Abstract
Autumn cultivation of desi-type chickpea in cold regions requires the introduction of cultivars tolerant to freezing stress. Hence, this study aimed to investigate the physiological response of desi-type chickpea germplasm during cold acclimation and its relationship with freezing tolerance (−12 °C) under controlled conditions. A genotype with a higher potential for accumulation of anthocyanins, proline, and DPPH (free radical scavenging activity) in cold acclimation duration was identified as a promising genotype with lower electrolyte leakage and 100% survival. In the germplasm, 81% of the genotypes kept 100% survival. No significant correlation was observed between the content of metabolites in cold acclimation duration and the survival percentage of genotypes after freezing stress. While the correlation between survival percentage and electrolyte leakage was negative and significant, except for one genotype, the other genotypes with the lowest EL had 100% survival. Principal component analysis indicated the first component screened genotypes with higher content of photosynthetic pigments and the second component screened genotypes with more survival and cell membrane stability after freezing stress. Morphological parameters explain the freezing tolerance better than metabolites. Based on the results of this experiment, it is recommended to evaluate the content of photosynthetic pigments before freezing stress and assess the stability of cell membranes after freezing stress to measure the tolerance to freezing stress in genetic engineering and breeding programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chickpea (Cicer arietinum L.) is one of the most important crops in the human food basket worldwide. It is a valuable source of protein, fat, fiber, and carbohydrate (Rachwa-Rosiak et al. 2015). As the human population grows, the demand for this protein source increases, and various approaches to its sustainable products are being developed (Grasso et al. 2022). Among pulses, chickpea is ranked second in the area under cultivation (15.3% of the total) and third in production (15.4%) (FAOSTAT 2018). In 2020, world production of chickpea reached 15 MT, among where Asian countries were ranked first with 86% of total world production (FAOSTAT 2020). The production of 78% of this crop by Low-Income Food-Deficit Countries (LIFDCs) (FAOSTAT 2020) indicates the unique role of this product as an alternative source of animal protein. Iran is also one of the largest producers of this crop, producing about 2% of the world’s chickpea (FAOSTAT 2020).
Compared to the Kabuli type chickpea, the desi-type has tiny, angular, dark-colored seeds that grow mainly in Asia and Africa (Rachwa-Rosiak et al. 2015). Desi-type is mainly processed and consumed as a split pea. Despite the extraordinary role of split pea in the diet of many countries, including Iran, the challenge of water scarcity has led to insufficient production of this plant, especially in arid and semi-arid regions. On the other hand, autumn cultivation of this crop to benefit from autumn and winter precipitations is impossible due to the lack of introduction of cold-tolerant cultivars (Karimzadeh Soureshjani et al. 2022). In addition, studies have found that autumn cultivation, due to escaping from the heat and drought stresses in late spring and early summer, increased the vegetative growth period. Hence, the production of more photosynthetic products, the yield and yield stability of this crop is greater than in spring cultivation (Hu et al. 2006).
The capacity of plant species to tolerate freezing stress under natural conditions varies. Thus, assessing the damage caused by freezing stress is particularly necessary to predict the survival of the plant species in winter and its regrowth in the spring of the following year (Hasanfard et al. 2021). This evaluation also effectively identifies cold-tolerant species plus cultivars and develops management strategies to improve plant yield under freezing stress. The adverse effects of physiological, cellular, metabolic, and molecular processes may be so severe that plant death occurs at low temperatures (Nabati et al. 2021). There are inherently physical, morphological, and molecular limitations to chickpea’s ability to respond to freezing stress. Studies indicated that sometimes freezing stress may not kill chickpea seedlings, although it affects plant regrowth during recovery and seed yield (Bhattacharya 2022). However, gradual rises in stress may regulate physiological and biochemical processes and protect plants from damage in the event of a sudden onset of freezing stress (Arslan et al. 2018); Thus, evaluating the mentioned parameters before freezing stress (BFS) and examining their relationship with plant survival after freezing stress (AFS) is one of the essential strategies for selecting tolerant genotypes in plants (Mir et al. 2021; Nabati et al. 2021). The present experiment was conducted to investigate the relationship between the response of germplasm metabolites of desi-type chickpeas during cold acclimation (CA) and its tolerance to freezing stress under controlled conditions.
