Abstract
Heat stress (HS) seriously affects crop growth, causing significant crop yield losses worldwide. The regulatory mechanisms controlling HS tolerance in plants are not well understood. Phytohormones are important molecules for coordinating myriad of phenomena related to plant growth and development. They are also essential endogenous signaling molecules that actively mediate numerous physiological responses under abiotic stress by triggering stress-responsive regulatory genes involved in plant growth. This review updates the central role of various phytohormones—indole acetic acid, gibberellic acid, abscisic acid, cytokinins, ethylene, salicylic acid, brassinosteroids, strigolactone, and jasmonic acid—in regulating the HS response so that plants can adapt to increasing temperature stress. We also reveal how these stress-responsive phytohormones switch on various regulatory gene(s) and genes encoding antioxidants and heat shock proteins (HSPs) to combat HS in various plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants, being immobile in nature, must respond to environmental perturbations, including heat stress (HS) (Zhu 2016). Plants have evolved coordinated genetic, molecular, biochemical, and physiological mechanisms to survive and adapt under abiotic stress (Mittler 2006; Wahid et al. 2007; Zhu 2016; Wani et al. 2018; Zaid et al. 2020). Given the rising global temperatures, tropical and semi-arid regions are facing frequent episodes of extreme temperatures, which are challenging crop yields (Lobell et al. 2011; Knox et al. 2012). High-temperature stress is becoming a serious problem for sustaining global crop production (Bita and Gerats 2013; Jha et al. 2014; Sadiq et al. 2020), affecting the entire plant life cycle, including physiological, biochemical, and metabolic processes (Bita and Gerats 2013; Hasanuzzaman et al. 2013; Sharma et al. 2019; Sadiq et al. 2020). Crop plants are most sensitive to HS during the reproductive stage, causing significant yield losses (Wheeler et al. 2000; Luo 2011; Gourdji et al. 2013; Deryng et al. 2014).
The effect of HS on various crops has been assessed using various simulation and prediction models (Swaminathan and Kesavan 2012; Tesfaye et al. 2016). Increasing HS associated with global climate change is predicted to reduce rainfed maize yields by 3.3–6.4% by 2030 and 5.2–12.2% by 2050 (Tesfaye et al. 2016, see Table 1). A 1 °C increase beyond normal air temperature is predicted to significantly reduce yields in two-thirds of African maize-growing regions (Lobell et al. 2011). Similarly, in South-East Asia, a 1 °C increase beyond normal air temperature reduced rice yields by 4–14% (Lobell et al. 2008). A 0.5 °C increase beyond normal winter temperatures will likely reduce rainfed wheat yields by 0.45 t ha–1 in India and 4–7% yield in China (Easterling et al. 2007). A 1 °C increase during summer is predicted to reduce soybean yields by up to 16% in Wisconsin in the United States of America (USA) (Kucharik and Serbin 2008). Table 1 shows further examples of crop yield losses due to HS.
Several strategies, including crop breeding, physiological and agronomic management, and genomics and transgenics approaches, have been suggested to ensure sustainable crop production under increasing HS events (Wahid et al. 2007; Bita and Gerats 2013; Jha et al. 2014). Phytohormones, including auxin/indole acetic acid (IAA), gibberellic acid (GA), abscisic acid (ABA), cytokinins (CTKs), ethylene (ET), salicylic acid (SA), brassinosteroids (BRs), strigolactone (SL), and jasmonic acid (JA) are important molecular players for regulating plant growth in response to various abiotic stress stimuli, including HS (Wani et al. 2016; Verma et al. 2016; Ahmad et al. 2019; Sharma et al. 2019). The above phytohormones significantly participate in various physiological and cellular processes, and molecular mechanisms involved in HS tolerance (Wani et al. 2016; Sharma et al. 2019). Upon perception of HS stimuli, plants release various phytohormones that contribute to plant development and physiological processes, including root development, stomatal movement, pollen development, photosynthesis, osmolyte accumulation, and curtailing reactive oxygen species (ROS) accumulation, thus helping plants to adapt under HS (Sakata et al. 2010; Franklin et al. 2011; Li et al. 2014; Thussagunpanit et al. 2015a; Wu et al. 2016, 2017; Jegadeesan et al. 2018; Rezaul et al. 2019; Raza et al. 2020).
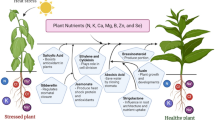
Thus, manipulating endogenous phytohormones or applying exogenous phytohormones could be promising approaches for plant adaptation and recovery from HS to minimize yield losses. The role of various phytohormones in regulating heat tolerance is summarized in Fig. 1. Here, we discuss the implications of various phytohormones and the plausible mechanistic roles in mediating HS tolerance in various plant species.
Role of phytohormones contributing to heat stress adaptations in plants. Phytohormones contribute to plant survival under heat stress by protecting photosynthesis apparatus (Ivanov et al. 1992; Sharma et al. 2019), rescuing pollen fertility (Firon et al. 2012; Zhang et al. 2018), improving grain filling (Yang et al. 2016), accumulating osmolytes (Robertson et al. 1994), activating antioxidant activity and reducing reactive oxygen species activity (Dat et al.1998a), and activating the transcription of various heat shock protein genes (Dhaubhadel et al. 1999; Makarova et al. 2018) and regulatory transcription factors, e.g., WRKY and ethylene response factor (Pan et al. 2012; Dang et al. 2013; Huang et al. 2016)
Indole Acetic Acid Homeostasis Maintains Reproductive Stability Under HS
IAA is the most abundant auxin in plants, orchestrating many essential developmental and physiological processes, including cell elongation and division, pollen development, anther dehiscence, and reproductive tissue development (Gray et al. 1998; Teale et al. 2006; Cecchetti et al. 2008; Sakata et al. 2010). Like other phytohormones, IAA plays a prominent role in plant adaptation to environmental stresses, including HS (Sakata et al. 2010; Franklin et al. 2011; Sun et al. 2012; Wit et al. 2014; Zheng et al. 2016; Zhang et al. 2018), by protecting photosynthesis and reducing photooxidation-mediated damage (Tognetti et al. 2012). The promotion of hypocotyl elongation through accumulated auxin is a well-established adaptive response by plants to HS (Gray et al. 1998). Subsequently, Franklin et al. (2011) shed mechanistic insight into the roles of PHYTOCHROME-INTERACTING FACTOR 4 (PIF4)—a transcription factor (Stavang et al. 2009)—and the SMALL AUXIN UP RNA (SUAR) genes (SAUR19–24 and SAUR61–68) that regulate auxin biosynthesis by upregulating related genes under high temperature (28 °C) and thus promoting hypocotyl elongation in Arabidopsis. The underlying genes involved in auxin biosynthesis upregulation were: YUC8, YUC9, TAA1, and CYP79B2 (Stavang et al. 2009; Franklin et al. 2011; Sun et al. 2012). Furthermore, Wang et al. (2016) demonstrated that auxin co-receptors, TIR1 and AFB2, and HEAT SHOCK PROTEIN 90 (HSP90) are involved in root and hypocotyl elongation and lateral root formation of Arabidopsis in response to HS. The authors also described the supportive role of SGT1, a co-chaperone of HSP90, in response to increasing temperature. However, the mutant SGTb1 protein encoded by the eta3 gene inhibited auxin and TIR1 accumulation, preventing auxin-mediated growth under HS. Likewise, auxin’s role in maintaining the function of male reproductive organs, especially pollen tube growth/elongation and anther dehiscence, under HS is worth mentioning (Chen and Zhao 2008; Wu et al. 2008, 2016, 2019; Sharma et al. 2018). Under high-temperature stress, inhibition of endogenous auxin biosynthesis genes YUCCA flavin monooxygenase (Sakata et al. 2010) and UDP-glucosyltransferase 74b1 (UGT74B1) (Grubb et al. 2004; Sharma et al. 2018) reduced pollen tube elongation, causing spikelet sterility and reduced pollen vigor in barley and rice and male sterility in Arabidopsis and cotton (Sakata et al. 2010; Fu et al. 2015; Song et al. 2015; Zhang et al. 2017, 2018; Wu et al. 2019). In contrast, IAA content declined at the tetrad stage and increased at the tapetal degradation and anther dehiscence stages in cotton anthers of H05, a heat-sensitive genotype, under HS (Min et al. 2014). These contrasting results suggest that anthers need an appropriate balance of IAA to confer heat tolerance. Further, in heat-stressed rice, spikelet fertility declined due to inhibited panicle expansion, which is likely the result of reduced accumulation of IAA and GA1, an active type of GA (Wu et al. 2016).
The damaging effect of HS could be counteracted/minimized by manipulating auxin biosynthesis gene(s) and exogenous supplementation of auxin to restore pollen development and pollen tube growth and reverse spikelet sterility in crop plants (Sakata et al. 2010; Zhang et al. 2017, 2018; Wu et al. 2019) (see Table 2). Several auxin-responsive genes participate in the pollen viability restoration mechanism, which has been investigated in plants under stress (Sakata et al. 2010; Pazhamala et al. 2020). Diminished expression of YUC2 and YUC6 auxin biosynthesis genes in barley and Arabidopsis anthers under HS resulted in short stamens and pollen abortion (Sakata et al. 2010). Further, external application of synthetic auxin restored anther development and pollen fertility through enhanced expression of the MCM5 gene mediating anther cell proliferation and development. External supplementation of 1 and 10 μmol L–1 NAA increased auxin content in high-temperature susceptible mutants of Nipponbare rice under HS, reducing spikelet sterility by promoting pollen tube growth in pistils (Zhang et al. 2018) (see Table 3). Interestingly, Nipponbare, a heat-tolerant rice cultivar, had higher auxin and ROS and lower peroxidase activity in pistils under HS than the heat-sensitive mutant. Reduced peroxidase activity increases ROS expression, increasing auxin in pollen tubes, which mediated HS tolerance in Nipponbare (Zhang et al. 2018). However, increased accumulation of ABA and IAA-tryptophan in KSG1177 (heat-sensitive wheat genotype) under HS resulted in pollen abortion and low seed set (Bheemanahalli et al. 2020).
Understanding the interaction of IAA with other phytohormones could reveal various complex regulatory networks orchestrating plant growth and development and plant adaptation mechanisms under various environmental cues. Recent investigations have revealed crosstalk between IAA, BRs, and GA3, an active type of GA, which regulates hypocotyl growth under temperature stress in Arabidopsis (Stavang et al. 2009; Maharjan and Choe 2011). However, the molecular mechanisms of IAA for rescuing pollen fertility, reducing spikelet sterility, and controlling root growth in response to HS need further investigation.
