Abstract
Understanding the physiological and biochemical basis of plant tolerance to freezing stress is very critical in in breeding plants tolerant to freezing stress. In this study, consisting of two independent experiments, kabuli type chickpea genotypes (701) were exposed to freezing stress (− 12 °C) at the seedling stage under controlled conditions. Thirty days after the stress, alive seedlings were transferred to the field, and at the end of the growing season, yield and yield-related variables were assessed. In general, 42% of the genotypes survived after the freezing stress, among which 38 had a survival rate above 75%. A significant and positive correlation was observed between survival percentage and maximum photochemical efficiency of photosystem II in the light (Fv′/Fm′), water soluble carbohydrates and ascorbate peroxidase (APX) activity after freezing stress. However, the survival percentage negatively correlated with electrolyte leakage. Principal component analysis showed that in the PC3 survival percentage, Fv′/Fm′ after freezing stress, water soluble carbohydrates after freezing stress, and APX activity after freezing stress had a high negative coefficient, while EL had a high positive coefficient. Stepwise regression analysis revealed that Fv′/Fm′ after freezing stress and APX activity after freezing stress described the most changes in survival percentage. In the field experiment, seed weight had the highest correlation coefficient with biomass and harvest index. In the principal component analysis, the coefficients of the PC1 for seed weight, biomass, and harvest index were − 0.92, − 0.87, and − 0.74, respectively. In conclusion, the chickpea genotypes with high antioxidant potential and water soluble carbohydrates were able to sustain carbon fixation and growth, and yielded well under freezing stress. The same traits may be used in mass screening of chickpea genotypes for tolerance to freezing stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chickpea (Cicer arietinum L.) is a highly nutritious grain legume crop and is an excellent source of protein, carbohydrates, essential fats, and dietary fiber (Grasso et al. 2022). Chickpea is used as a substitute for meat to provide protein to the growing population due to its high protein quality. This plant plays a vital role in biological nitrogen fixation, soil fertility, and animal fodder, which adds to the particular importance of its production (Jukanti et al. 2012). The domesticated chickpea has been divided into two major distinct chickpeas: desi and kabuli. The seeds of kabuli chickpeas are usually large with a beige color seed coat, and aerial plant parts are green and lack anthocyanin pigmentation with white flowers (Upadhyaya et al. 2008).
The freezing stress suppresses plant growth response by altering physiological, biochemical, and morphological processes (Rani et al. 2021). In extreme cases, freezing stress may also lead to complete plant mortality (Rastgoo et al. 2022). Freezing stress causes significant reduction in the carbon influx and assimilation due to decrease in stomatal conduatance in metabolic limitations (Goswami et al. 2022). In this regard, photosystem II is more sensitive to low temperature stress than the other components of the thylakoid membrane (Østrem et al. 2018). However, activation of antioxidant defense system may help cope with the freezing stress-induced oxidative damages. Thus, the evaluation of freezing stress-induced injuries and the mechanisms involved in tolerance to freezing stress is crucial to predicting the survival of plant species in winter and its recovery capacity (Hasanfard et al. 2021).
Autumn cultivation of plants often leads to proper benefits from precipitations, no exposure to heat stress in late spring and early summer, and no interference of sensitive stages of plants with diseases and pests. Despite the advantages of autumn cultivation, unfavorable environmental factors such as freezing stress decrease plants’ winter survival, yield, and yield components (Nabati et al. 2020, 2021a). In this regard, Nezami et al. (2023) reported that autumn planting of some chickpea genotypes in the cold region had lower seed yield compared to other region. These researchers reported the damage caused by cold stress in autumn is the main reason for low chickpea yield in cold regions. However, exposing plants to a gradual decrease in temperature may lead to the regulation of physiological and biochemical processes and protect them from damage during freezing stress (Arslan et al. 2018). In this regard, evaluating the relationship between grain yield and yield-related variables with physiological, biochemical, and morphological attributes to determine promising genotype(s) under freezing stress is one of the critical approaches for selecting freezing-tolerant genotypes (Mir et al. 2021; Boinot et al. 2022).