Materials and Methods
Plant Material and Growing Conditions
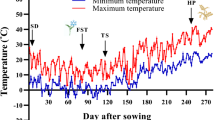
An experiment based on a Completely Randomized Design (CRD) with three replications was conducted in 2019 to evaluate the effect of freezing temperatures on desi-type chickpea genotypes. The experiment was performed in the Research Center for Plant Science (RCPS) at the Ferdowsi University of Mashhad, Iran (Lat 36° 15′ N, Long 59° 28 E; 985 m Altitude). Experimental factors included 31 selected genotypes of desi-type, one cold-tolerant cultivar (cv. Saral) (Table 1), and three temperature treatments, including 0, −12, and −15 °C. Seeds were provided from Mashhad chickpea collection RCPS. Plastic pots (13 cm high, 14 cm diameter) were filled with sand, soil, and peat moss (1:1:1, v/v), and seeds were planted at 4–5 cm depth below the soil surface. To obtain CA, the pots were kept outdoors and grown in natural conditions (Fig. 1). During the growing season, all seedlings were irrigated daily with potable water.
Measurement of Traits After Cold Acclimation
After the CA duration and BFS, the youngest fully expanded leaves were harvested for the biochemical assays. Content of photosynthetic pigments (CPPs) (Dere et al. 1998), anthocyanins (Wagner 1979), water soluble carbohydrates (WSCs) (Dubois et al. 1951), Proline (Bates et al. 1973), free radical scavenging activity by DPPH (Brand-Williams et al. 1995), total phenol content (Singleton and Rossi 1965) were measured by spectrophotometer (Jenway UV-Visible Spectrophotometer Model 6305, UK). The leaf relative water content (RWC) was also calculated using Eq. 1 (Ritchie et al. 1990).
Where FW is the fresh weight, TW is the turgid weight measured after 24 h of saturation on deionized water at 4 °C in the dark, and DW is the dry weight determined after 48 h in an oven at 70 °C.
Freezing Treatment
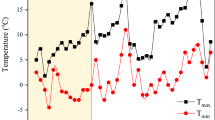
Chickpea seedlings were exposed to freezing treatment after CA duration at the true six-leaf stage. Freezing treatment was performed using a thermogradient freezer. The initial temperature of the thermogradient freezer was +5 °C; however, the freezer was cooled down in a stepwise trend at a rate of 2 °C per hour. The plant samples were kept at the intended temperatures for one h (Fig. 2), and instantly after the freezing treatment, the samples were transferred to the growth chamber at ~ 5 °C for about 24 h to decrease the thawing rate.
Measurement of Traits After Freezing Stress
After applying stress, freezing stress damage to the cell membrane was measured by electrolyte leakage (EL) using an electrical conductivity meter (Jenway Model 4510, UK). The percentage of EL was calculated using Eq. 2 (Hasanfard et al. 2021; Waalen et al. 2011).
Where EC1 is the initial conductivity, EC2 is the conductivity of the killed samples.
After four weeks of the freezing treatment, survival percentage (Eq. 3), plant height and dry weight (DW) were evaluated. Since the plant samples survived completely at 0 °C and were almost destroyed at −15 °C due to the intensity of the freezing stress, the results of these traits were found at −12 °C (the minimum average temperature at the examination location) was reported.
Where n1 and n2 are the number of alive plants four weeks after freezing treatment, and the number of alive plants before freezing treatment, respectively.
Statistical Analysis
Data were analyzed using the SAS 9.4 software (v. 9.4, SAS Institute Inc, Cary, NC, USA) and their mean comparison was performed by Duncan Multiple Range Test (DMRT) at a 5% probability level. Cluster analysis (based on Euclidean distance) and principal component analysis (PCA) were also performed by Statistica software (v. 8.0, StatSoft Inc., Tulsa, USA).
Results
Physiological Traits
Content of Photosynthetic Pigments (CPPs)
MCC32 and MCC913 had the most elevated CPPs (chlorophyll a, chlorophyll b, and carotenoids) (Fig. 3). However, the Saral (control cultivar) had only more chlorophyll a among the mentioned photosynthetic pigments. In 22% of genotypes (seven genotypes), the chlorophyll a/b ratio was higher than in other genotypes (Fig. 3). The highest total pigments were MCC32, MCC913, MCC914, and Saral cultivar with 3.11 to 3.57 mg gfw−1.