CTKs Contribute Plant Thermal Acclimation Beyond Growth and Development
CTKs are multifaceted phytohormones contributing to basic plant development, including cell division, breaking seed dormancy, chloroplast biogenesis, apical dominance, leaf senescence, shoot differentiation, photomorphogenic development, and nucleic acid metabolism (Sakakibara 2010; for details, see Hwang et al. 2012). The role of CTK signaling, metabolism, and its crosstalk with other phytohormones for alleviating plants from the adverse effects of various environmental cues, including HS, has been investigated (Veselova et al. 2005; Jeon et al. 2010; Rivero et al. 2010; Ghanem et al. 2011; Hwang et al. 2012; Reguera et al. 2013; Fahad et al. 2015a; Yang et al. 2016). CTKs protect plants from the deleterious effects of HS by activating antioxidant mechanisms, protecting photosynthetic apparatus, or maintaining stay green and delayed senescence (He and Jin 1999; Thomas and Howarth 2000; Liu and Huang 2002; Zavaleta-Mancera et al. 2007). The role of kinetin in scavenging free radicals and switching on the antioxidant mechanism to prevent purine breakdown is worth noting (Chakrabarti and Mukherji 2003). Liu and Huang (2002) reported that CTKs play a role in alleviating HS in creeping bentgrass by activating antioxidant mechanisms and reducing lipid peroxidation activity. During the reproductive phase, HS reduced CTKs in rice panicles due to inhibited CTK biosynthesis, interrupted CTK transport from roots to shoots, and enhanced CTK catabolism (Skalák et al. 2016; Wu et al. 2016, 2017; Wu and Yang 2019). Maintaining sufficient CTK content under HS by stabilizing CTK biosynthesis and lowering CTK oxidase and dehydrogenase activity likely increases spikelet number per panicle in rice (Ha et al. 2012; Wu et al. 2016, 2017). High-temperature stress impaired CTK transport from roots to shoots in heat-sensitive rice genotypes—LYPJ, HHZ, and N22—reducing spikelet number per panicle due to the higher activity of CTK oxidase/dehydrogenase (Wu et al. 2017). However, exogenous application of CTKs maintained spikelet number per panicle in heat-sensitive rice cultivars under HS, indicating that CTKs play a pivotal role in stabilizing spikelet numbers under HS (Wu et al. 2017). Likewise, Wu et al. (2016) advocated that the external application of CTKs retained spikelet fertility and enhanced grain size in heat-sensitive rice cultivars. External application of 60 mg L–1 6-benzylaminopurine, a synthetic CTK (see Table 3), strengthened the wheat grain sink under HS by increasing endosperm cell number and carbohydrate accumulation during grain filling in the presence of elevated levels of IAA and zeatin riboside, a naturally occurring CTK (Yang et al. 2016). Banowetz et al. (1999) also implicated CTKs for improving grain weight in wheat under HS. Similarly, improved HS tolerance by maintaining a balance between ABA and CTK has been reported in maize (Cheikh and Jones 1994). An abundance of ABA and paucity of CTK impaired maize kernel development during HS (Cheikh and Jones 1994). Thus, the application of benzyladenine to maize under HS maintained a balance between ABA and CTK, ultimately preventing kernel abortion (Cheikh and Jones 1994). Recently, the inhibitory action of CTK degradation by INCYDE-F—an inhibitor of CTK oxidase/dehydrogenase—was investigated for mediating heat tolerance in HS-acclimated Arabidopsis plants (Prerostova et al. 2020; see Table 2). Interestingly, the external application of melatonin significantly ameliorated leaf senescence in perennial ryegrass under HS by enhancing CTK biosynthesis and limiting ABA biosynthesis (Zhang et al. 2017). Further insight into the underlying molecular mechanisms mediated by CTK controlling the grain-filling process and grain weight under HS will improve grain yield. Moreover, investigation of the key gene(s) controlling CTK biosynthesis and its regulation under HS will provide novel insights into the HS tolerance mechanism in plants.
ABA Regulates Plant Response to HS
ABA is an important phytohormone with a central role in regulating various processes from plant development and seed dormancy to myriad of biotic and abiotic stress responses (Verslues and Zhu 2007; Huang et al. 2017; Kang et al. 2017; Cho et al. 2018). It is an important signaling molecule for plants exposed to various environmental stresses, including water, cold, salinity, heavy metal toxicity, and heat, and well recognized in concert with other phytohormones regulating complex gene networks (Hsu and Kao 2003; Narusaka et al. 2003; Suzuki et al. 2016; Zhang et al. 2017; Rubio et al. 2018; Albertos et al. 2019). Importantly, ABA’s mechanistic role in regulating plant water status by controlling stomatal aperture closure during water stress is well-established (Daszkowska-Golec and Szarejko 2013). However, the mechanisms of ABA’s contribution to HS tolerance in plants are not fully understood (Suzuki et al. 2016; Zhang et al. 2017). Concerted efforts in recent decades have suggested that ABA contributes to both basal and acquired thermotolerance by preventing photoinhibition and improving photosystem II (PSII) efficiency, minimizing the damaging effects of chloroplast ultrastructure, activating various antioxidant mechanisms by producing various osmolytes, upregulating heat shock transcription factors (HSFs), and switching on various HSP gene(s) and genes actively participating in maintaining ‘energy homeostasis’ in plants under HS (Ivanov et al. 1992; Robertson et al. 1994; Gong et al. 1998; Larkindale and Knights 2002; Larkindale and Huang 2004; Larkindale et al. 2005; De Block and Lijsebettens 2011; Wang et al. 2017; Rezaul et al. 2018, 2019; Li et al. 2020). ABA-treated bromegrass (Bromus inermis) enhanced HS tolerance by producing various proteins and osmolytes (Robertson et al. 1994). Likewise, maize seedlings treated with ABA and Ca2+ increased HS tolerance by enhancing antioxidant enzyme activities and reducing lipid peroxidation activity (Gong et al. 1998). Zandalinas et al. (2016) revealed that the increased activity of ascorbate peroxidase 1 and multiprotein bridging factor 1 proteins, attributing plant acclimation under combined drought and HS in response to increased ABA, safeguarded plants against HS damage. Abscisic acid enables plants to withstand HS by enhancing H2O2 activity, activating antioxidant mechanisms, and accumulating HSPs (Li et al. 2014; Rezaul et al. 2019). In contrast, increased sensitivity to HS due to impaired ABA biosynthesis has been reported (Ding et al. 2010; Kumar et al. 2012) and supported in ABA-deficient mutants displaying higher sensitivity to HS damage (Wang et al. 2014a; Wu et al. 2017). The role of ABA in mediating the thermotolerance response by regulating HSF networks and downstream HSP encoding genes is well-established (Pareek et al. 1998; Campbell et al. 2001; Larkindale and Knights 2002; Larkindale et al. 2005; Huang et al. 2016; Suzuki et al. 2016). Considering this, how HSFA6b—an HSF acting as a hub for ABA signaling and serving in thermotolerance in association with ABA—has been investigated in Arabidopsis (Huang et al. 2016). The upregulation of FaHSFAc2 encoding HSPs in tall fescue (Festuca arundinacea Schreb) treated with ABA improved HS tolerance (Wang et al. 2017) (see Table 4). Likewise, Hu et al. (2018a) advocated that overexpression of TaHsfC2a regulatory genes, mediated by ABA under HS, conferred heat tolerance in wheat by switching on various HSPs and osmoprotective genes, viz., TaHSP16.9b, TaHSP62.4, HSP101b, TaGalSyn, TaHSA32, TaRof1, and TaβAmy1. An increased abundance of ABA in the embryo, relative to the endosperm, during grain filling, contributed to heat tolerance by inducing HSP accumulation (Walker-Simmons 1987). Further investigation by Walker-Simmons and Sesing (1990) provided evidence of higher ABA accumulation in wheat grains under high-temperature stress (25 °C) during grain filling.
The role of ABA for maintaining energy homeostasis under HS has been explored (De Block and Lijsebettens 2011). Rezaul et al. (2018, 2019) and Chen et al. (2019) reported evidence of ABA’s participatory role in heat tolerance by enhancing sugar metabolism by switching on sucrose transporter genes, sucrose synthase (SUS) genes, and invertase (INV) genes to maintain ATP formation, and allocating sugars to alleviate the HS effect in rice spikelets, thus improving pollen fertility. Increased availability of carbohydrates, expression of HSP genes (HSP71.1 and HSP24.1), and ATP and NAD(H) production in Nipponbare (wild-type with flat leaf) rice subjected to exogeneous ABA application under HS revealed a significant role of ABA in enhancing heat tolerance (Li et al. 2020). However, the external application of ABA in an hts mutant of Nipponbare, featuring semi-rolled leaves, exhibited lower heat tolerance than its counterpart wild-type Nipponbare due to enhanced leaf temperature and respiration rate, which increased carbohydrate consumption and resulted in energy deficiency (Li et al. 2020). Furthermore, ABA-mediated drought priming enhanced HS tolerance in Festuca arundinacea by upregulating various regulatory genes, viz., CDPKs, DREBs, MYB, MYC, HSFs, and HSPs, but their role in conferring HS tolerance needs further investigation (Zhang et al. 2019). Unveiling the complex pathways of ABA signaling and its crosstalk with other phytohormones in response to HS and the regulatory gene network involved in inducing HS tolerance could provide insight into the role of ABA in plant HS tolerance.
Gibberellic Acid Regulates Plant Responses to HS
Gibberellic acid belongs to a group of natural diterpenoids. It is an essential phytohormone contributing to various developmental processes, including seed germination, stem elongation, and flower and fruit development (Sun and Gubler 2004; Yamaguchi 2008), and a key mediator of various abiotic stresses, including HS, in plants (Ko et al. 2007; Alonso-Ramírez et al. 2009). Vettakkorumakankav et al. (1999) illustrated the involvement of GA3 in regulating HS tolerance by treating normal and dwarf barley seedlings with GA3 and paclobutrazol (GA3 inhibitor) under HS. The GA3-treated normal and dwarf plants had more ion leakage and fewer photosynthetic pigments than the paclobutrazol-treated plants under HS; that is, the normal and dwarf plants treated with GA3 exhibited heat sensitivity (Vettakkorumakankav et al. 1999). Likewise, barley seedlings treated with a triazole, a GA3 inhibitor, had increased thermotolerance due to reduced GA3 and plant height (Sarkar et al. 2004). In rice, the incidence of HS during panicle initiation inhibited spikelet fertility by repressing panicle opening, which was ascribed to the depletion of IAA and GA1 (Wu et al. 2016). Inhibition of GA biosynthesis due to lower expression of various genes, viz., GA20ox1, GA20ox2, GA3ox1, and GA3ox2, regulating GA biosynthesis under HS was examined in Arabidopsis (Toh et al. 2012). In rice, Tang et al. (2008) documented the contributory role of GA3, IAA, and proline on pollen fertility and spikelet fertility damage imposed by high-temperature stress. Exogenous application of GA3 improved spikelet fertility in rice under HS, suggesting a significant role in protecting male reproductive organs under HS (Kwon and Paek 2016). Likewise, a GA3-mediated mechanism imparting HS tolerance in wheat by enhancing invertase activities increased grain sink activity under HS (Asthir and Bhatia 2014). Alonso-Ramírez et al. (2009) established that external addition of GA3 alleviated the inhibitory effects imposed by HS, oxidative, and salinity stresses by modulating SA biosynthesis and switching on transgene FsGASA4 (introduced from beechnut Fagus sylvatica), a GA3-responsive gene, in Arabidopsis seedlings. A recent investigation by Khan et al. (2020) demonstrated the basis of HS tolerance in date palm using externally applied GA3 in conjunction with silicon that modulated various genes, viz., GPX2, CAT, Cyt-Cu/Zn SOD, and glyceraldehyde3-phosphate dehydrogenase engaged in antioxidant mechanisms, HsfA3 encoding heat shock transcription factor, and the diminishing action of PYL4, PYL8, and PYR1 ABA signaling-related genes. Various gene(s) contributing to the HS response and tolerance due to phytohormone signaling are listed in Table 4.
Further investigation into genes related to GA biosynthesis, GA-signaling pathways and networks, and crosstalk of GA with other phytohormones using a systems biology approach could expand our understanding of GA for defending HS in crop plants (Verma et al. 2016).