During the last few decades, research has been carried out on the adaptation and tolerance of plants to freezing stress. Since the primary mechanisms for understanding freezing tolerance are complex, recognition of the physiological and biochemical processes related to the tolerance to freezing stress and the yield of crops requires more comprehensive studies. This study was conducted to identify the key physiological, biochemical, and morphological parameters involved in freezing tolerance in kabuli type chickpea genotypes.
Materials and Methods
Controlled Trial
Trial Setup
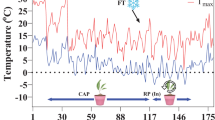
A total of 701 kabuli type chickpea genotypes, of diverse origin and morphology, collected from the Mashhad Chickpea Collection at the Research Center for Plant Science, Mashhad, Iran, were used in this study. Seeds of chickpea genotypes were planted in 6 × 12 seedling trays (23 × 45 × 5 cm3; 6 genotypes per tray), at 3 cm depth, filled with a mixture of sand, soil and peat moss (1:1:1, w/w). during first week of November 2021. The experiment was conducted in a Completely Randomized Design with three replications (12 plants per replication) in an open environment at the Research Center for Plant Science, Ferdowsi University of Mashhad, Iran (Lat 36° 15′ N, Long 59° 28 E; 985 m Altitude). To obtain cold acclimation (CA), the seedling trays were kept outdoors under natural conditions (Fig. 1). No fertilizer was added to the seedlings.
Application of Freezing Stress
After CA duration, chickpea seedlings were exposed to freezing stress at the true four-leaf stage (80 days after sowing). Freezing stress was applied using a thermo-gradient freezer with the ability to adjust the duration and intensity of freezing. The relative humidity inside the freezer was 40–50% with complete darkness. Considering the long-term weather data of the region, the freezer’s temperature decreased at a rate of 2 °C per hour and reached the minimum temperature (− 12 °C) (Hasanfard et al. 2021). The plant samples were treated at − 12 °C for two hours (Fig. 2) and were immediately transferred to a cool chamber at ~ 5 °C for about 12 h to decrease the thawing rate. Afterward, the seedling trays were moved to the natural conditions for four weeks.
Measurement of Attributes (Controlled Trial)
Twenty-four hours after freezing stress, the damage to the cell membrane was measured by leaf electrolyte leakage (EL) using an electrical conductivity meter (Jenway Model 4510, UK), and the EL% was calculated using Eq. 1 (Liu et al. 2013). For this purpose, EL was determined with the two youngest fully developed leaves removed from each sample and placed in the vials containing 50 ml of double-distilled water and kept at the laboratory temperature for 24 h. Then, the initial conductivity (EC1) was measured using a conductivity meter. The samples were autoclaved at 110 °C at a pressure of 1.2 atm for 30 min to destroy the cell membrane. After 24 h, the conductivity of the killed samples (EC2) was determined.
The maximum photochemical efficiency of photosystem (PS) II in the light (Fv′/Fm′) before and six days after freezing stress (AFS) was measured using a pulse-modulated fluorometer (model OS1-FL, Opti-Sciences, Hudson, NH, USA) from 10:00 to 12:00 (Murchie and Lawson 2013). The youngest fully expanded leaves were sampled before and after freezing stress to assay metabolites. Content of photosynthetic pigments (CPPs) (Dere et al. 1998), water soluble carbohydrates content (WSCs) (Dubois et al. 1951), free radical scavenging activity by DPPH (Brand-Williams et al. 1995), total phenol contents (Singleton and Rossi 1965), flavonoids (Chun et al. 2003), and activity of ascorbate peroxidase (APX), peroxidase (POD), and catalase (CAT) (Lee and Lee 2000) were measured by spectrophotometer (Jenway UV-Visible Spectrophotometer Model 6305, UK). The leaf osmotic potential was measured using an osmometer (OM802-D; Vogel, Germany).
After four weeks, the seedlings’ survival percentage (SU%) was calculated using Eq. 2. Accordingly, 294 genotypes survived and were used for evaluation in the study (biochemical and physiological attributes).
Where N1 and N0 are the number of alive seedlings 4 weeks AFS, and the number of alive seedlings before freezing stress (BFS), respectively.