Anthocyanin
The anthocyanin content in the tested genotypes varied and ranged from 0.16 to 0.65 mmol gfw−1 (Table 2). MCC40 with 0.65 mmol gfw−1 had higher anthocyanin content compared to other genotypes. So the anthocyanin content in this genotype was 2.1 times higher than the total average. Also, the anthocyanins content in the Saral cultivar was 51% lower than the mentioned genotype.
Water Soluble Carbohydrates (WSCs)
MCC259 and MCC291 with 89 and 76 mg gfw−1 had more WSCs than other genotypes (Table 2). Therefore, the average content of WSCs in these two genotypes was 2.3 times higher than the total average in the genotypes.
Proline
Maximum proline content was observed in MCC100 and MCC40, with 8.1 and 6.9 mg.gfw−1, respectively (Table 2). The mean proline content of these two genotypes was 2.6 times higher than the total mean of the genotypes. Proline content in the Saral cultivar was 68% lower than the average of the mentioned genotypes.
Free Radical Scavenging Activity by DPPH
The DPPH of MCC755, MCC914, MCC212, MCC885, and MCC40 varied from 2.65 to 3.34 mg gfw−1 and was higher than other genotypes (Table 2). The mean of DPPH in these genotypes was 1.8 times higher than the average of the genotypes. Free radical scavenging activity by DPPH was at its lowest level in the Saral cultivar. Its DPPH was 81% lower than the average in the mentioned genotypes.
Phenol
According to Table 2, six genotypes and the Saral cultivar had higher phenol content than other genotypes. The range of phenol in the mentioned germplasm varied from 360 to 423 mg.gfw−1, and the mean of these genotypes was 1.2 times higher than the total mean.
Leaf Relative Water Content (RWC)
The RWC in the studied germplasm varied from 63 to 84% (Table 2). MCC885, MCC751, MCC29, MCC613, and the Saral cultivar with 82 to 84% had higher RWC than other genotypes (Table 2). Hence, their average RWC was 1.1 times higher than the total average.
Electrolyte Leakage (EL)
Exposure of chickpea seedlings to −12 °C led to EL in 43 to 78% (Fig. 4). MCC600, MCC603, MCC40, MCC901, MCC29, MCC884, and the Saral cultivars with a range of 43 to 51% had less EL than other genotypes. Simply put, the average leaf cell membrane stability in these genotypes was 20% higher than the total average.
Survival Percentage
Survival of 26 genotypes AFS (−12 °C) was more than 95% (Fig. 4). Among alive seedlings, MCC884 had a lower survival percentage (33%) than others. Also, MCC199 was the only genotype completely killed by exposure to −12 °C.
Plant Height
In this experiment, 24 genotypes (75%) with a height of 10.3 to 15.3 cm were placed in the same statistical group and had the highest height (Table 2). Among alive seedlings, MCC884 had the lowest height (4.33 cm). The height of the Saral cultivar was 44% lower than the total average.
Plant Dry Weight (DW)
The DW of 24 genotypes ranged from 143 to 250 mg plant−1. These genotypes were placed in the same statistical group (Table 2). MCC884 with 43 mg plant−1 had the lowest DW. Also, the DW of the Saral cultivar was 49% lower than the total average, and this cultivar was among the genotypes with the lowest plant DW.
Correlation Matrix and Principal Component Analysis (PCA)
Based on the results of the Pearson’s correlation coefficient, a significant positive correlation was observed between the content of chlorophyll a with chlorophyll b (r2 = 0.74**) and the total pigment with the content of chlorophyll a, chlorophyll b, and carotenoids (r2 = 0.95**, r2 = 0.87**, and r2 = 0.73**) (Fig. 5a). Also, a significant positive correlation between plant height with survival and dry weight (r2 = 0.71** and r2 = 0.79**) showed that there is a high relationship between these parameters (Fig. 5a). The five main components accounted for 78.01% of the data diversity (Table 3). The first component, which explains 27.15% of the total changes, had a high positive coefficient for photosynthetic pigments (Fig. 5b). The second component with 20.78% of the total changes had a high positive coefficient for survival, plant height, and DW and a high negative coefficient for EL. Consequently, the top 10 genotypes (dashed line) with high tolerance capacity were identified based on the two-dimensional cartesian coordinate system and how they are distributed (Fig. 5c).