SA Improves Plant Performance By Boosting Plant Defense Mechanisms Against HS
SA is a multifunctional hormone contributing to plant growth, seed germination, development, photosynthesis, transpiration, leaf senescence, redox homeostasis, and defense responses to various biotic and abiotic stresses, including HS (Javid et al. 2011; Vicente and Plasencia 2011; Fahad and Bano 2012; Bastam et al. 2013; Khanna et al. 2016; Zhang et al. 2017; Ahmad et al. 2018a; Kohli et al. 2019; van Butselaar and Van den Ackerveken 2020; Ding and Ding 2020). There is accumulating evidence of SA contributing to thermotolerance in various plants by activating antioxidant mechanisms, accumulating osmoprotectants and HSPs, and inducing excessive H2O2 production that mediates signal transduction (Dat et al. 1998a, b; Lopez-Delgado et al. 1998; Wang and Li 2006; Hayat et al. 2009; Wang et al. 2010, 2014b; Khan et al. 2013; Kohli et al. 2018a, b; Kaya et al. 2020a). The ameliorative role of SA in mediating HS tolerance by improving key processes, viz., photosynthesis and nitrogen assimilation activity, and increasing proline content by enhancing γ-glutamyl kinase and reducing proline oxidase enzyme activity has been established in wheat (Khan et al. 2013). External application of SA enhanced photosynthetic activity by improving net photosynthesis rate, Rubisco and PSII efficiency, chlorophyll content, proline content, soluble protein, HSPs, and sugar content, and reducing membrane damage, attributing to heat tolerance in wheat (Kousar et al. 2018), brassica (Kaur et al. 2009; Hayat et al. 2009), cotton (Galani et al. 2016), grapevine (Wang and Li 2007; Wang et al. 2010), tall fescue (Festuca arundinacea Schreb) (Pirnajmedin et al. 2020), and alfalfa (Wassie et al. 2020). Likewise, SA can alleviate plant injury from HS by activating the antioxidant defense system in various plant species (Dat et al. 1998a; Saleh et al. 2007; Kaur et al. 2009; Li 2015; Khanna et al. 2016; Zhang et al. 2017; Janda et al. 2020). Exogenous application of SA (50 ppm) along with H2O2 (30 ppm), ascorbic acid (70 ppm), and moringa leaf extract enhanced net photosynthetic rate and yield-contributing traits in cotton and protected plants from heat-induced injury by modulating the antioxidant enzyme mechanism (Sarwar et al. 2018; see Table 3). Similarly, the synergistic effect of SA and H2S on HS tolerance induced proline, betaine, and trehalose accumulation and enhanced antioxidant activity in maize under HS (Li 2015; Li et al. 2015). Moreover, SA treatment in rice at 40 °C alleviated spikelet damage by inducing higher activity of ROS-scavenging enzymes and accumulating soluble sugars, proline and IAA, ABA, and BR (Zhang et al. 2017). Feng et al. (2018) argued that H2O2 induced higher accumulation of SA, which improved pollen viability, minimized ROS activity, and inhibited tapetum programmed cell death in rice under HS. Brachypodium treated with 0.5 mM NaSA had elevated levels of glutathione-S-transferase under HS, which supports the role of SA in activating the antioxidant mechanism for ameliorating HS injury (Janda et al. 2020). Furthermore, various gene(s) have been induced by SA that contribute to HS tolerance (Wen et al. 2008; Feng et al. 2018; Janda et al. 2020). An SA treatment under high-temperature stress reduced excessive ROS in rice anthers and tapetum degradation by repressing caspase-3, thus preventing programmed cell death in anthers (Feng et al. 2018). Moreover, the authors postulated the role of EAT1, MIL2, and DMT1 genes (see Table 4), related to tapetum development, in SA-mediated protection of pollen abortion from HS; the molecular mechanism remains elusive. Increased expression of the phenylalanine ammonia-lyase enzyme contributing to phenylpropanoid metabolism due to external application of SA in grape berry increased the accumulation of phenolics, thus imparting HS tolerance in grape berry (Wen et al. 2008). Dang et al. (2013) speculated that the CaWARKY40 gene has an important role in regulating HS tolerance mediated by SA, JA, and ET in transgenic tobacco. Under HS, overexpression of CaWRKY40 enhanced expression of SA‐dependent NtPR1a/c, NtPR2 genes, JA‐responsive NtPR1b gene, JA biosynthesis‐associated NtLOX1 gene, and ET synthesis genes, viz., NtACS6 and NtEFE26.Therefore, ET-, SA-, and JA-mediated regulation of the CaWRKY40 gene could enhance downstream genes related to HS tolerance. Likewise, positive overexpression of the regulatory gene AtWRKY39 in cooperation with SA, JA, and calcium signaling pathways imparting heat tolerance has been established in Arabidopsis thaliana (Li et al. 2010). A model proposed by Divi et al. (2010) depicted the interplay of BR with SA, ABA, and ET mediating various stress tolerance mechanisms. They also explained the positive regulation of BR with NPR1, a regulator of SA-mediated defense genes, which plays a pivotal role in conferring HS tolerance. Thus, modulation of SA responsive gene(s) or SA signaling is an attractive approach for protecting plants from HS damage. However, the molecular mechanisms involved in SA minimizing ROS activity, protecting pollen grains from HS-induced damage, and inducing various regulatory genes under HS remain elusive.
Brassinosteroids as Emerging Phytohormones: Their Possible Roles in Regulating HS Tolerance
BRs are a novel class of polyhydroxylated plant steroid hormones synthesized in the endoplasmic reticulum (Kim et al. 2006; Northey et al. 2016; Nolan et al. 2017, 2020; Ahanger et al. 2018, 2020). They comprise brassinolide (BL), castasterone (CS), and various derivatives involved in regulating plant growth and development, seed germination, pollen tube growth, flower and fruit production, protein synthesis, nucleic acid synthesis, and various environmental stress responses, including HS (Grove et al. 1979; Clouse and Sasse 1998; Nakaya et al. 2002; Ogweno et al. 2007; Hayat et al. 2010; Zhang et al. 2013; Fahad et al. 2015b; Lozano-Durán and Zipfel 2015; Sharma et al. 2016; Ahmad et al. 2018b, c, 2019; Jan et al. 2020; Kaya et al. 2020b; Nolan et al. 2020). The working mechanism of BR signaling, from binding to cell surface receptors to switching on TFs in response to various stresses, has been investigated (Krishna 2003; Nolan et al. 2020). BR-mediated HS tolerance has been reported in B. inermis (Wilen et al. 1995), brassica (Brassica napus) (Dhaubhadel et al. 1999, 2002), tomato (Solanum lycopersicum) (Dhaubhadel et al. 1999; Singh and Shono 2005; Ogweno et al. 2010; Mazorra et al. 2011), Vigna radiata (Hayat et al. 2010), and barley (Hordeum vulgare) (Janeczko et al. 2011). The counteractive effect of BRs against HS in rice enhanced chlorophyll content, photosynthetic activity, stomatal conductivity, and filled seed numbers (Thussagunpanit et al. 2015a, b). The mechanism of BR that contributes to plant HS tolerance is mediated by various essential physiological and biochemical processes, viz., photosynthetic efficiency by maximizing the carboxylation rate of Rubisco and improving the efficiency of PSII photochemistry, enhancing chlorophyll content, stomatal conductivity, membrane stability, and proline content, reducing lipid peroxidation, activating antioxidant mechanisms, and maintaining redox homeostasis (Cao and Zhao 2008; Ogweno et al. 2007, 2010; Hayat et al. 2010; Kaur et al. 2018; Kaya et al. 2019). The ameliorative effects of trihydroxylated spirotane, an analog of BR, by decreasing the negative effect of HS on photosynthetic activity and promoting growth has been reported for banana under HS (Gonzalez-Olmedo et al. 2005). Thussagunpanit et al. (2015a) reported that EBR and 7,8-dihydro-8α-20-hydroxyecdysone (DHECH), a mimic compound of BR, impart HS tolerance. Rice plants treated separately with 1 nM EBR and 1 nM DHECH at the flowering stage at 40/30 °C (day/night temperature) protected photosynthetic apparatus by enhancing stomatal conductance and increasing the PSII quantum yield, improving plant yield (Thussagunpanit et al. 2015a). Likewise, exogenous application of EBR in Cucumis melo L. aided the recovery of chlorophyll content and improved photosynthesis and photochemical activity of PSI under high-temperature stress (Zhang et al. 2013, 2014). The role of BRs in minimizing ROS activity and maintaining plant cellular redox homeostasis by activating antioxidant and glyoxylase mechanisms under HS has been reported (Hussain et al. 2019). Likewise, Cao and Zhao (2008) revealed that BR contributed to HS tolerance in contrasting heat-tolerant and heat-sensitive rice cultivars by enhancing antioxidant enzymes and diminishing the lipid peroxidation process. Furthermore, Ogweno et al. (2007, 2010) reported HS tolerance at 40/30 °C in EBR-treated tomato plants due to improved photosynthetic activity that activated antioxidant mechanisms and concomitantly prevented photoinhibition and lipid oxidation activity. EBR in association with Si improved HS tolerance in a heat-sensitive wheat cultivar (PBW343) by switching on antioxidant mechanisms and producing excessive proline (Hussain et al. 2019) (see Table 1). Brassinosteroids induced the expression of various HSPs, viz., hsp100, hsp90, and hsp70, in tomato and B. napus, protecting the cellular protein synthesis machinery from HS (Dhaubhadel et al. 1999, 2002; Singh and Shono 2005). Crosstalk of BRs with JA, ABA, ET, and SA signaling pathways mediating HS tolerance has been demonstrated (Divi et al. 2010); however, the precise molecular mechanism remains poorly understood. A thorough understanding of BR-responsive BRASSINAZOLE RESISTANCE1 (BZR1)/BRI1-EMS SUPPRESSOR 1 (BES1) transcription factors controlling BR-targeted genes regulating the HS response in plants could help to develop HS-tolerant plants (Anwar et al. 2018).
Research is needed to clarify the participatory role of BRs and their crosstalk with other phytohormones for modulating the defense response of plants to HS.