Field Trial
Land Preparation and Planting
The field was prepared by plowing with a reversible plow and breaking and smoothing the soil with a power harrow. The experimental soil was silt loam soil having organic matter 0.64%, nitrogen 0.068%, phosphorus 16.83 mg kg−1, and potassium 112 mg kg−1. On 15 February 2022, seedlings (294 genotypes) at the six-leaf stage were transferred to the research field of Ferdowsi University of Mashhad (35.16°N, 59.38°E). The seedlings were planted on a cloudy day with a temperature above 0 °C (Fig. 2). Planting was done manually in 55 cm spaced rows with a density of 30 plants per m2. Irrigation was applied three times, including immediately after planting, 14 days after the first irrigation, and during flowering through surface irrigation. Weed control was done manually using hoeing in the middle of May 2022.
Measurement of Attributes (Field Trial)
From the planted genotypes, 223 genotypes survived in the field and entered the reproductive phase and seed production, and their characteristics were evaluated. In the middle of July 2022, plant height, 100 seed weight, seed yield (SY) and biological yield (BY) were recorded. The harvest index (HI) was calculated following Eq. 3.
Data Analysis
The analysis of variance (ANOVA) was performed using the SAS 9.4 software (v. 9.4, SAS Institute Inc, Cary, NC, USA). Duncan Multiple Range Test (DMRT) was used to mean comparison (p ≤ 0.05). Cluster analysis (based on Euclidean distance) and principal component analysis (PCA), and distribution of genotypes were performed by Statistica software (v. 8.0, StatSoft Inc., Tulsa, USA). The effect of independent variables on the dependent variable (SU%) was determined through stepwise regression analysis (SRA) using the JMP software (v. 8.0, SAS Institute, USA). The graphs were drawn using GraphPad Prism software (v. 8.00; GraphPad, CA, USA).
Results
Controlled Trial
Correlation Matrix and Principal Component Analysis (PCA)
The controlled experiment’s descriptive statistics and ANOVA results showed a significant difference between the studied traits (Table 1S). Pearson’s correlation results showed that the SU% had a significant and negative correlation (− 0.50**) with the EL%, whereas the SU% was positively correlated with Fv′/Fm′ AFS, WSCs AFS, and APX activity AFS (0.53**, 0.40**, and 0.37**, respectively) (Fig. 3). The EL had a significant and negative correlation with Fv′/Fm′ AFS, carotenoid content AFS, and WSCs AFS (− 0.66**, − 0.16**, − 0.26**, respectively). Further, a significant and positive correlation was recorded between Fv′/Fm′ AFS and WSCs AFS, and APX activity AFS (0.22** and 0.16**, respectively). A significant and strong positive correlation was also observed between the content of total pigment (CTP) AFS and the content of chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoids, and cha/chb AFS (0.98**, 0.79**, 0.86**, and 0.76**, respectively). The WSCs AFS had a significant and positive correlation with the total phenol content AFS, the flavonoid content AFS, and the APX activity AFS (0.16**, 0.14*, and 0.21**, respectively). The results further showed the correlation of total phenol contents AFS with the flavonoid content AFS (0.33**) and APX activity AFS with POD activity AFS (0.13*) was significant and positive.
Pearson correlation coefficient matrix between different biochemical and physiological attributes of chickpea genotypes. BFS: Before freezing stress; AFS: After freezing stress). 1-Survival, 2-Electrolyte leakage, 3-Fv′/Fm′ (BFS), 4-Fv′/Fm′ (AFS), 5-Chlorophyll a (AFS), 6-Chlorophyll b (AFS), 7-Carotenoids (AFS), 8-cha/chb (AFS), 9-Total pigment (AFS), 10-Chlorophyll a (BFS), 11-Chlorophyll b (BFS), 12-Carotenoids (BFS), 13-cha/chb (BFS), 14-Total pigment (BFS), 15-DPPH (AFS), 16-DPPH (BFS), 17-Soluble carbohydrates (AFS), 18-Soluble carbohydrates (BFS), 19-Phenol (AFS), 20-Phenol (BFS), 21-Flavonoids (AFS), 22-Flavonoids (BFS), 23-Ascorbate peroxidase (AFS), 24-Ascorbate peroxidase (BFS), 25-Catalase (AFS), 26-Catalase (BFS), 27-Peroxidase (AFS), 28-Peroxidase (BFS), 29-Osmotic potential (AFS), 30-Osmotic potential (BFS). There were 701 chickpea genotypes; each with three replications and 12 plants per replication
Twelve PCs explained 78.2% of the total variability (Table 2S). The PC1, which explains about 14% of the total changes, had a high negative coefficient for CPPs AFS, and the PC2 that explains 12% of the total changes, had a high positive coefficient with the Chl a, Chl b, carotenoids, CTP BFS (Table 2S and Fig. 4a). The PC3, which explains 8.54% of the total changes, had a high negative coefficient for SU%, Fv′/Fm′ AFS, WSCs AFS, and APX activity AFS. Interestingly, EL% had a high coefficient of positive (Table 2S and Fig. 4b).