Pearson correlation coefficient matrix between biochemical, physiological, and morphological characteristics of chickpea genotypes (a), principal component analysis (PCA) related to observations (b), and distribution of chickpea genotypes based on the two first components (c). Car Carotenoids, TP Total pigment, Ant Anthocyanin, SC Soluble carbohydrates, Pro Proline, Phe Phenol, H Height, DW Dry weight, EL Electrolyte leakage
Cluster Analysis and Canonical Correlation Analysis (CCA)
Cluster analysis based on the studied traits grouped chickpea germplasm into seven distinct groups (Fig. 6 and Table 4). The second group with nine genotypes included the highest, and the groups of three, five, six, and seven with three genotypes had the lowest number of genotypes. The deviation from the total mean for the traits was calculated to evaluate the share of each group’s experimented trait (Table 4). Hence, the average survival percentage of groups four and seven were lower than the total mean. Except for groups four and six, the other groups had a higher group average in eight traits than the total average. Also, according to Table 4, the Saral cultivar and six genotypes were in group four. This group had a higher mean in only four traits than the total mean. Analysis of variance of the groups also showed a significant difference between the groups in terms of soluble carbohydrate and phenol in the CA duration and survival, height, and DW after freezing stress (Table 5). In other words, between group variation in each of the mentioned traits is 3, 25, 7.4, 9.8, and 24 times higher than within group variation, respectively. Multivariate Analysis of Variance (MANOVA) based on an unbalanced completely randomized design to confirm the differences between the groups showed that the Wilks’ lambda test in the first and second functions was significant, with 0.001 and 0.009, respectively (p ≤ 0.01).
In canonical discriminate analysis, it was found that the first two canonical variables were significant and had canonical correlations of 0.967 and 0.944, respectively (Table 6). In the first canonical discriminate equation, the highest standard canonical coefficients belonged to chlorophyll b, chlorophyll a/b, and phenol. The highest coefficients in the second canonical discriminate equation were chlorophyll a and DW (Fig. 7). Also, in the study of grouping genotypes based on Mahalanobis distances (the distance between two points in multivariate space), the maximum distance between groups five to seven and the lowest between groups one to two was observed (Fig. 8).
Canonical function coefficients of trait measured in chickpea genotypes. *Most significant absolute correlation between each variable and any discriminant function. Variables failing tolerance test: Minimum tolerance level is 0.001. Tolerance level for total pigment ≤ 0.001. Car Carotenoids, Ant Anthocyanin,SC Soluble carbohydrates, Pro Proline, Phe Phenol, H Height, DW Dry weight, EL Electrolyte leakage
Discussion
The adaptation process in the germplasm of plant species is different under environmental conditions, leading to different plant metabolism and ultimately different tolerance to freezing stresses. However, no significant correlation was observed between photosynthetic pigments and survival percentage in the present experiment, Meanwhile, 67% of the genotypes with lower survival (66.7% ≥ survival) had the lowest total concentration of photosynthetic pigments. The CPPs determine leaf photosynthetic capacity (Fürtauer et al. 2019). Thus, genotypes with higher CPPs are expected to have better conditions for receiving light, electron transfer in photosystems, and ultimately overwintering.
Anthocyanins are colored water-soluble pigments belonging to the flavonoid group that plays a crucial role in plant protection mechanisms. The plant response after freezing stress is the generation of reactive oxygen species (ROSs), which are utilized as signaling molecules to activate the stress tolerance mechanism. Higher ROS levels are generated during severe stress, causing oxidative damage to the plant (Naing and Kim 2021). Under such conditions, the biosynthesis of anthocyanins increases, leading to the plant’s stability with its antioxidant activity and elimination of excess ROS. The MCC40 had more significant potential for anthocyanin biosynthesis during the CA duration in this experiment. Hence, one of the probable causes for its 100% survival after freezing stress is its defense mechanism via antioxidant activities.