ET and Its Crosstalk with Other Phytohormones Regulating HS Tolerance in Plants
ET is an important gaseous phytohormone involved in controlling various plant developmental processes and regulating various biotic and abiotic stresses, including HS (Larkindale and Knight 2002; Larkindale and Huang 2004; Larkindale et al. 2005; Muller and Munne-Bosch 2015; Abdelrahman et al. 2017). However, the regulatory mechanisms contributing to heat tolerance remain under investigation. Larkindale and Knight (2002) and Larkindale et al. (2005) reported ET-mediating basal thermotolerance after studying mutant, wild and transgenic Arabidopsis. Larkindale and Knight (2002) illustrated the involvement of Ca2+, SA, ABA, and ET in protecting plants from HS-induced oxidative damage by comparing seedling survival of an ET-insensitive mutant (etr-1), ABA-insensitive mutant (abi-1), nahG transgenic line with a depleted level of SA, and the corresponding wild-type Columbia, Landsberg erecta, and Columbia background Arabidopsis lines treated with HS. The significant role of these phytohormones conferring HS tolerance was confirmed in the mutants defective in these phytohormones, which had lower survival rates than the wild types. Larkindale et al. (2005) also confirmed the participatory role of ET, SA, and ABA in HS tolerance because the abi1 and abi2 mutants defective in ABA signaling had impaired acquired thermotolerance. However, ein-2 and etr-1 mutants defective in ET signaling had greater impairment in basal thermotolerance than acquired thermotolerance in Arabidopsis (Larkindale and Knight 2002). Recently, ET’s role in safeguarding plant cells from oxidative stress damage by switching on various antioxidant systems and HSF genes has been investigated in rice under HS through the external application of 1-aminocyclopropane-1-carboxylic acid (ACC), an ET precursor (Wu and Yang 2019). The upregulatory activity of Oryza sativa EIN2 (OsEIN2) and OsEIL1 genes, contributing to ET signaling pathways, and HsfA2b, HSFA2a, c, d, e, and f HSF genes protecting rice seedlings under HS was investigated (Wu and Yang 2019). ACC plays a role in the HS tolerance of creeping bentgrass (Agrostis stolonifera var. palustris) by increasing superoxidase dismutase (SOD), peroxidase (POX), and ascorbate peroxidase (APX) activities by protecting plants against HS-induced oxidative damage (Larkindale and Huang 2004). Jegadeesan et al. (2018) uncovered both upregulatory and downregulatory genes participating in increased ET biosynthesis and signaling that contribute to HS tolerance in tomato pollen grains (Table 4), which agrees with the findings of Firon et al. (2012), who reported that tomato plants pre-treated with ET releaser retained high-quality fertile pollen grain under HS. The role of ethylene-responsive factor (ERF) TFs, acting as a regulatory gene for controlling various abiotic stresses, is well recognized (Mizoi et al. 2012). Upregulation of various ERF genes, including Arabidopsis thaliana ERF1 (AtERF1) in Arabidopsis (Cheng et al. 2013), Cicer arietinum ERF116 (CarERF116) in chickpea (Deokar et al. 2015), and Solanum lycopersicon ERF5 (SlERF5) in tomato (Pan et al. 2012), reportedly confer HS tolerance. An increased abundance of ET during HS reduced wheat yields due to low spikelet fertility and reduced final grain weights (Cheng and Lur 1996; Yang et al. 2004; Hays et al. 2007; Huberman et al. 2013). A genome-wide association study elucidated five and 22 marker-trait associations for spike ET content and its association with spike dry weight, respectively, under HS in the field in a panel of 130 wheat genotypes (Valluru et al. 2017). The authors also found a negative correlation between spike ET content and spike dry weight and suggested exploring genes that control the reduced ET effect on plant yield under HS for improving plant yield. Likewise, inhibition of ET biosynthesis by downregulating ET signaling components, viz., ACS6 and ETR2, could improve grain yield under drought stress in rice and maize (Young et al. 2004; Wuriyanghan et al. 2009; Habben et al. 2014). Moreover, crosstalk of ET with other phytohormones, viz., ABA, SA, and JA, for controlling various abiotic stresses, including HS, by inducing ERF genes has been reported (Muller and Munne-Bosch 2015). Crosstalk of various phytohormones and phytohormone-responsive genes mediating HS tolerance is depicted in Fig. 2. Complete molecular understanding of ET-mediating basal thermotolerance and thermopriming, signaling pathway, induction of antioxidant mechanism and expression of ERFs viz., ERF95 and ERF97, and HSFs especially, HSFA2 could help improve HS tolerance in plant (Wu and Yang 2019; Huang et al. 2020; Singh et al. 2021).
Phytohormones regulate various genes controlling heat stress tolerance in plants. In response to heat stress, plants adapt by activating various transcription factors (TFs) that regulate various phytohormones. Abscisic acid (ABA) and jasmonic acid (JA) positively regulate DREB2A (Zhang et al. 2019; Tian et al. 2020) and ethylene (ET) positively regulates ethylene response factor (ERF) for attributing heat tolerance in plants (Cheng et al. 2013; Muller and Munne-Bosch 2015). In contrast, ABA negatively regulates ERF1 (Cheng et al. 2013) and jasmonic acid positively regulates bZIP3, WRKY40 for controlling heat tolerance (Dang et al. 2013; Balfagón et al. 2019). Several gene(s) are involved in mediating heat tolerance through their upregulation by exogeneous application of various phytohormones contributing heat tolerance: HSP101 and HSP70 are induced by JA and ET (Muller and Munne-Bosch 2015), FsGASA4 is induced by gibberellic acid (Alonso-Ramírez et al. 2009), SUT, SUS, INV, and RBOH1 are induced by ABA (Rezaul et al. 2018, 2019; Chen et al. 2019; Li et al. 2020), YUCCA, YUC1, and YUC9 are induced by auxin (Stavang et al. 2009; Franklin et al. 2011; Sun et al. 2012), CKX1 is induced by cytokinin (Macková et al. 2013), ERF1, EIN2, EIN3, HsfA1a, and HsfA2a induced by ET (Wu and Yang 2019), and EAT1, MIL2, DMT1, and MBF1c are induced by salicylic acid (Feng et al. 2018). However, PIN1, PIN2, and PIN5 are negatively regulated by strigolactone (Hu et al. 2018b)
Strigolactones: Novel Players Mediating Plant HS Tolerance
Strigolactones (SLs) are a group of terpenoid lactones regulating plant architecture, mainly shoot branching and mesocotyl, hypocotyl, internode, and root elongation, to improve plant abiotic stress tolerance, including HS (Umehara et al. 2008; Toh et al. 2012; Brewer et al. 2013; de Saint Germain et al. 2013; Jia et al. 2014; Van Ha et al. 2014; Hu et al. 2018a, b). How SLs regulate HS tolerance in plants is unclear. Hu et al. (2018a, b) revealed that SLs applied externally to tall fescue (Festuca arundinacea Schreb) controlled HS tolerance by promoting root elongation through the upregulation of cell division and cell-cycle-related genes, such as PCNA, CycD2, and CDKB, and downregulation of the auxin receptor TIR1 gene and transport genes, such as PIN1, PIN2, and PIN5 (see Table 4). Subsequently, Hu et al. (2019) highlighted the role of SLs in conferring HS tolerance in tall fescue through leaf elongation by enhancing the activity of cell-cycle-related genes and diminishing the activity of auxin transport-related genes. Future research on the contribution of SLs to HS tolerance could advance our understanding of the mechanistic role of SLs in HS tolerance in plants.
Jasmonates: a Multifunctional Phytohormone Functioning Beyond Plant Biotic Stress Defense
Jasmonates (JAs) play a pivotal role in the plant defense response to various pathogens (Vijayan et al. 1998; Mei et al. 2006) and serve as multifunctional phytohormones contributing to plant development, growth, and reproduction processes (Wasternack 2007; Browse 2009). Their participatory role in controlling various abiotic stresses, including salinity, cold, light, heavy metals, and HS, in plants has been investigated (Dar et al. 2015; Kazan 2015; Sharma and Laxmi 2016; Per et al. 2018; Balfagón et al. 2019; Savchenko et al. 2019; Ali and Baek 2020). While JA plays an assertive role in response to various biotic and abiotic stresses in plants, it adversely affects plant growth and photosynthesis (Kazan and Manners 2012; Yang et al. 2012; Wasternack 2014).
The role of JA in heat tolerance in Arabidopsis was demonstrated by Clarke et al. (2009) by assessing electrolyte leakage in cell membrane treated with methyl jasmonate (MeJA) under HS—an external application of 5 µm MeJA protected cell viability under HS, while application > 5 µm reversed this result by enhancing membrane damage, indicating that its ability to ameliorate the negative effect of HS is concentration specific (Clarke et al. 2009). Shahzad et al. (2015) compared the effect of MeJA by treating pea plants with various concentrations of MeJA, viz., 50, 100, and 200 µM under HS (40 °C), cold stress (4 °C), and control (20 °C) conditions—200 µM of MeJA inhibited plant growth and reduced photosynthesis, stomatal conductance, and chlorophyll content under HS. To dissect the role of various regulatory TFs controlled by JA in response to HS, Dang et al. (2013) showed that external application of JA enhanced CaWRKY40 gene activity, conferring HS tolerance in pepper plants. Similarly, JA alleviated the negative effects of combined high light and HS by inducing several regulatory genes, viz., bZIP3, BHLH114, BHLH137, and WRKY8, which might allow plants to withstand heat and high light stress (Balfagón et al. 2019). Recently, Tian et al. (2020) reported the role of JAs in mediating heat tolerance through the DREB2A regulatory gene. Interplay/crosstalk of JA with other phytohormones mediating plant growth and development, complex signaling, and various biotic and abiotic stresses has been established (Raza et al. 2020). Synergistic and antagonistic effects of JA with other phytohormones play an essential role in providing abiotic and biotic stress tolerance in plants (Wang et al. 2020). The molecular role of JA in concert with SA imparting basal thermotolerance has been established in Arabidopsis by assessing electrolyte leakage in HS-treated double mutants, cpr5‐1 npr1‐1 cpr5‐1 jar1‐1, and the corresponding single mutant (Clarke et al. 2009). Similarly, Tsai et al. (2019) advocated that JA is associated with SA-enhanced heat tolerance and tomato yellow leaf curl virus resistance in tomato. Nevertheless, JA acts as an antagonist to ET for governing the HS response (Sharma and Laxmi 2016). The molecular mechanism and signaling of JAs and their crosstalk with other phytohormones controlling abiotic stresses, including HS responses, remain unknown (Yang et al. 2019). Furthermore, insights into the regulatory mechanisms of JAZ and JAV1 repressor proteins involved in regulating the transcription of various JA-responsive genes under various stresses, including HS, could improve our understanding of how JA is involved in plant HS adaptation (Raza et al. 2020).
Embracing Genetic Engineering for Manipulating Phytohormone-Responsive Gene(s) Regulating Heat Tolerance
Genetic engineering/transgenics is a powerful approach for deciphering the functional roles of various regulatory and phytohormone-responsive gene(s) contributing to HS tolerance (Cheng et al. 2013; Muller and Munne-Bosch 2015). Manipulation of phytohormone-responsive gene(s) could be a novel avenue for designing heat-tolerant crops. Previously, Alonso-Ramírez et al. (2009) monitored overexpression of the beechnut (Fagus sylvatica) GA-responsive gene FsGASA4 that induces endogenous SA, imparting higher thermotolerance in transgenic Arabidopsis. Likewise, in transgenic Arabidopsis, overexpression of ERF1, an ERF TF involved in ET signaling and response-mediated HS tolerance, upregulated the AtHsfA3 heat shock factor and various heat shock protein genes, viz., HSP23.6, HSP70, and HSP17.6A (Cheng et al. 2013). Youm et al. (2008) advocated the overexpression role of CaPF1, a pepper transcription factor ERF gene contributing to higher heat tolerance in transgenic potato. Similarly, overexpression of HSFA6b, serving as a positive regulator for ABA signaling, exhibited increased heat tolerance in transgenic Arabidopsis (Huang et al. 2016). Overexpression of the AtDWF4 gene involved in BR biosynthesis conferred HS tolerance in transgenic B. napus (Sahni et al. 2016). Overexpression of the Triticum aestivum BR-insensitive1 (TaBRI1) gene imparts thermotolerance in transgenic Arabidopsis by increasing membrane stability under HS (Singh et al. 2016). However, transgenic Arabidopsis containing the NahG (encoding salicylate hydrolase) gene is depleted of SA under HS, increasing electrolyte leakage due to heat shock and oxidative stress-mediated damage, indicating the essential role of SA in plant basal thermotolerance (Clarke et al. 2004). Increased expression of the tomato NAC transcription factor (SIJA2) in transgenic tobacco decreased heat tolerance due to a reduction in SA accumulation (Liu et al. 2017), suggesting a vital role of SA in conferring heat tolerance. Technological innovations, viz., next-generation sequencing, could unveil phytohormone-responsive candidate regulatory genes/noncoding RNAs regulating HS tolerance across genomes (Wang et al. 2019). Furthermore, augmentation CRISPR/Cas9-based genome editing is a promising approach for precisely modifying phytohormone-responsive TFs/genomic regions controlling the HS response for the future design of heat-tolerant crop plants (LeBlanc et al. 2018; Debbarma et al. 2019).