Principal component analysis (PCA) related to observations of chickpea genotypes based on the two first components (a) and second and third components (b). B before freezing stress, A after freezing stress, SU survival, EL electrolyte leakage, Chl a chlorophyll a, Chl b chlorophyll b, WSCs water soluble carbohydrates, APX ascorbate peroxidase, CAT catalase, POD peroxidase, OP osmotic potential. There were 701 chickpea genotypes; each with three replications and 12 plants per replication
Cluster Analysis
Of the 294 genotypes surviving AFS (− 12 °C), 38 genotypes with SU higher than 75% were selected for cluster analysis (Fig. 5). The cluster analysis divided chickpea germplasm into four distinct groups (Fig. 1S). The third group with 13 genotypes had the highest, and the fourth group with three genotypes had the lowest number of genotypes. The deviation from the total mean for the attributes was calculated to evaluate the share of each group’s studied trait. The mean of groups was compared using DMRT at 5% (Table 1). Based on this, the genotypes of the second group with six traits, including EL%, Fv′/Fm′ BFS and AFS, WSCs AFS, total phenol content BFS and flavonoid content BFS were more appropriate than the other groups. ANOVA of the groups also showed a significant difference in the values of some traits (Table 2). In this way, the value of variance between groups in the EL%, Fv′/Fm′ BFS and AFS, WSCs AFS, the total phenol content BFS and the flavonoid contents BFS and CAT activity BFS and AFS were 5, 3, 34, 5, 13, 3, 22 and 34 times higher than the value of variance within groups, respectively.
Stepwise Regression Analysis (SRA)
In the SRA, SU% was considered a function variable (Y), and other attributes as independent variables. The results of SRA showed that the seven traits, including EL%, Fv′/Fm′ AFS, WSCs AFS, APX activity BFS and AFS, CAT activity BFS and AFS explained the SU% of chickpea seedlings (Prob>|t|) (Tables 3 and 4). Meanwhile, the more estimated SRA coefficients (t ratio) belonged to Fv′/Fm′ AFS (5.63) and APX activity AFS (4.48) (Table 4).
Field Trial
Correlation Matrix and Principal Component Analysis (PCA)
The field experiment’s descriptive statistics and ANOVA results showed a significant difference between the studied traits (Table 3S). A significant and positive correlation was observed between all studied traits in the field (Fig. 6). Seed weight had a very strong and positive correlation with biomass (0.89**) and HI (0.68**). The PC1 (eigenvalue > 1) explains 56% of the data variance (Table 4S). The studied traits had high and negative coefficients in this component (Table 4S, Fig. 7a). Meanwhile, seed weight, biomass, and HI had coefficients of − 0.92, − 0.87, and − 0.74, respectively. Three genotypes, MCC2003, MCC2009, and MCC2090 (dashed line), had high yield, biomass, and HI potential based on the two-dimensional cartesian coordinate system and their distribution (Fig. 7b).
Cluster Analysis
Cluster analysis of field traits was done using genotypes with more than 75% SU (Fig. 5). Cluster analysis based on the studied attributes in the field grouped chickpea germplasm into four distinct groups (Tables 5 and 6, and Fig. 2S). The fourth group with 17 genotypes had the highest, and the third group with three genotypes had the lowest number of genotypes. The comparison between groups in the studied traits using DMRT showed that the mean genotypes in the groups were significant regarding seed weight, biomass, and HI (Table 5). In the current study, the mean of the genotypes of the third group (MCC2003, MCC2009, MCC2090) in all three mentioned traits was significantly higher than in other groups.