Accumulating WSCs in plants act as cellular protective compounds that can prevent or restrict the formation of ice crystals (Morin et al. 2007). In this experiment, MCC259 and MCC291 had the highest concentrations of WSCs, which were placed in group five based on cluster analysis results. The genotypes of this group included the highest and lowest deviations from the total mean in terms of survival and EL, respectively, compared to other groups. Freezing stress causes the intercellular water potential to be more negative than intracellular water potential. Water always moves from its higher to its lower potential, where intracellular water is transferred to the intercellular space, and the plant’s leaves become plasmolyzed. Hence, increasing the biosynthesis of carbohydrates, which effectively regulate the osmotic pressure and protect cell membranes, can reduce damage (Landry et al. 2017). In this experiment, the high accumulation of WSCs during the CA duration may have reduced cellular dehydration in these two genotypes (MCC259 and MCC291) and maintained the structure of the plant cell membrane function in the face of freezing stress and hence their complete survival. In addition to carbohydrates, CA in plants causes proline biosynthesis. It protects the plants from various stresses and helps plants recover from stress more rapidly. As a stress-related messenger, this compound acts as an osmotic regulator and preserves intracellular structures when freezing stress occurs (Mansour and Salama 2020). Likewise, high levels of ROSs cause oxidative changes in macromolecules, inhibition of protein function, and cell death, through which proline plays an influential protective role. In this experiment, genotypes with a high proline content probably survived with the mechanism of reducing osmotic potential and eliminating excess ROS against freezing stress.
A significant positive correlation between DPPH and anthocyanin content suggests similar changes. Genotypes with a high content of these compounds have a similarly higher antioxidant capacity that can successfully inhibit free radicals. Phenolic compounds also contain many secondary metabolites that diminish the adverse effects of freezing stress with their antioxidant properties. In the current study, all genotypes with the most elevated content of DPPH and phenol kept 100% survival after exposure to freezing stress. The results of the PCA also indicated that the mentioned characteristics, albeit being lower in weight, were in the dimension affecting the survival percentage. Although all genotypes with the highest RWC had complete survival, based on the first and second PCA and correlation between traits, this index had a negligible effect on the results of this experiment.
Evaluation of cell membrane damage after freezing stress has been introduced as one of the most valid and efficient methods of assessing freezing tolerance in plants (Thalhammer et al. 2014; Wei et al. 2022). In this investigation, the EL in 60% of the genotypes was lower than the total average. In other words, the tested germplasm had excellent potential to keep the stability of the cell membrane after exposure to freezing stress. A negative and significant correlation of EL with proline and DPPH also has indicated that less accumulation of these compounds was associated with more damage to cell membranes. Conversely, increased biosynthesis of these compounds has led to maintaining plant cell membrane integrity and function. EL in the second PCA was in the opposite dimension of survival percentage and with a relatively large distance from the center (negative weight). Hence, in this experiment, EL was recognized as one of the crucial factors affecting the survival of chickpea seedlings AFS.
The studied germplasm kept acceptable survival in the face of freezing stress. Specifically, 25 chickpea genotypes tolerated −12 °C well, and their survival after recovery was 100%. After the recovery period, a positive and significant correlation between survival and height as well as DW revealed that seedlings, in addition to maintaining survival, had also promising potential in improving morphological characteristics such as dry matter accumulation. Furthermore, as the most critical attribute for the second principal component, the survival further justified the variability of this component. In a similar experiment, a significant positive correlation was reported between the survival of lentil (Lens culinaris Medik) genotypes and plant height plus DW (Nabati et al. 2020).
According to the first and second functions of MANOVA, the genotypes within the groups were more similar to each other than the genotypes of the other groups in terms of the studied traits. In the present experiment, no significant correlation was observed between biochemical factors in the CA duration and the survival percentage of genotypes after freezing stress. However, the correlation between survival percentage and EL was negative and significant (r2 = −0.22*), indicating that cell membranes’ stability is a suitable criterion for maintaining survival AFS. Except for the MCC884, other genotypes with the most inferior EL had 100% survival. Although ten genotypes had the promising potential to withstand freezing stress according to the two-dimensional cartesian coordinate system, due to the importance of survival and EL, group five genotypes (MCC259, MCC291, and MCC911) with 100% survival and average leakage of fewer electrolytes than the total average (compared to other groups) were found to be more suitable for selection. Furthermore, the genotypes of this group were statistically at the highest level in the mean value of five traits, which were superior in this regard compared to other groups.
