Abstract
In order to explore the possible physiological mechanism of high temperature induced sterility in rice, we examined the floret sterility and endogenous plant growth regulator contents in pollens of two hybrid rice cultivars Shanyou63 and Teyou559 that are tolerant and susceptible to high temperature, respectively. Indexes of floret sterility, pollen activity, and variation of endogenous indole-3-acetic acid (IAA), gibberellic acids (GAs), abscisic acid (ABA), free proline and soluble proteins in anthers were measured. We found that during the course of high temperature treatment, both cultivars exhibited a marked decrease in pollen activity, pollen germination and floret fertility; however, the high temperature tolerant Shanyou63 showed a much slower rate of decrease than the high temperature susceptible Teyou559. In addition, anthers of both cultivars displayed a decrease in the contents of IAA, GAs, free proline and soluble proteins but an increase in the ABA content. Yet compared to Teyou559, Shanyou63 retained significantly higher levels of free praline and GAs and a lower level of ABA, along with higher pollen vigour and pollen germination rate even after prolonged high temperature treatment. Our study suggests a possible correlation between pollen viability/floret sterility and high temperature-caused changes in IAA, GAs, ABA, free proline and soluble protein contents. The severity in these changes may reflect the variation of rice cultivars in their heat stress sensitivities for floret development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is grown mainly in tropical and subtropical zones. Heading and flowering stage of rice is most sensitive to high temperatures (Satake and Yoshida 1978; Tan et al. 1985). During this stage, heat stress can most likely cause floret sterility, fecundity decrease and yield loss, largely due to decrease in pollen activity and pollen germination, limited growth of pollen tube, low anther dehiscence, lack of pollen reaching to chapiter, and incomplete pollination (Li et al. 2002; Mackill et al. 1982; Matsui et al. 2001; Matsushima et al. 1982; Satake and Yoshida 1978; Tan et al. 1985; Tang et al. 2006).
There are obvious differences of sensitivity to high temperatures among rice cultivars (Smirnoff 1993; Tan et al. 1985). High temperature-tolerant cultivars exhibit higher floret fertility, fecundity and anther dehiscence than susceptible cultivars under the high temperature stress (Matsui et al. 2000, 2001; Satake and Yoshida 1978). In addition, tolerant cultivars experience far less severe decrease of pollen activity and pollen germination at high temperatures than susceptible cultivars (Tang et al. 2006). The main cause of anther indehiscence was well understood, but physiological and biochemical mechanisms for decreasing pollen activity and pollen germination induced by heat are still not clear (Matsui et al. 2000, Matsui and Omasa 2002).
It has been reported that endogenous hormones play an important role in modulating male fertility. Insufficient IAA and GAs and excessive ABA may induce pollen abortion (Nakajima et al. 1991; Shimizu and Kuno 1967; Tang et al. 1996; Yang et al. 1990). Free proline and soluble proteins in anthers are important materials for normal pollen development and germination. Disruption of their normal accumulations due to stresses may lead to drastic loss of pollen activity and even to sterility (Plif 1981; Song et al. 1999). High temperature (heat) stress is an often occurring event during rice growth season. Yet different rice cultivars display marked difference in heat sensitivity. We report here our studies on high temperature induced changes in anthers of endogenous phytohormones, free proline and soluble proteins, in connection with pollen activity, pollen germination and fecundity of two rice cultivars of distinct high temperature tolerance. Our results suggest a possible link between changes in hormones, free proline and soluble proteins in rice anthers and floret sterility during heat stress.
Materials and methods
Plant materials
Two hybrid rice cultivars Shanyou63 and Teyou559 (Oryza sativa ssp. indica) were used in this study. Shanyou63 is tolerant and Teyou559 susceptible to high temperatures. The experiments were arranged as a complete randomized-block design with two replications. Each replication consisted of four plants per cultivar. Seeds were sown on April 29th, 2006 and seedlings were transplanted on May 28th, 2006 in the plastic boxes with four seedlings per box. During different growing stages, urea containing rapeseed meal (organic fertilizer) and the nitrogen/phosphorus/potassium compound fertilizer were applied as scheduled. Imidacloprid, Fipronil, and Validamycin were sprayed to control Chilo suppressalis, Baliothrips biformis, plant hopper, Cnaphalocrocis medinalis and sheath blight as needed.
High temperature treatments
Phytotron was used for high-temperature treatments. Plants at flowering stage were exposed to 39 ± 0.5°C for 4 h (10:00–14:00, Beijing time) for 1, 3 or 5 days. The control plants were grown in the same conditions as treated plants except for at the average temperature of 32 ± 0.5°C instead of 39°C.
Pollen activity was measured immediately after the treatments. At the same time, anthers were collected, frozen in liquid nitrogen and kept in −20°C until use for assays of endogenous hormones, free proline and soluble proteins. Pollen germination rate was measured on the next day of each treatment.
Assaying methods
The methods for extraction and purification of IAA, GAs (GA1 and GA4), and ABA were modified from those described by He (1993). Anthers were ground with 80% methanol containing 1 mM butylated hydroxytoluene and extracted with 100 mg of polyvinylpyrrolidone (PVP) per gram fresh material for 4 h at 4°C. The supernatant was passed through Chromosep C18 columns (C18 Sep-Park Cartridge, Waters Corp., Millford, MA), prewashed with 10 ml 100% (w/v) and 5 ml 80% (v/v) methanol, respectively. The hormone fractions were eluted with 10 ml 100% (v/v) methanol and 10 ml ether from the columns and dried under N2, and dissolved in 2 ml phosphate buffer saline (PBS) containing 0.1% (v/v) Tween 20 and 0.1% (w/v) gelatin (pH 7.5) for analysis by ELISA.