Discussion
Freezing stress affects the growth and yield of plants by disrupting the metabolism as well as altering the stability and integrity of cell membranes. The adaptation of plants to the environment and their capacity to overwinter depend on the biochemical and physiological responses caused by their cold acclimation duration (Nezami et al. 2022). This process, called adaptation, seeks to reprogram intracellular metabolism, leading to enhanced tolerance to freezing stress in plants (Oberschelp et al. 2020). The adaptation process in plant species is different under environmental conditions, which leads to different metabolism contents and, finally, tolerance to different freezing stress. Maintaining the integrity of the cell membrane and the hardening of the leaf cells are crucial factors in the SU of plants under freezing stress. In the current study, a negative correlation was observed between the EL% and the SU% of chickpea germplasm. Hence, the decline in cell turgidity AFS and the increase in EL clearly indicate the function of the cell membrane in protecting chickpea seedlings against the damage of freezing stress. In similar studies, the direct relationship between SU% and EL% indicates that studying damage to plant cell membranes after freezing stress determined the extent of plant damage and freezing tolerance thresholds (Wąsek et al. 2021). Interestingly, the EL% was negatively associated with Fv′/Fm′ AFS, carotenoids AFS, and WSCs AFS. Thus, the destruction of the cell membrane AFS has disturbed the electron transport and photochemical efficiency of PS II. Recent studies have also reported that the electron transport chain from PS II to PS I is blocked a decrease in temperature due to the excessive reduction of the primary electron acceptor of PS II (Zeng et al. 2022; Nabati et al. 2018). This damage to the photosynthetic apparatus has presumably caused ROS accumulation and disturbance in plant metabolism, including the accumulation of WSCs. Supplying adequate carbohydrates to regulate the osmotic pressure and protect the cell membrane effectively would mitigate the damage of freezing stress (Landry et al. 2017). In this study, the high capacity of accumulation of WSCs AFS has led to the reduction of cell shrinkage, preservation of plant cell membrane structure and function, and hence, their high SU.
The SU’s significant and positive correlation with Fv′/Fm′ AFS, WSCs AFS, and APX activity AFS reveals their similar change. In other words, the improvement of the mentioned characteristics AFS was directly influential in the SU% of the studied germplasm.
The results of the PC3 of the trial also confirmed the results of the correlation between traits (Table 2S). Subsequently, the SU%, EL%, Fv′/Fm′ AFS, WSCs AFS, and APX activity AFS explain the variability of this component agreeably. These attributes with the highest weight and the most significant distance from the center were the most critical factors of this component (Fig. 4b). The different strong coefficient of SU% with EL% and its similar strong coefficient with other mentioned traits prove the importance of applying these characteristics in chickpea germplasm breeding and selection programs.
The significant intergroup variance of the EL%, Fv′/Fm′ AFS, WSCs AFS, and CAT activity AFS revealed that the groups’ mean was not the same, which confirms the importance of cluster analysis to determine superior genotypes. The high value of variance between groups for the mentioned attributes compared to the variance within groups (error effects) showed the appropriate explanation of the effect of the mentioned traits in the study and the impact of less error. In the controlled trial, the genotypes of the second group of cluster analysis had the highest number of superior traits (Table 1). Interestingly, the EL%, Fv′/Fm′ AFS, and WSCs AFS were among the traits that were also effective based on Pearson’s correlation coefficient and PCA results.