Perspective and Conclusion
Increasing HS events is a major issue for overall plant growth, causing great concern for global food security (Wahid et al. 2007). Among the various strategies for developing heat-tolerant crops, the role of phytohormones in mitigating the effect of HS deserves attention.
Exogenous application of various phytohormones could improve plant performance by protecting key physiological processes, viz., photosynthesis by minimizing photoinhibition, lowering lipid peroxidation, and stimulating antioxidant mechanisms under HS (Sharma et al. 2019). However, the complex molecular mechanisms of various phytohormones contributing to morpho-physiological adaptations in plants, such as minimizing ROS activity, rescuing pollen fertility, and protecting spikelet fertility, grain-filling process, and grain weights under HS need greater attention. Phytohormones regulate various biological regulatory pathways and processes related to HS. Phytohormones can switch on the signaling of various regulatory gene(s) under HS. Simultaneously, crosstalk/interactions of various phytohormones play a pivotal role in regulating the HS response in plants; however, understanding their complete molecular mechanisms controlling HS tolerance remains elusive. Among the phytohormone-based strategies for developing HS-tolerant crop plants, phytohormone priming could improve plant’s adaptation to HS. However, the bioformulations need rigorous testing under laboratory conditions before applying in the field. Likewise, manipulating phytohormone-responsive TFs/genomic regions controlling the HS response through genetic engineering/genome editing technologies is an emerging approach for designing HS-tolerant crops. As the role of phytohormone signaling and crosstalk in regulating plant growth in response to HS is complex, it merits further investigation (Verma et al. 2016). Moreover, the underlying regulatory genes and gene networks stimulated by the phytohormones in response to HS need further research.
References
Abdelrahman M, El-Sayed M, Jogaiah S, Burritt DJ, Tran L-SP (2017) The “STAY- GREEN” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep 36:1009–1025
Ahanger MA, Ashraf M, Bajguz A, Ahmad P (2018) Brassinosteroids regulate growth in plants under stressful environments and crosstalk with other potential phytohormones. J Plant Growth Regul 37:1007–1024
Ahanger MA, Mir RA, Alyemeni MN, Ahmad P (2020) Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol Biochem 147:31–42
Ahmad P, Alyemeni MN, Ahanger MA, Egamberdieva D, Wijaya L, Alam P (2018a) Salicylic acid (SA) induced alterations in growth, biochemical attributes and antioxidant enzyme activity in faba bean (Vicia faba L.) seedlings under NaCl toxicity. Russian J Plant Physiol 65:104–114
Ahmad P, Abd-Allah EF, Alyemeni MN, Wijaya L, Alam P, Bhardwaj R, Siddique KH (2018b) Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci Rep 8:1–5
Ahmad P, Ahanger MA, Egamberdieva D, Alam P, Alyemeni MN, Ashraf M (2018c) Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J Plant Growth Regul 37:309–322
Ahmad B, Zaid A, Sadiq Y, Bashir S, Wani SH (2019) Role of selective exogenous elicitors in plant responses to abiotic stress tolerance. Plant abiotic stress tolerance. Springer, Cham, pp 273–290
Albertos P, Wagner K, Poppenberger B (2019) Cold stress signalling in female reproductive tissues. Plant Cell Environ 42:846–853
Ali MS, Baek KH (2020) Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int J Mol Sci 21:621
Alonso-Ramírez A, Rodríguez D, Reyes D, Jiménez JA, Nicolás G, López-Climent M, Gómez-Cadenas A, Nicolás C (2009) Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol 150:1335–1344
An Y, Zhou P, Liang J (2014) Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci 65:274–286
Anwar A, Liu Y, Dong R, Bai L, Yu X, Li Y (2018) The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol Res 51:46
Asthir B, Bhatia S (2014) In vivo studies on artificial induction of thermotolerance to detached panicles of wheat (Triticum aestivum L.) cultivars under heat stress. J Food Sci Technol 51:118–123
Balfagón D, Sengupta S, Gómez-Cadenas A, Fritschi FB, Rajeev K (2019) Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol 181:1668–1682
Banowetz GM, Ammar K, Chen DD (1999) Postanthesis temperatures influence cytokinin accumulation and wheat kernel weight. Plant Cell Environ 22:309–316
Bastam N, Baninasab B, Ghobadi C (2013) Improving salt tolerance by exogenous application of salicylic acid in seedlings of pistachio. Plant Growth Regul 69:275–284
Bheemanahalli R, Impa SM, Krassovskaya I, Vennapusa AR, Gill KS, Obata T, Jagadish SVK (2020) Enhanced N-metabolites, ABA and IAA-conjugate in anthers instigate heat sensitivity in spring wheat. Physiol Plant. https://doi.org/10.1111/ppl.13109
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273
Brewer PB, Koltai H, Beveridge CA (2013) Diverse roles of strigolactones in plant development. Mol Plant 6:18–28
Browse J (2009) Jasmonate passes muster: a receptor and targets for the defence hormone. Annu Rev Plant Biol 60:183–205
Campbell JL, Klueva NY, Zheng H, Nieto-Sotelo J, Ho THD, Nguyen HT (2001) Cloning of new members of heat shock protein HSP101 gene family in wheat (Triticum aestivum (L.) Moench) inducible by heat, dehydration, and ABA. Biochim Biophys Acta Gene Struct Expr 1517:270–277
Cao YY, Zhao H (2008) Protective roles of brassinolide in rice seedlings under heat stress. Rice Sci 15:63–68
Cecchetti V, Altamura MM, Falasca G, Costantino P, Cardarelli M (2008) Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 20:1760–1774
Cerny M, Jedelsky PL, Novak J, Schlosser A, Brzobohaty B (2014) Cytokinin modulates proteomic, transcriptomic and growth responses to temperature shocks in Arabidopsis. Plant Cell Environ 37:1641–1655. https://doi.org/10.1111/pce.12270
Chakrabarti N, Mukherji S (2003) Alleviation of NaCl stress by pretreatment with phytohormones in Vigna radiata. Biol Plant 46:589–594
Cheikh N, Jones RJ (1994) Disruption of maize kernel growth and development by heat stress (role of cytokinin/abscisic acid balance). Plant Physiol 106:45–51
Chen T, Li G, Islam MR et al (2019) Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biol 19:525
Chen D, Zhao J (2008) Free IAA in stigmas and styles during pollen germination and pollen tube growth of Nicotiana tabacum. Physiol Plant 134:202–215
Cheng C-Y, Lur H-S (1996) Ethylene may be involved in abortion of the maize caryopsis. Physiol Plant 98:245–252
Cheng MC, Liao PM, Kuo WW, Lin TP (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol 162:1566–1582
Cho SH, Von Schwartzenberg K, Quatrano R (2018) The role of abscisic acid in stress tolerance. Ann Plant Rev 36:282–297
Clarke SM, Mur LA, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38:432–447
Clarke SM, Cristescu SM, Miersch O, Harren FJ, Wasternack C, Mur LA (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182:175–187
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Ann Rev Plant Physiol Plant Mol Biol 49:427–451
Dang FF, Wang YN, Yu L, Eulgem T, Lai Y, Liu ZQ et al (2013) CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ 36:757–774
Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H (2015) Jasmonates counter plant stress: a review. Environ Exp Bot 115:49–57
Daszkowska-Golec A, Szarejko I (2013) Open or close the gate: stomata action under the control of phytohormones in drought stress conditions. Front Plant Sci 4:138
Dat JF, Foyer CH, Scott IM (1998a) Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol 118:455–461
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998b) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116:1351–1357
Debbarma J, Sarki YN, Saikia B, Boruah HP, Singha DL, Chikkaputtaiah C (2019) Ethylene response factor (ERF) family proteins in abiotic stresses and CRISPR–Cas9 genome editing of ERFs for multiple abiotic stress tolerance in crop plants: a review. Mol Biotechnol 61:153–172
Deokar AA, Kondawar V, Kohli D, Aslam M, Jain PK, Karuppayil SM, Varshney RK, Srinivasan R (2015) The CarERF genes in chickpea (Cicer arietinum L.) and the identification of CarERF116 as abiotic stress responsive transcription factor. Funct Integr Genomics 15:27–46
Deryng D, Conway D, Ramankutty N, Price J, Warren R (2014) Global crop yield response to extreme heat stress under multiple climate change futures. Environ Res Lett 9:034011
De Block M, Van Lijsebettens M (2011) Energy efficiency and energy homeostasis as genetic and epigenetic components of plant performance and crop productivity. Curr Opin Plant Biol 14:275–282
de Saint GA, Ligerot Y, Dun EA, Pillot JP, Ross JJ, Beveridge CA, Rameau C (2013) Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol 163:1012–1025
Dhaubhadel S, Browing KS, Gillic DR, Krishna P (2002) Brassinosteroid function to protect the translational machinery and heat shock protein synthesis following thermal stress. Plant J 29:681–691
Dhaubhadel S, Chaudhary S, Dobinson KF et al (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342
Ding W, Song L, Wang X, Bi Y (2010) Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis. Biol Plant 54:607–613
Ding P, Ding Y (2020) Stories of salicylic acid: a plant defense hormone. Trend Plant Sci 25:549–565
Divi UK, Rahman T, Krishna P (2010) Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol 10:151–165
Easterling WE, Aggarwal PK, Batima P et al (2007) Food, fibre and forest products. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (eds) Climate change 2007: impacts, adaptation and vulnerability contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 273–313
Fahad S, Bano A (2012) Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak J Bot 44:1433–1438
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Huang J (2015a) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul. https://doi.org/10.1007/s10725-014-0013-y
Fahad S, Nie L, Chen Y, Wu C, Xiong D, Saud S, Hongyan L, Cui K, Huang J (2015b) Crop plant hormones and environmental stress. Sustain Agric Rev 15:371–400
FAO Report (2009) http://en.wikipedia.org/wiki/Climate_change_and_agriculture. Accessed 18 June 2020
Feng BH, Zhang CX, Chen TT, Zhang XF, Tao LX, Fu GF (2018) Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol 18:245
Firon N, Pressman E, Meir S, Khoury R, Altahan L (2012) Ethylene is involved in maintaining tomato (Solanum lycopersicum) pollen quality under heat-stress conditions. AoB Plants. https://doi.org/10.1093/aobpla/pls024
Franklin KA, Sang HL, Patel D, Kumar SV, Spartz AK, Gu C, Ye S, Yu P, Breen G, Cohen JD, Wigge PA, Gray WM (2011) Phytochrome-interacting factor 4 (pif4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci 108:20231–20235
Fu G, Zhang C, Yang X, Yang Y, Chen T, Zhao X, Fu W, Feng B, Zhang X, Tao L, Jin Q (2015) Action mechanism by which SA alleviates high temperature induced inhibition to spikelet differentiation. Chin J Rice Sci 29:637–647
Galani S, Hameed S, Ali MK (2016) Exogenous application of salicylic acid: inducing thermotolerance in cotton (Gossypium hirsutum L.) seedlings. Intl J Agric Food Res 5:9–18
