Maqbool A, Shafiq S, Lake L (2010) Radiant frost tolerance in pulse crops—a review. Euphytica 172:1–12
Maqbool MA, Aslam M, Ali H (2017) Breeding for improved drought tolerance in Chickpea (Cicer arietinum L.). Plant Breed 136:300–318
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Merga B, Haji J (2019) Economic importance of chickpea: production, value, and world trade. Cogent Food Agric 5:1615718. https://doi.org/10.1080/23311932.2019.1615718
Moellering ER, Muthan B, Benning C (2010) Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330:226–228
Morsy MR, Jouve L, Hausman J-F et al (2007) Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J Plant Physiol 164:157–167
Murray GA, Eser D, Gusta LV, Eteve G (1988) Winterhardiness in pea, lentil, faba bean and chickpea. World crops: cool season food legumes. Springer, New York, pp 831–843
Muzi C, Camoni L, Visconti S, Aducci P (2016) Cold stress affects H+-ATPase and phospholipase D activity in Arabidopsis. Plant Physiol Biochem 108:328–336
Nazari M, Amiri RM, Mehraban FH, Khaneghah HZ (2012) Change in antioxidant responses against oxidative damage in black chickpea following cold acclimation. Russ J Plant Physiol 59:183–189
Nezami A, Bandara MS, Gusta LV (2012) An evaluation of freezing tolerance of winter chickpea (Cicer arietinum L.) using controlled freeze tests. Can J Plant Sci 92:155–161
Nunes MES, Smith GR (2003) Electrolyte leakage assay capable of quantifying freezing resistance in rose clover. Crop Sci 43:1349–1357
Prasad TK, Anderson MD, Stewart CR (1994) Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol 105:619–627
Rizza F, Pagani D, Stanca AM, Cattivelli L (2001) Use of chlorophyll fluorescence to evaluate the cold acclimation and freezing tolerance of winter and spring oats. Plant Breed 120:389–396
Rurek M, Woyda-Ploszczyca AM, Jarmuszkiewicz W (2015) Biogenesis of mitochondria in cauliflower (Brassica oleracea var. botrytis) curds subjected to temperature stress and recovery involves regulation of the complexome, respiratory chain activity, organellar translation and ultrastructure. Biochim Biophys Acta 1847:399–417
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51:121–124
Shulaev V, Cortes D, Miller G, Mittler R (2008) Metabolomics for plant stress response. Physiol Plant 132:199–208
Si T, Wang X, Wu L et al (2017) Nitric oxide and hydrogen peroxide mediate wounding-induced freezing tolerance through modifications in photosystem and antioxidant system in wheat. Front Plant Sci 8:1284
Singh KB, Malhotra RS, Saxena MC, Bejiga G (1997) Superiority of winter sowing over traditional spring sowing of chickpea in the Mediterranean region. Agron J 89:112–118
Srinivas ND, Rashmi KR, Raghavarao K (1999) Extraction and purification of a plant peroxidase by aqueous two-phase extraction coupled with gel filtration. Process Biochem 35:43–48
Srinivasan A, Johansen C, Saxena NP (1998) Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): characterization of stress and genetic variation in pod set. F Crop Res 57:181–193
Srinivasan A, Saxena NP, Johansen C (1999) Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): genetic variation in gamete development and function. F Crop Res 60:209–222
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol (USA) 35:543–584
Su L, Dai Z, Li S, Xin H (2015) A novel system for evaluating drought–cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol 15:82
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Taïbi K, Del Campo AD, Vilagrosa A et al (2018) Distinctive physiological and molecular responses to cold stress among cold-tolerant and cold-sensitive Pinus halepensis seed sources. BMC Plant Biol 18:1–11
Tan W-J, Yang Y-C, Zhou Y et al (2018) Diacylglycerol acyltransferase and diacylglycerol kinase modulate triacylglycerol and phosphatidic acid production in the plant response to freezing stress. Plant Physiol 177:1303–1318
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Biol 50:571–599
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Welti R, Li W, Li M et al (2002) Profiling membrane lipids in plant stress responses: role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J Biol Chem 277:31994–32002
Wood JA, Grusak MA (2007) Nutritional value of chickpea. In: Yadav SS, Chen W (eds) Chickpea breeding and management. CABI, England, pp 101–142
Yamaguchi K, Mori H, Nishimura M (1995) A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol 36:1157–1162
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yu Q, Rengel Z (1999) Drought and salinity differentially influence activities of superoxide dismutases in narrow-leafed lupins. Plant Sci 142:1–11
Zhou R, Hyldgaard B, Yu X et al (2018) Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 214:68
Zhu J-K (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324
Zouari M, Ben AC, Elloumi N et al (2016) Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf 128:195–205
Author information
Authors and Affiliations
Contributions
HKS: Software, formal analysis, writing—original draft, sampling, and data collecting. AN: Validation, investigation, resources, and funding. JN: Supervision, conceptualization, methodology, investigation, project administration, review & editing. EO: Methodology, investigation, review & editing. MJA: Sampling and data collecting, writing—review & editing. HKS: Wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Handling Editor: Rhonda Peavy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karimzadeh Soureshjani, H., Nezami, A., Nabati, J. et al. The Physiological, Biochemical, and Molecular Modifications of Chickpea (Cicer arietinum L.) Seedlings Under Freezing Stress. J Plant Growth Regul 41, 1109–1124 (2022). https://doi.org/10.1007/s00344-021-10369-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10369-4