Without considering interactions between SU% and other traits, simple correlation analysis may mislead plant breeders from achieving their primary purpose. Thus, it is necessary to use multivariate analysis, such as SRA. “Independent” and “dependent” variables are the two fundamental variables in statistical modeling and experimental designs. In SRA, all independent variables are included in the model, and independent variables that do not significantly affect the dependent variable are removed from the model. In this experiment, stepwise multiple regression was employed to determine the role of biochemical and physiological attributes in improving tolerance to freezing stress, increasing speed, and boosting efficiency in selecting a limited number of traits essential for achieving breeding objectives. The changes in the regression model for SU% were explained by traits that had a functional role in the study (based on correlation coefficient and PCA). The activity of APX and CAT among the components influencing the SU% (Table 4) revealed that the activity of the aforementioned antioxidant enzymes had an influential role in the successful overwintering of chickpea seedlings. Through their role in plant defense mechanism, these enzymes scavenge ROS (CAT) or modulate their levels (APX) in the signaling pathway (Sofo et al. 2015). In plants, CAT is found predominantly in peroxisomes and converts hydrogen peroxide (H2O2) into H2O and O2. APX decomposes H2O2 by oxidizing co-substrates such as phenolic compounds and/or antioxidants (Dąbrowska et al. 2007; Willekens et al. 1995). Therefore, the high activity of the enzymes, as mentioned earlier, was recognized as one of the essential mechanisms in the SU of chickpea germplasm.
Changes in seed weight were consistent with other characteristics evaluated in the field. Likewise, seed weight was strongly associated with biomass and HI. In PC1 (eigenvalue = 2.78), all traits had similar factor loadings, which confirms the results of the correlation coefficients (Table 4S). Seed weight, biomass, and HI had the highest coefficients of the PC1 and explained the variability of this component more (Fig. 7a). The higher HI indicates the high capacity of genotypes in allocating photosynthetic materials to seeds (Sandana and Calderini 2019), which is considered one of the superiority factors of the studied germplasm. In other words, genotypes with a higher HI do not have sink limitations and can receive photosynthetic materials in the seed. The significant correlation of seed weight with plant height and 100 seed weight also confirmed that the mentioned traits highly influence crop yield. Accordingly, modifying these traits will ultimately lead to more seed yield. As such, it is necessary to breed freezing-tolerant plants through the mentioned techniques (correlation between traits and PCA) and determine the traits affecting seed yield, especially in the germplasm of species with high genetic diversity (Nabati et al. 2021b; Tiwari et al. 2022). Comparing the mean between groups for cluster analysis revealed that the genotypes of group three (MCC2003, MCC2009, and MCC2090) were at the highest level in all three significant traits, including seed weight, biomass, and HI, compared to other groups. According to the determination of the mentioned traits as influential factors in the field study, it can be concluded that these genotypes had a favorable response to the most crucial traits determined. In addition, based on the PC1, two-dimensional Cartesian coordinate system, and the distribution of genotypes, the three mentioned genotypes with the lowest negative factor loadings had high seed yield, biomass, and HI (Fig. 7).
High SU% is the most important factor in screening genotypes tolerant to freezing stress. Thus, it is necessary to select genotypes with a high SU% and breed them based on traits that affect winter SU. In this experiment, genotypes with SU > 75% were selected to determine the characteristics affecting chickpea yield in the field. Overall, MCC2003 and MCC2090, with the appropriate metabolic response, high potential in tolerance to freezing stress, and high grain yield, biomass, and HI, were considered promising genotypes.
Conclusions
The tested chickpea genotypes varied for their responses to freezing stress. The chickpea genotypes with high antioxidant potential and water soluble carbohydrates were able to survive, sustain growth, and produce fair grains under freezing stress. The same traits may be used in mass screening of chickpea genotypes for tolerance to freezing stress.