The results of the PCA revealed that the screening of genotypes based on the first component to sift genotypes with more CPPs led to the screening of genotypes based on the second component to sift genotypes with survival ability and other superior morphological characteristics AFS. Thus, according to the results of this experiment, group five genotypes and their selection based on the second component would lead to their successful overwintering.
Traits with the highest canonical discriminate coefficients had the best effect on the diversity across the studied genotypes. In this experiment, the significant intergroup variance of survival percentage, as the most critical factor in screening AFS, revealed that the groups mean was not the same, which confirms the importance of cluster analysis to determine superior genotypes in terms of the mentioned trait. The high value of variance between groups for survival percentage compared to the variance within groups (as error effects) indicated a suitable explanation of the impact of the trait in the experiment and the effect of a minor error.
In the study of biochemical characteristics of chickpea genotypes, it was observed that the MCC40 had a higher potential in generating most of these compounds in CA duration. Thus, considering its 100% survival and less EL, regardless of the result of cluster analysis, it can be regarded as a promising genotype in the screening for tolerating freezing stress.
Conclusions
Due to the importance of survival AFS and its significant negative correlation with EL, group five genotypes with 100% survival and average EL less than the total average compared to other groups are more suitable for freezing tolerance. Selection for freezing tolerance of desi-type chickpea genotypes based on the second component will screen chickpea genotypes with high survival and other superior morphological characteristics and far better membrane stability AFS. Accordingly, selecting group five genotypes of cluster analysis based on the second component for freezing stress tolerance is recommended. Although survival of all three genotypes (group five) was 100%, what distinguished MCC259 and MCC291 was their high concentration of WSCs and phenols and their high cell membrane integrity based on EL index. According to the results of this experiment, selection for successful overwintering of desi-type chickpea genotypes in cold regions is recommended based on the mentioned characteristics in breeding programs. The chickpea genotypes and morpho-physiological traits determined from this study may be helpful for genetic engineering and breeding programs that integrate chickpea freezing tolerance.
References
Arslan Ö, Eyidoğan F, Ekmekci Y (2018) Freezing tolerance of chickpea: biochemical and molecular changes at vegetative stage. Biol plant 62(1):140–148. https://doi.org/10.1007/s10535-017-0760-5
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207. https://doi.org/10.1007/BF00018060
Bhattacharya A (2022) Effect of low-temperature stress on germination, growth, and phenology of plants: a review. Physiol Process Plants Under Low Temp Stress. https://doi.org/10.1007/978-981-16-9037-2_1
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lwt-food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Dere Ş, Güneş T, Sivaci R (1998) Spectrophotometric determination of chlorophyll‑A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 22(1):13–18
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith FAJN (1951) A colorimetric method for the determination of sugars. Nature 168(4265):167–167. https://doi.org/10.1038/168167a0
FAOSTAT (2018) Food and agriculture organization. Statistical database of the united nations food and agriculture organization (FAO) statistical division
FAOSTAT (2020) Food and agriculture organization. Statistical database of the united nations food and agriculture organization (FAO) statistical division
Fürtauer L, Weiszmann J, Weckwerth W, Nägele T (2019) Dynamics of plant metabolism during cold acclimation. Int J Mol Sci 20(21):5411. https://doi.org/10.3390/ijms20215411
Grasso N, Lynch NL, Arendt EK, O’Mahony JA (2022) Chickpea protein ingredients: a review of composition, functionality, and applications. Compr Rev Food Sci Food Saf 21(1):435–452. https://doi.org/10.1111/1541-4337.12878