Ghanem ME, Albacete A, Smigocki AC, Frébort I, Pospísilová H, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Lutts S, Dodd IC, Pérez-Alfocea F (2011) Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 62:125–140
Gong M, Li Y-J, Chen S-Z (1998) Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol 153:488–496
Gonzalez-Olmedo JL, Cordova A, Aragon CE, Pina D, Rivas M, Rodrıguez R (2005) Effect of an analogue of brassinosteroid on FHIA-18 plantlets exposed to thermal stress. InfoMusa 14:18–20
Gourdji SM, Sibley AM, Lobell DB (2013) Global crop exposure to critical high temperatures in the reproductive period: historical trends and future projections. Environ Res Lett 8:024041
Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyls elongation in Arabidopsis. Proc Natl Acad Sci USA 95:7197–7202
Grove MD, Spencer GF, Rohwedder WK, Mandava N et al (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217
Grubb CD, Zipp BJ, Ludwig-Müller J, Masuno MN, Molinski TF, Abel S (2004) Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J 40:893–908
Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran L-SP (2012) Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci 17:172–179
Habben JE, Bao X, Bate NJ, DeBruin JL, Dolan D, Hasegawa D, Helentjaris TG, Lafitte RH, Lovan N, Mo H, Reimann K (2014) Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol J 12:685–693
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Intl J Mol Sci 14:9643–9684
Hayat S, Masood A, Yusf M, Fariduddin Q, Ahmad A (2009) Growth of Indian mustard (Brassica juncea L.) in response to salicylic acid under high-temperature stress. Braz J Plant Physiol 21:187–195
Hayat S, Mori M, Fariduddin Q, Bajguz A, Ahmad A (2010) Physiological role of brassinosteroids: an update. Indian J Plant Physiol 15:99–109
Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci 172:1113–1123
He P, Jin JY (1999) Relationships among hormone changes, transmembrane flux of Ca2+ and lipid peroxidation during leaf senescing in spring maize. Acta Bot Sin 41:1221–1225
Hsu YT, Kao CH (2003) Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 26:867–874
Hu XJ, Chen D, Mclntyre CL, Dreccer MF, Zhang ZB, Drenth J, Sundaravelpandian K, Chang H, Xue GP (2018a) Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ 41:79–98
Hu Q, Zhang S, Huang B (2018b) Strigolactones and interaction with auxin regulating root elongation in tall fescue under different temperature regimes. Plant Sci 271:34–39
Hu Q, Zhang S, Huang B (2019) Strigolactones promote leaf elongation in tall fescue through upregulation of cell cycle genes and downregulation of auxin transport genes in tall fescue under different temperature regimes. Int J Mol Sci 20:1836
Huang YC, Niu CY, Yang CR, Jinn TL (2016) The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol 172:1182–1199
Huang Y, Sun MM, Ye Q, Wu XQ, Wu WH, Chen YF (2017) Abscisic acid modulates seed germination via ABA INSENSITIVE5-mediated PHOSPHATE1. Plant Physiol 175:1661–1668
Huang J, Zhao X, Bürger M, Wang Y, Chory J (2020) Two interacting ethylene response factors regulate heat stress response. Plant Cell. https://doi.org/10.1093/plcell/koaa026
Huberman M, Riov J, Goldschmidt EE, Apelbaum A, Goren R (2013) The novel ethylene antagonist, 3-cyclopropyl-1-enyl-propanoic acid sodium salt (CPAS), increases grain yield in wheat by delaying leaf senescence. Plant Growth Regul 73:249–255
Hussain M, Khan TA, Yusuf M, Fariduddin Q (2019) Silicon-mediated role of 24-epibrassinolide in wheat under high-temperature stress. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-04938-0
Hwang I, Sheen J, Müller B (2012) Cytokinin signaling networks. Annu Rev Plant Biol 63:353–380
Ivanov AG, Kitcheva MI, Christov AM, Popova LP (1992) Effects of abscisic acid treatment on the thermostability of the photosynthetic apparatus in barley chloroplasts. Plant Physiol 98:1228–1232
Jahan MS, Wang Y, Shu S, Zhong M, Chen Z, Wu J, Sun J, Guo S (2019) Exogenous salicylic acid increases the heat tolerance in tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci Hortic 247:421–429
Jan S, Noman A, Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) 24-Epibrassinolide alleviates the injurious effects of Cr (VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity and regulation of ascorbate–glutathione and glyoxalase cycles. J Plant Growth Regul 39:1587–1604
Janda T, Lejmel MA, Molnar AB, Majlath I, Pal M, Nguyen QT et al (2020) Interaction between elevated temperature and different types of Na-salicylate treatment in Brachypodium dystachion. PLoS ONE 15:e0227608
Janeczko A, Oklešťková J, Pociecha E, Kościelniak J, Mirek M (2011) Physiological effects and transport of 24-epibrassinolide in heat-stressed barley. Acta Physiol Plant 33:1249–1259
Javid MG, Sorooshzadeh A, Moradi F, Sanavy SAMM, Allahdadi I (2011) The role of phytohormones in alleviating salt stress in crop plants. Aust J Crop Sci 5:726–734
Jegadeesan S, Beery A, Altahan L et al (2018) Ethylene production and signaling in tomato (Solanum lycopersicum) pollen grains is responsive to heat stress conditions. Plant Reprod 31:367–383
Jeon J, Kim NY, Kim S, KangNY NO, Ku SJ, Cho C, Lee DJ, Lee EJ, Strnad M, Kim JA (2010) subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J Biol Chem 285:23371–23386
Jha UC, Bohra A, Singh NP (2014) Heat stress in crop plants: its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed 133:679–701
Jia KP, Luo Q, He SB, Lu XD, Yang HQ (2014) Strigolactone-regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis. Mol Plant 7:528–540
Kang M, Lee S, Abdelmageed H, Reichert A, Lee HK, Fokar M, Mysore KS, Allen RD (2017) Arabidopsis stress associated protein 9 mediates biotic and abiotic stress responsive ABA signaling via the proteasome pathway. Plant Cell Environ 40:702–716
Kaur P, Ghai N, Sangha MK (2009) Induction of thermotolerance through heat acclimation and salicylic acid in Brassica species. Afr J Biotech 8:619–625
Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KH, Ahmad P (2018) 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt-and temperature-induced oxidative stress in Brassica juncea. Sci Rep 8:1–3
Kaya C, Ashraf M, Wijaya L, Ahmad P (2019) The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol Biochem 143:119–128
Kaya C, Ashraf M, Alyemeni MN, Corpas FJ, Ahmad P (2020a) Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J Hazard Mater 399:123020
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020b) Nitrate reductase rather than nitric oxide synthase activity is involved in 24-epibrassinolide-induced nitric oxide synthesis to improve tolerance to iron deficiency in strawberry (Fragaria × annassa) by up-regulating the ascorbate-glutathione cycle. Plant Physiol Biochem 151:486–499
Kazan K, Manners JM (2012) JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci 17:22e31
Kazan K (2015) Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 20:219–229
Khan MI, Iqbal N, Masood A, Per TS, Khan NA (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav 8:e26374. https://doi.org/10.4161/psb.26374
Khan A, Bilal S, Khan AL, Imran M et al (2020) Silicon and gibberellins: synergistic function in harnessing aba signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 9:620. https://doi.org/10.3390/plants9050620
Khanna P, Kaur K, Gupta AK (2016) Salicylic acid induces differential anti-oxidant response in spring maize under high temperature stress. Indian J Exp Biol 54:386–393
Kim HB, Kwon M, Ryu H, Fujioka S, Takatsuto S, Yoshida S, An CS et al (2006) The regulation of DWARF4 expression is likely a critical mechanism in maintaining the homeostasis of bioactive brassinosteroids in Arabidopsis. Plant Physiol 140:548–557
Knox J, Hess T, Daccache A, Wheeler T (2012) Climate change impacts on crop productivity in Africa and South Asia. Environ Res Lett 7:034032
Ko CB, Woo YM, Lee DJ, Lee MC, Kim CS (2007) Enhanced tolerance to heat stress in transgenic plants expressing the GASA4 gene. Plant Physiol Biochem 45:722–728
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Wijaya L, Alyemeni MN, Ahmad P (2018a) Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ Sci Pollut Res 25:15159–15173
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2018b) Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 255:11–24
Kohli SK, Bali S, Tejpal R, Bhalla V, Verma V, Bhardwaj R, Alqarawi AA, Abd-Allah EF, Ahmad P (2019) In-situ localization and biochemical analysis of bio-molecules reveals Pb-stress amelioration in Brassica juncea L. by co-application of 24-epibrassinolide and salicylic acid. Sci Rep 9:1–5
Kousar R, Qureshi R, Jalal-Ud-Din MM, Shabbir G (2018) Salicylic acid mediated heat stress tolerance in selected bread wheat genotypes of Pakistan. Pak J Bot 50:2141–2146
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297
Kucharik CJ, Serbin SP (2008) Impacts of recent climate change on Wisconsin corn and soybean yield trends. Environ Res Lett 3:034003
Kumar S, Kaushal N, Nayyar H, Gaur P (2012) Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiol Plant 34:1651–1658
Kumar RR, Sharma SK, Goswami S, Verma P, Singh K, Dixit N, Pathak H, Viswanathan C, Rai RD (2015) Salicylic acid alleviates the heat stress-induced oxidative damage of starch biosynthesis pathway by modulating the expression of heat-stable genes and proteins in wheat (Triticum aestivum). Acta Physiol Plant 37:1–12
Kwon C-T, Paek N-C (2016) Gibberellic acid: a key phytohormone for spikelet fertility in rice grain production. Intl J Mol Sci 17:794
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:682–695
Larkindale J, Huang B (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161:405–413
Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138:882–897
LeBlanc C, Zhang F, Mendez J, Lozano Y, Chatpar K, Irish VF, Jacob Y (2018) Increased efficiency of targeted mutagenesis by CRISPR/Cas9 in plants using heat stress. Plant J 93:377–386
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529:84–87
Li S, Zhou X, Chen L, Huang W, Yu D (2010) Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol Cells 29:475–483
Li H, Liu SS, Yi CY, Wang F, Zhou J, Xia XJ, Shi K, Zhou YH, Yu JQ (2014) Hydrogen peroxide mediates abscisic acid-induced hsp70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ 37:2768–2780
Li Z-G, Xie L-R, Li X-J (2015) Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J Plant Physiol 177:121–127. https://doi.org/10.1016/j.jplph.2014.12.018
Li ZG (2015) Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal Behav 10:e105-1278
Li GY, Zhang CX, Zhang GH, Fu WM, Feng BH, Chen TT et al (2020) Abscisic acid negatively modulates heat tolerance in rolled leaf rice by increasing leaf temperature and regulating energy homeostasis. Rice 13:18
Liu XZ, Huang BR (2002) Cytokinin effects on creeping bentgrass response to heat stress: II. leaf senescence and antioxidant metabolism. Crop Sci 42:466–472
Liu ZM, Yue MM, Yang DY, Zhu SB, Ma NN, Meng QW (2017) Over-expression of SlJA2 decreased heat tolerance of transgenic tobacco plants via salicylic acid pathway. Plant Cell Rep 36:529–542
Lobell DB, Field CB (2007) Global scale climate–crop yield relationships and the impacts of recent warming. Environ Res Lett 2:014002
Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, Naylor RL (2008) Prioritizing climate change adaptation needs for food security in 2030. Science 319:607–610
Lobell DB, Banziger M, Magorokosho C, Vivek B (2011) Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat Clim Chang 1:42–45
Lopez-Delgado H, Dat JF, Foyer CH, Scott IM (1998) Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J Exp Bot 49:713–720
Lozano-Durán R, Zipfel C (2015) Trade-off between growth and immunity: role of brassinosteroids. Trends Plant Sci 20:12–19
Luo Q (2011) Temperature thresholds and crop production: a review. Clim Chang 109:583–598
Maharjan PM, Choe S (2011) High temperature stimulates DWARF4 (DWF4) expression to increase hypocotyl elongation in Arabidopsis. J Plant Biol 54:425–429
Macková H, Hronková M, Dobrá J, Turečková V, Novák O, Lubovská Z, Motyka V, Haisel D, Hájek T, Prášil IT, Gaudinová A (2013) Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J Expt Bot 64:2805–2815
Makarova S, Makhotenko A, Spechenkova N, Love AJ, Kalinina NO, Taliansky M (2018) Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with potato virus Y. Front Microbiol 9:2582
Mazorra LM, Holton N, Bishop GJ, Núñez M (2011) Heat shock response in tomato brassinosteroid mutants indicates that thermotolerance is independent of brassinosteroid homeostasis. Plant Physiol Biochem 49:1420–1428
Mei C, Qi M, Sheng G, Yang Y (2006) Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact 19:1127–1137
Min L, Li Y, Hu Q, Zhu L, Gao W, Wu Y et al (2014) Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol 164:1293–1308
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819:86–96
Muller M, Munne-Bosch S (2015) Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol 169:32–41
Nakaya M, Tsukaya H, Murakami N, Kato M (2002) Brassinosteroids control the proliferation of leaf cells of Arabidopsis thaliana. Plant Cell Physiol 43:239
Narusaka Y, Nakashima K, Shinwari ZK, Sakuma Y, Furihata T, Abe H, Narusaka M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J 34:137–148
Navneet K, Jagmeet K, Kaur GS, Inderjit S (2019) Effect of heat stress on antioxidative defense system and its amelioration by heat acclimation and salicylic acid pre-treatments in three pigeonpea genotypes. Indian J Agric Biochem 32:106–110
Neill EM, Byrd MCR, Billman T et al (2019) Plant growth regulators interact with elevated temperature to alter heat stress signaling via the unfolded protein response in maize. Sci Rep 9:10392
Nolan T, Chen J, Yin Y (2017) Cross-talk of brassinosteroid signaling in controlling growth and stress responses. Biochem J 474:2641–2661
Nolan T, Vukasinovic N, Liu D, Russinova E, Yin Y (2020) Brassinosteroids: multi-dimensional regulators of plant growth, development, and stress responses. Plant Cell. https://doi.org/10.1105/tpc.19.00335
Northey JGB, Liang S, Jamshed M, Deb S, Foo E, Reid JB et al (2016) Farnesylation mediates brassinosteroid biosynthesis to regulate abscisic acid responses. Nat Plants 2:16114
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S (2007) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57
Ogweno JO, Hu WH, Song XS, Shi K, Mao WH, Zhou YH, Yu JQ (2010) Photoinhibition-induced reduction in photosynthesis is alleviated by abscisic acid, cytokinin and brassinosteroid in detached tomato leaves. Plant Growth Regul 60:175–182
Oshino T, Miura S, Kikuchi S, Hamada K, Yano K, Watanabe M, Higashitani A (2011) Auxin depletion in barley plants under high-temperature conditions represses DNA proliferation in organelles and nuclei via transcriptional alterations. Plant Cell Environ 34:284–290
Pan Y, Seymour GB, Lu C, Hu Z, Chen X, Chen G (2012) An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep 31:349–360
Pan C, Zhang H, Ma Q et al (2019) Role of ethylene biosynthesis and signaling in elevated CO2-induced heat stress response in tomato. Planta 250:563–572
Pareek A, Sigla SL, Grover A (1998) Protein alterations associated with salinity, desiccation, high and low temperature stresses and abscisic acid application in seedlings of Pusa 169, a high-yielding rice (Oryza sativa L.) cultivar. Curr Sci 75:1023–1035
Pazhamala LT, Chaturvedi P, Bajaj P, Srikanth S, Ghatak A, Chitikineni A, Bellaire A, Hingane A, Kumar CS, Saxena KB, Weckwerth W (2020) Multiomics approach unravels fertility transition in a pigeonpea line for a two-line hybrid system. Plant Genome 13:e20028
Per TS, Khan MIR, Anjum NA, Masood A, Hussain SJ, Khan NA (2018) Jasmonates in plants under abiotic stresses: crosstalk with other phytohormones matters. Environ Exp Bot 145:104–120
Pirnajmedin F, Majidi MM, Taleb H et al (2020) Amelioration of high temperature stress by exogenously applied salicylic acid: genotype-specific response of physiological traits. Agron J. https://doi.org/10.1002/agj2.20150
Prerostova S, Dobrev PI, Kramna B et al (2020) Heat acclimation and inhibition of cytokinin degradation positively affect heat stress tolerance of Arabidopsis. Front Plant Sci 11:87
Raza A, Charagh S, Zahid Z, Mubarik MS, Javed R, Siddiqui MH, Hasanuzzaman M (2020) Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. https://doi.org/10.1007/s00299-020-02614-z
Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E (2013) Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol 163:1609–1622
Rezaul IM, Baohua F, Tingting C, Weimeng F, Caixia Z, Longxing T et al (2019) Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol Plant 165:644–663
Rezaul IM, Baohua F, Tingting C, Longxing T, Guanfu F (2018) Role of abscisic acid in thermal acclimation of plants. J Plant Biol 61:255–264
Rivero RM, Gimeno J, VanDeynze A, Walia H, Blumwald E (2010) Enhanced cytokinin synthesis in tobacco plants expressing PSARK::IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol 51:1929–1941
Robertson AJ, Ishikawa M, Gusta LV, MacKenzie SL (1994) Abscisic acid-induced heat tolerance in Bromus inermis leyss cell-suspension cultures. Heat-stable, abscisic acid-responsive polypeptides in combination with sucrose confer enhanced thermostability. Plant Physiol 105:181–190
Rubio S, Noriega X, Pérez FJ (2018) Abscisic acid (ABA) and low temperatures synergistically increase the expression of CBF/DREB1 transcription factors and cold-hardiness in grapevine dormant buds. Ann Bot 20:1–9
Sadiq Y, Zaid A, Khan MM (2020) Adaptive physiological responses of plants under abiotic stresses: role of phytohormones. Plant ecophysiology and adaptation under climate change: mechanisms and perspectives. Springer, Singapore, pp 797–824
Sadura I, Pociecha E, Dziurka M et al (2019) Mutations in the HvDWARF, HvCPD and HvBRI1 genes-involved in brassinosteroid biosynthesis/signalling: altered photosynthetic efficiency, hormonal homeostasis and tolerance to high/low temperatures in barley. J Plant Growth Regul 38:1062–1081
Sahni S, Prasad BD, Liu Q, Grbic V, Sharpe A, Singh SP, Krishna P (2016) Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci Reps 6:1–4
Sakakibara H (2010) Cytokinin biosynthesis and metabolism. In: Davies PJ (ed) The plant hormones: biosynthesis, signal transduction, action, 3rd edn. Springer, Dordrecht, pp 95–114
Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A (2010) Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci USA 107:8569–8574
Saleh AAH, Abdel-Kader DZ, El Elish AM (2007) Role of heat shock and salicylic acid in antioxidant homeostasis in mungbean (Vigna radiata L.) plant subjected to heat stress. Am J Plant Physiol 2:344–355
Sarkar S, Perras MR, Falk DE, Zhang R, Pharis RP, Fletcher RA (2004) Relationship between gibberellins, height, and stress tolerance in barley (Hordeum vulgare L.) seedlings. J Plant Growth Regul 42:125–135
Sarwar M, Saleem MF, Ullah N, Rizwan M, Ali S, Shahid MR, Alamri SA, Alyemeni MN, Ahmad P (2018) Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci Rep 8:17086
Savchenko TV, Rolletschek H, Dehesh K (2019) Jasmonates-mediated rewiring of central metabolism regulates adaptive responses. Plant Cell Physiol 60:2613–2620
Schlenker W, Roberts MJ (2009) Nonlinear temperature effects indicate severe damages to US crop yields under climate change. Proc Natl Acad Sci USA 106:15594–15598
Shahzad R, Waqas M, Khan AL, Hamayun M, Kang S-M, Lee I-J (2015) Foliar application of methyl jasmonate induced physio- hormonal changes in Pisum sativum under diverse temperature regimes. Plant Physiol Biochem 96:406–416
Sharma M, Laxmi A (2016) Jasmonates: emerging players in controlling temperature stress tolerance. Front Plant Sci 6:1129
Sharma L, Dalal M, Verma RK, Kumar SVV, Yadav SK, Pushkar S, Kushwaha SR, Bhowmik A, Chinnusamy V (2018) Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ Expt Bot 150:9–24
Sharma L, Priya M, Kaushal N, Bhandhari K, Chaudhary S, Dhankher PO, Vara Prasad PV, Siddique HMK, Nayyar H (2019) Plant growth regulating molecules as thermoprotectants: functional relevance and prospects for improving heat tolerance in food crops. J Exp Bot 71:569–594
Sharma A, Thakur S, Kumar V, Kanwar MK, Kesavan AK, Thukral AK, Bhardwaj R, Alam P, Ahmad P (2016) Pre-sowing seed treatment with 24-epibrassinolide ameliorates pesticide stress in Brassica juncea L. through the modulation of stress markers. Front Plant Sci 7:1569
Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. Plant Growth Regul 47:111–119
Singh A, Breja P, Khurana JP, Khurana P (2016) Wheat brassinosteroid-insensitive1 (TaBRI1) interacts with members of TaSERK gene gamily and cause early flowering and seed yield enhancement in Arabidopsis. PLoS ONE 11:e0153273
Singh G, Sarkar NK, Grover A (2021) Tango between Ethylene and HSFA2 Settles Heat Tolerance. Trends in Plant Science. Trends Plant Sci S1360–1385:00062–5
Skalák J, Černý M, Jedelský P, Dobrá J, Ge E, Novák J, Hronková M, Dobrev P, Vanková R, Brzobohatý B (2016) Stimulation of ipt overexpression as a tool to elucidate the role of cytokinins in high temperature responses of Arabidopsis thaliana. J Expt Bot 67:2861–2873
Song G, Wang M, Zeng B, Zhang J, Jiang C, Hu Q, Geng G, Tang C (2015) Anther response to high-temperature stress during development and pollen thermotolerance heterosis as revealed by pollen tube growth and in vitro pollen vigor analysis in upland cotton. Planta 241:1271–1285
Stavang JA, Gallego-Bartolomé J, Gómez MD, Yoshida S, Asami T, Olsen JE, García-Martínez JL, Alabadí D, Blázquez MA (2009) Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J 60:589–601
Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55:197–223
Sun J, Qi L, Li Y, Chu J, Li C (2012) PIF4–mediated activation of YUCCA8, expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet 8:e1002594
Suzuki N, Bajad S, Shuman J et al (2008) The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J Biol Chem 283:9269–9275
Suzuki N, Bassil E, Hamilton JS, Inupakutika MA, Zandalinas SI, Tripathy D et al (2016) ABA is required for plant acclimation to a combination of salt and heat stress. PLoS ONE 11:e0147625. https://doi.org/10.1371/journal.pone.0147625
Swaminathan MS, Kesavan PC (2012) Agricultural research in an era of climate change. Agric Res 1:3–11