References
Arslan O, Eyidoğan F, Ekmekci Y (2018) Freezing tolerance of chickpea: biochemical and molecular changes at vegetative stage. Biol Plant 62(1):140–148. https://doi.org/10.1007/s10535-017-0760-5
Boinot M, Karakas E, Koehl K, Pagter M, Zuther E (2022) Cold stress and freezing tolerance negatively affect the fitness of Arabidopsis thaliana accessions under field and controlled conditions. Planta 255(2):1–18. https://doi.org/10.1007/s00425-021-03809-8
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Chun OK, Kim DO, Lee CY (2003) Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem 51(27):8067–8072. https://doi.org/10.1021/jf034740d
Dąbrowska G, Kata A, Goc A, Szechyńska-Hebda M, Skrzypek E (2007) Characteristics of the plant ascorbate peroxidase family. Acta Biol Crac Ser Bot 49(1):7–17
Dere S, Gunes T, Sivaci R (1998) Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 22(1):13–18
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith FAJN (1951) A colorimetric method for the determination of sugars. Nature 168(4265):167–167. https://doi.org/10.1038/168167a0
Goswami AK, Maurya NK, Goswami S, Bardhan K, Singh SK, Prakash J, Pradhan S, Kumar A, Chinnusamy V, Kumar P, Sharma RM, Sharma S, Bisht DS, Kumar C (2022) Physio-biochemical and molecular stress regulators and their crosstalk for low-temperature stress responses in fruit crops: a review. Front Plant Sci. https://doi.org/10.3389/fpls.2022.1022167
Grasso N, Lynch NL, Arendt EK, O’Mahony JA (2022) Chickpea protein ingredients: a review of composition, functionality, and applications. Compr Rev Food Sci Food Saf 21(1):435–452. https://doi.org/10.1111/1541-4337.12878
Hasanfard A, Rastgoo M, Darbandi EI, Nezami A, Chauhan BS (2021) Regeneration capacity after exposure to freezing in wild oat (Avena ludoviciana Durieu.) and turnipweed (Rapistrum rugosum (L.) all.) In comparison with winter wheat. Environ Exp Bot 181:104271. https://doi.org/10.1016/j.envexpbot.2020.104271
Jukanti AK, Gaur PM, Gowda CLL, Chibbar RN (2012) Nutritional quality and health benefits of chickpea (Cicer arietinum L.): a review. Br J Nutr 108(1):S11–S26. https://doi.org/10.1017/S0007114512000797
Landry EJ, Fuchs SJ, Bradley VL, Johnson RC (2017) The effect of cold acclimation on the low molecular weight carbohydrate composition of safflower. Heliyon 3(9):e00402. https://doi.org/10.1016/j.heliyon.2017.e00402
Lee DH, Lee CB (2000) Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci 159(1):75–85. https://doi.org/10.1016/S0168-9452(00)00326-5
Liu W, Yu K, He T, Li F, Zhang D, Liu J (2013) The low temperature induced physiological responses of Avena nuda L., a cold-tolerant plant species. Sci World J. https://doi.org/10.1155/2013/658793
Mir AH, Bhat MA, Dar SA, Sofi PA, Bhat NA, Mir RR (2021) Assessment of cold tolerance in chickpea (Cicer spp.) grown under cold/freezing weather conditions of North-Western Himalayas of Jammu and Kashmir, India. Physiol Mol Biol 27(5):1105–1118. https://doi.org/10.1007/s12298-021-00997-1
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64(13):3983–3998. https://doi.org/10.1093/jxb/ert208
Nabati J, Nezami A, Hasanfard A, Haghighat Sheshvan Z (2018) The trend of changes in chlorophyll fluorescence parameters in two Vicia faba ecotype during freezing stresses. Iran J Pulses Res 9(2):139–150. https://doi.org/10.22067/ijpr.v9i2.59524
Nabati J, Nezami A, Mirmiran SM, Hasanfard A, Hojjat SS, Bagheri AR (2020) Freezing tolerance in some lentil genotypes under controlled conditions. Seed Plant J 36(2):183–205. https://doi.org/10.22092/SPPI.2020.123186
Nabati J, Hasanfard A, Nezami A, Ahmadi-Lahijani MJ, Boroumand Rezazadeh E (2021a) Gas exchange variables as promising criteria for screening freezing‐tolerant faba bean (Vicia faba L.) landraces at early growth stages. Legum Sci 3(1):e72. https://doi.org/10.1002/leg3.72
Nabati J, Nezami A, Hasanfard A, Zare Mehrjerdi M, Rastgoo M (2021b) Selection of lentil (Lens culinaris medik.) Genotypes by assessing phenological, morphological, yield and yield attributes. Iran Agri Res 40(1):51–60