Hasanfard A, Rastgoo M, Darbandi EI, Nezami A, Chauhan BS (2021) Regeneration capacity after exposure to freezing in wild oat (Avena ludoviciana Durieu.) and turnipweed (Rapistrum rugosum (L.) All.) in comparison with winter wheat. Environ Exp Bot 181:104271. https://doi.org/10.1016/j.envexpbot.2020.104271
Hu WH, Zhou YH, Du YS, Xia XJ, Yu JQ (2006) Differential response of photosynthesis in greenhouse- and field-ecotypes of tomato to long-term chilling under low light. J Plant Physiol 163:1238–1246. https://doi.org/10.1016/j.jplph.2005.10.006
Karimzadeh Soureshjani H, Nezami A, Nabati J, Oskoueian E, Ahmadi-Lahijani MJ (2022) The physiological, biochemical, and molecular modifications of chickpea (Cicer arietinum L.) seedlings under freezing stress. J Plant Growth Regul 41(3):1109–1124. https://doi.org/10.1007/s00344-021-10369-4
Landry EJ, Fuchs SJ, Bradley VL, Johnson RC (2017) The effect of cold acclimation on the low molecular weight carbohydrate composition of safflower. Heliyon 3(9):e402. https://doi.org/10.1016/j.heliyon.2017.e00402
Mansour MMF, Salama KHA (2020) Proline and abiotic stresses: responses and adaptation. In: Plant ecophysiology and adaptation under climate change: mechanisms and perspectives II. Springer, Singapore, pp 357–397
Mir AH, Bhat MA, Dar SA, Sofi PA, Bhat NA, Mir RR (2021) Assessment of cold tolerance in chickpea (Cicer spp.) grown under cold/freezing weather conditions of North-Western Himalayas of Jammu and Kashmir, India. Physiol Mol Biol Plants 27(5):1105–1118. https://doi.org/10.1007/s12298-021-00997-1
Morin X, Améglio T, Ahas R, Kurz-Besson C, Lanta V, Lebourgeois F, Miglietta F, Chuine I (2007) Variation in cold hardiness and carbohydrate concentration from dormancy induction to bud burst among provenances of three European oak species. Tree Physiol 27(6):817–825. https://doi.org/10.1093/treephys/27.6.817
Nabati J, Nezami A, Mirmiran SM, Hasanfard A, Hojjat SS, Bagheri AR (2020) Freezing tolerance in some lentil genotypes under controlled conditions. Seed Plant J 36(2):183–205. https://doi.org/10.22092/SPPI.2020.123186
Nabati J, Hasanfard A, Nezami A, Ahmadi-Lahijani MJ, Boroumand Rezazadeh E (2021) Gas exchange variables as promising criteria for screening freezing-tolerant faba bean (Vicia faba L.) landraces at early growth stages. Legum Sci 3(1):e72. https://doi.org/10.1002/leg3.72
Naing AH, Kim CK (2021) Abiotic stress-induced anthocyanins in plants: their role in tolerance to abiotic stresses. Physiol Plant 172(3):1711–1723. https://doi.org/10.1111/ppl.13373
Rachwa-Rosiak D, Nebesny E, Budryn G (2015) Chickpeas-composition, nutritional value, health benefits, application to bread and snacks: a review. Crit Rev Food Sci Nutr 55:1137–1145. https://doi.org/10.1080/10408398.2012.687418
Ritchie SW, Nguyen HT, Holaday AS (1990) Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci 30(1):105–111. https://doi.org/10.2135/cropsci1990.0011183X003000010025x
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158
Thalhammer A, Hincha DK, Zuther E (2014) Measuring freezing tolerance: electrolyte leakage and chlorophyll fluorescence assays. In: Plant cold acclimation. Humana Press, New York, pp 15–24
Waalen WM, Tanino KK, Olsen JE, Eltun R, Rognli OA, Gusta LV (2011) Freezing tolerance of winter canola cultivars is best revealed by a prolonged freeze test. Crop Sci 51:1988–1996. https://doi.org/10.2135/cropsci2011.02.0098
Wagner GJ (1979) Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol 64(1):88–93. https://doi.org/10.1104/pp.64.1.88
Wei Y, Chen H, Wang L, Zhao Q, Wang D, Zhang T (2022) Cold acclimation alleviates cold stress-induced PSII inhibition and oxidative damage in tobacco leaves. Plant Signal Behav 17(1):2013638. https://doi.org/10.1080/15592324.2021.2013638
Acknowledgments
We would like to express our genuine gratitude and appreciation to Vice-President for Research and Technology, Ferdowsi University of Mashhad for providing financial and material support of research project number 51847.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Nezami, J. Nabati, S.M. Mirmiran, A. Hasanfard and M. Mohammadi declare that they have no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nezami, A., Nabati, J., Mirmiran, S.M. et al. How Does the Freezing Stress in the Seedling Stage Affect the Chickpea’s Morpho-Physiological and Biochemical Attributes?. Gesunde Pflanzen 75, 1107–1119 (2023). https://doi.org/10.1007/s10343-022-00771-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-022-00771-7