Tang RS, Zheng JC, Jin ZQ, Zhang DD, Huang YH, Chen LG (2008) Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54:37–43
Teale WD, Paponov IA, Palme K (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7:847–859
Teixeira EI, Fischer G, van Velthuizen H, Walter C, Ewert F (2013) Global hot-spots of heat stress on agricultural crops due to climate change. Agric Forest Meteor 170:206–215
Tesfaye K, Zaidi PH, Gbegbelegbe S, Boeber C et al (2016) Climate change impacts and potential benefits of heat-tolerant maize in South Asia. Theor Appl Climatol 130:959–970
Thomas H, Howarth CJ (2000) Five ways to stay green. J Exp Bot 51:329–337
Thussagunpanit J, Jutamanee K, Sonjaroon W, Kaveeta L, Chai-Arree W, Pankean P, Suksamrarn A (2015a) Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynth 53:312–320
Thussagunpanit J, Jutamanee K, Kaveeta L, Chai-arree W, Pankean P, Homvisasevongsa S, Suksamrarn A (2015b) Comparative effects of brassinosteroid and brassinosteroid mimic on improving photosynthesis, lipid peroxidation, and rice seed set under heat stress. J Plant Growth Regul 34:320–331
Tian X, Wang F, Zhao Y et al (2020) Heat shock transcription factor A1b regulates heat tolerance in wheat and Arabidopsis through OPR3 and jasmonate signalling pathway. Plant Biotech J18:1109–1111
Tognetti VB, Mühlenbock PE, Van Breusegem F (2012) Stress homeostasis–the redox and auxin perspective. Plant Cell Environ 35:321–333
Toh S, Kamiya Y, Kawakami N, Nambara E, McCourt P, Tsuchiya Y (2012) Thermo inhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol 53:107–117
Tsai WA, Weng SH, Chen MC, Lin JS, Tsai WS (2019) Priming of plant resistance to heat stress and tomato yellow leaf curl Thailand virus with plant-derived materials. Front Plant Sci 10:906
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K et al (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195
Valluru R, Reynolds MP, Davies WJ, Sukumaran S (2017) Phenotypic and genome-wide association analysis of spike ethylene in diverse wheat genotypes under heat stress. New Phytol 214:271–283
van Butselaar T, Van den Ackerveken G (2020) Salicylic acid steers the growth-immunity tradeoff. Trends Plant Sci 25:566–576
Van Ha C, Leyva-González MA, Osakabe Y, Tran UT, Nishiyama R et al (2014) Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci USA 111:851–856
Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:86
Verslues PE, Zhu JK (2007) New developments in abscisic acid perception and metabolism. Curr Opin Plant Biol 10:447–452
Veselova SV, Farhutdinov RG, Veselov SY, Kudoyarova GR, Veselov DS, Hartung W (2005) The effect of root cooling on hormone content, leaf conductance and root hydraulic conductivity of durum wheat seedlings (Triticum durum L.). J Plant Physiol 162:21–26
Vettakkorumakankav NN, Falk D, Saxena P, Fletcher RA (1999) A crucial role for gibberellins in stress protection of plants. Plant Cell Physiol 40:542–548
Vicente R-S, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Vijayan P, Shockey J, Lévesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95:7209–7214
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Walker-Simmons M (1987) ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol 84:61–66
Walker-Simmons M, Sesing J (1990) Temperature effects on embryonic abscisic acid levels during development of wheat grain dormancy. J Plant Growth Regul 9:51–56
Wang LJ, Li SH (2006) Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci 170:685–694
Wang L, Li S (2007) The effects of salicylic acid on distribution of 14C-assimilation and photosynthesis in young grape plants under heat stress. Acta Hortic 738:779–7851
Wang R, Zhang Y, Kieffer M, Yu H, Kepinski S, Estelle M (2016) HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat Commun 7:10269
Wang LJ, Fan L, Loescher W et al (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10:34–44
Wang K, Zhang X, Ervin E (2013) Effects of nitrate and cytokinin on creeping bentgrass under supraoptimal temperatures. J Plant Nutr 36:1549–1564
Wang Y, Zhang H, Hou P, Su X, Zhao P, Zhao H, Liu S (2014a) Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul 73:289–297
Wang Y, Chang H, Hu S, Lu X, Yuan C, Zhang C, Wang P, Xiao W, Xiao L, Xue GP, Guo X (2014b) Plastid casein kinase2 knockout reduces abscisic acid (ABA) sensitivity, thermotolerance, and expression of ABA-and heat-stress-responsive nuclear genes. J Exp Bot 65:4159–4175
Wang X, Zhuang L, Shi Y (2017) Up-regulation of HSFA2c and HSPs by ABA contributing to improved heat tolerance in tall fescue and Arabidopsis. Int J Mol Sci 18:E1981
Wang A, Hu J, Gao C, Chen G, Wang B, Lin C, Song L, Ding Y, Zhou G (2019) Genome-wide analysis of long noncoding RNAs unveils the regulatory roles in the heat tolerance of Chinese cabbage (Brassica rapa ssp. chinensis). Sci Rep 9:5002
Wang J, Song L, Gong X, Xu J, Li M (2020) Functions of jasmonic acid in plant regulation and response to abiotic stress. Int J Mol Sci 21:1446
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176
Wani W, Masoodi KZ, Zaid A, Wani SH, Shah F, Meena VS, Wani SA, Mosa KA (2018) Engineering plants for heavy metal stress tolerance. Rendiconti Lincei. Sci Fis Nat 29:709–723
Wassie M, Zhang W, Zhang Q, Ji K et al (2020) Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicagosativa L.). Ecotox Environ Saf 191:110206
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Wasternack L (2014) Action of jasmonates in plant stress responses and development and applied aspects. Biotechnol Adv 32:31e39
Wen PF, Chen JY, Wan SB, Kong WF, Zhang P, Wang W et al (2008) Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. Plant Growth Regul 55:1–10
Wheeler TR, Craufurd PQ, Ellis RH et al (2000) Temperature variability and the yield of annual crops. Agric Ecosyst Environ 82:159–167
Wilen RW, Sacco M, Gusta LV, Krishna P (1995) Effects of 24-epibrassinolide on freezing and thermotolerance of bromegrass (Bromus inermis) cell cultures. Physiol Plant 95:195–202
Wit MD, Lorrain S, Fankhauser C (2014) Auxin-mediated plant architectural changes in response to shade and high temperature. Physiol Plant 151:13–24
Wu JZ, Lin Y, Zhang XL, Pang DW, Zhao J (2008) IAA stimulates pollen tube growth and mediates the modification of its wall composition and structure in Torenia fournieri. J Exp Bot 59:2529–2543
Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Huang J, Nie L, Mohapatra PK, Peng S (2017) Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in Rice. Front Plant Sci 8:371
Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Huang J, Nie L, Peng S (2016) Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci Rep 6:34978
Wu YS, Yang CY (2019) Ethylene-mediated signaling confers thermotolerance and regulates transcript levels of heat shock factors in rice seedlings under heat stress. Bot Stud 60:23
Wu C, Tang S, Li G, Wang S, Fahad S, Ding Y (2019) Roles of phytohormone changes in the grain yield of rice plants exposed to heat: a review. PeerJ 7:e7792
Wuriyanghan H, Zhang B, Cao W-H, Ma B, Lei G, Liu Y-F, Wei W, Wu H-J, Chen L-J, Chen H-W et al (2009) The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell 21:1473–1494
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Ann Rev Plant Physiol 59:225–251
Yang JC, Zhang JH, Ye YX, Wang ZQ, Zhu QS, Liu LJ (2004) Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant Cell Environ 27:1055–1064
Yang DL, Yao J, Mei CS, Tong XH, Zeng LJ, Li Q, Xiao LT, Sun TP, Li J, Deng XW, Lee CM (2012) Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci 109:E1192–E1200
Yang D, Li Y, Shi Y, Cui Z, Luo Y, Zheng M, Chen J, Li Y, Yin Y, Wang Z (2016) Exogenous cytokinins increase grain yield of winter wheat cultivars by improving stay-green characteristics under heat Stress. PLoS ONE 11:e0155437
Yang J, Duan G, Li C, Liu L, Han G, Zhang Y, Wang C (2019) The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front Plant Sci 10:1349
You L, Rosegrant MW, Wood S, Sun D (2009) Impact of growing season temperature on wheat productivity in China. Agric For Meteorol 149:1009–1014
Youm JW, Jeon JH, Choi D, Yi SY, Joung H, Kim HS (2008) Ectopic ex- pression of pepper CaPF1 in potato enhances multiple stresses tolerance and delays initiation of in vitro tuberization. Planta 228:701–708
Young TE, Meeley RB, Gallie DR (2004) ACC synthase expression regulates leaf performance and drought tolerance in maize. Plant J 40:813–825
Zaid A, Bhat JA, Wani SH (2020) Influence of metalloids and their toxicity impact on photosynthetic parameters of plants. Metall Plants 14:113–124
Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A, Inupakutika MA, Mittler R (2016) ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot 67:5381–5390
Zavaleta-Mancera HA, Lopez-Delagdo H, Loza-Tavera H, Mora-Herrera M, Trevilla-Garcia C, Vargas- Suarez M et al (2007) Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J Plant Physiol 164:1572–1582
Zhang YP, Zhu XH, Ding HD et al (2013) Foliar application of 24-epibrassinolide alleviates high-temperature-induced inhibition of photosynthesis in seedlings of two melon cultivars. Photosynthesis 51:341–349
Zhang YP, He J, Yang SJ et al (2014) Exogenous 24-epibrassinolide ameliorates high temperature-induced inhibition of growth and photosynthesis in Cucumis melo. Biol Plant 58:311–318
Zhang J, Shi Y, Zhang X, Du H, Xu B, Huang B (2017) Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ Exp Bot 138:36–45
Zhang CX, Li GY, Chen TT, Feng BH, Fu WM, Yan JX, Islam MR, Jin QY, Tao LX, Fu GF (2018) Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice 11:14
Zhang X, Wang X, Zhuang L, Gao Y, Huang B (2019) Abscisic acid mediation of drought priming-enhanced heat tolerance in tall fescue (Festuca arundinacea) and Arabidopsis. Physiol Plant 167:488–501
Zheng Z, Guo Y, Novák O, Chen W, Ljung K, Noel JP, Chory J (2016) Local auxin metabolism regulates environment-induced hypocotyl elongation. Nat Plants 2:16025
Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ (2014) H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot 65:4371–4383
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:P313–P324
Acknowledgements
UCJ conceived the idea and wrote the MS with HN and KHMS. UCJ acknowledges support from the Indian Council of Agricultural Research (ICAR), New Delhi, India. HN is thankful to DBT, DST, MOA, UWA (Perth), ICARDA, and World Vegetable Center for supporting research work on heat stress in food crops.
Funding
No funds were required for writing this article.
Author information
Authors and Affiliations
Contributions
UCJ conceived the idea and written the MS along with HN and KHMS. HN gave assisted in giving concept of Fig.1 and Fig.2. KHMS edited the MS and improved the quality of MS. All authors read and approved the MS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Rhonda Peavy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jha, U.C., Nayyar, H. & Siddique, K.H.M. Role of Phytohormones in Regulating Heat Stress Acclimation in Agricultural Crops. J Plant Growth Regul 41, 1041–1064 (2022). https://doi.org/10.1007/s00344-021-10362-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10362-x