Nezami A, Nabati J, Mirmiran SM, Hasanfard A, Mohammadi M (2022) How does the freezing stress in the seedling stage affect the chickpea’s morpho-physiological and biochemical attributes? Gesunde Pflanz. https://doi.org/10.1007/s10343-022-00771-7
Nezami A, Mahmoodi AA, Nabati J, Mohammadi M, Hasanfard A (2023) Feasibility study of cultivating desi-type chickpea genotypes in cold and temperate regions. Legume Sci e179. https://doi.org/10.1002/leg3.179
Oberschelp GPJ, Guarnaschelli AB, Teson N, Harrand L, Podesta FE, Margarit E (2020) Cold acclimation and freezing tolerance in three Eucalyptus species: a metabolomic and proteomic approach. Plant Physiol Biochem 154:316–327. https://doi.org/10.1016/j.plaphy.2020.05.026
Østrem L, Folkestad J, Solhaug KA, Brandsæter LO (2018) Frost tolerance, regeneration capacity after frost exposure and high photosystem II efficiency during winter and early spring support high winter survival in Juncus spp. Weed Res 58(1):25–34. https://doi.org/10.1111/wre.12277
Rani A, Kiran A, Sharma KD, Prasad PV, Jha UC, Siddique KH, Nayyar H (2021) Cold tolerance during the reproductive phase in chickpea (Cicer arietinum L.) s associated with superior cold acclimation ability involving antioxidants and cryoprotective solutes in anthers and ovules. Antioxidants 10(11):1693. https://doi.org/10.3390/antiox10111693
Rastgoo M, Nezami A, Hasanfard A, Nabati J, Ahmadi-Lahijani MJ (2022) Freezing stress induces changes in the morphophysiological of chickpea and wild mustard seedlings. Legum Sci. https://doi.org/10.1002/leg3.173
Sandana P, Calderini DF (2019) Source-sink relationships in cereals and legumes. Crop Sci. https://doi.org/10.1007/978-1-4939-8621-7_1037
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16(6):13561–13578. https://doi.org/10.3390/ijms160613561
Tiwari S, Singh Y, Upadhyay PK, Koutu GK (2022) Principal component analysis and genetic divergence studies for yield and quality-related attributes of rice restorer lines. Indian J Genet Plant Breed 82(01):94–98. https://doi.org/10.31742/IJGPB.82.1.13
Upadhyaya HD, Dwivedi SL, Baum M, Varshney RK, Udupa SM, Gowda CL, Hoisington D, Singh S (2008) Genetic structure, diversity, and allelic richness in composite collection and reference set in chickpea (Cicer arietinum L.). BMC Plant Biol 8(1):1–12. https://doi.org/10.1186/1471-2229-8-106
Wąsek I, Dyda M, Gołębiowska G, Tyrka M, Rapacz M, Szechyńska-Hebda M, Wędzony M (2021) Quantitative trait loci and candidate genes associated with freezing tolerance of winter triticale (× Triticosecale Wittmack). J Appl Genet. https://doi.org/10.1007/s13353-021-00660-1
Willekens H, Inze D, Van Montagu M, Van Camp W (1995) Catalases in plants. Mol Breed 1(3):207–228. https://doi.org/10.1007/BF02277422
Zeng JJ, Hu WH, Hu XH, Tao HM, Zhong L, Liu LL (2022) Upregulation of the mitochondrial alternative oxidase pathway improves PSII function and photosynthetic electron transport in tomato seedlings under chilling stress. Photosynthetica 60(2):271–279. https://doi.org/10.32615/ps.2022.019
Acknowledgements
We are grateful to Ferdowsi University of Mashhad, Iran, for providing financial support for the present research.
Author information
Authors and Affiliations
Contributions
AH: Conceptualization, Methodology, Laboratory work, Data collection, Data analysis, Writing. JN: Conceptualization, Methodology, Supervision, Reviewing. AN: Supervision, Reviewing. MF: Conceptualization, Supervision, Reviewing, Editing.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Handling Editor: Saddam Hussain.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasanfard, A., Nabati, J., Nezami, A. et al. Antioxidant Potential and Osmotic Adjustment Modulate Growth and Yield Formation in Kabuli-Type Chickpea Genotypes Under Freezing Stress. J Plant Growth Regul 42, 7649–7659 (2023). https://doi.org/10.1007/s00344-023-11040-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11040-w