The mouse monoclonal antigens and antibodies against IAA, GAs and ABA, and IgG horseradish peroxidase used in ELISA were produced at the Phytohormones Research Institute (China Agricultural University; He 1993). Each well on the microtitration plate was coated with 100 μL coating buffer (1.5 g/l Na2CO3, 2.93 g/l NaHCO3, and 0.02 g/l NaN3, pH 9.6) containing 0.25 μg/ml antigens against the hormones. The coated plates were incubated for 4 h at 37°C for GAs, and ABA, and overnight at 4°C for IAA, and then kept at room temperature for 30 to 40 min. After washing four times with PBS + 0.1% Tween 20 buffer (pH 7.4), each well was filled with 50 μl of either pollen grain extracts or IAA, GAs, and ABA standards (0–2,000 ng/ml dilution range), and 50 μl of 20 μg/ml antibodies against IAA, GAs, and ABA, respectively. The plate was incubated for 3 h at 28°C for GAs, ABA, and overnight at 4°C for IAA, and then washed as above. One hundred microliters of 1.25 μg/ml IgG-horseradish peroxidase substrate were added to each well and incubated for 1 h at 30°C. The plate was rinsed five times with the above PBS + Tween 20 buffer, and 100 μl color-appearing solution containing 1.5 mg/ml 0-phenylenediamine and 0.008% (v/v) H2O2 was added to each well. The reaction was stopped by addition of 50 μl 6N H2SO4 per well when the 2,000 ng/ml standard had a pale color, and the 0 ng/ml standard had a deep color in the wells. Color development in each well was recorded using an ELISA Reader (model EL310, Bio-TEK, Winooski, VT) at optical density A490. IAA, GAs, and ABA contents were calculated following Weiler et al. (1981).
Acid Ninhydrin method was used for free proline assaying. Anthers were ground in 3% sulfosalicylic acid and incubated at 100°C for 15 min. Supernatant collected after centrifuge at 3,000 g for 10 min was mixed with an equal volume of acetic acid and acid Ninhydrin and incubated at 100°C for 0.5 h. Toluene was added into solution and color development of extract was measured at A520.
Soluble proteins were extracted according to Zhang and Tang (1992). Anthers were ground with 30 mM Tris-HCl (pH 8.7) containing 1 mM 1,4-dithiothreitol, 1 mM Vc, 1 mM ethylene- diaminetetraacetic Acid, 5 mM MgCl2 and 1 mM phenyl methyl sulfonyl fluoride. Supernatant collected after centrifuge at 4,000 g for 15 min was stained with Coomassie Brilliant Blue G250 for measuring soluble protein with colorimetric method at a wavelength of 595 nm.
Pollen from different florets was assayed for pollen activity with benzidine staining test. Pollen for germination test was placed on the glass slide covered with 0.6% agar medium containing 18% sucrose, 60 mg/l H3BO3 and 300 mg/l Ca(NO3)2 and artificially germinated by incubating at 29 ± 0.5°C for 5 min. Pollen from 8 to 10 spikes were scored as germination rate for each treatment.
At maturity seeds were differentiated as plump and shriveled once soaked in 95% ethanol.
Results
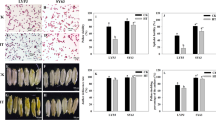
Effect of high temperature stress on seed setting in indica hybrid rice
The percentage of fertility was decreased significantly in both Shanyou63 and Teyou559 under 39°C at flowering stage (Fig. 1). However, the pattern of the decrease differed significantly according to cultivars and treatment periods. As the temperature treatments prolonged from 1 day to 3 days or 5 days, the effect on fecundity was less severe with the high temperature tolerant Shanyou63 than with the susceptible Teyou559 (Fig. 1). Nonetheless, longer duration of temperature treatment decreased fertility percentage in both cultivars.
Effect of high temperature stress on pollen activity
Pollen activity was reduced significantly in both Shanyou63 and Teyou559 after only one treatment of 4 h at 39°C (Fig. 2A). The longer the plants were exposed to the high temperature, the more pollen activity decreased. In vivo pollen germination also decreased after one 4-h 39°C treatment for both cultivars (Fig. 2B). The degrees of decreases in both pollen activity and pollen germination were more severe in Teyou559 than in Shanyou63, indicating that Shanyou63 was more tolerant to high temperature than Teyou559, which is in accordance with the change of fertility.
Effect of high temperature stress on the content of free proline and soluble proteins in anthers
The amounts of free proline (Fig. 3A) and soluble proteins (Fig. 3B) in anthers of both Shanyou63 and Teyou559 decreased dramatically under high temperature stress. The decrease, which was positively correlated with the period of the treatment, was much more obvious in anthers of Teyou559 than that in those of Shanyou63 under the same treatment, again reflecting the property of Shanyou63 as being heat tolerant. It is worth noting that after a 4-h heat treatment for 5 days, the soluble protein content in anthers was reduced to about the same level between these two cultivars (Fig. 3B), even though Shanyou63 still contained a much higher level of free proline (Fig. 3A).
Effect of heat stress on the endogenous hormone contents in anthers
Contents of IAA and GAs decreased notably in anthers of both Shanyou63 and Teyou559 under high temperature stress (Fig. 4). Particularly for Teyou559, a 4-h treatment at 39°C resulted in approximately 46% drop in IAA content (Fig. 4A). In contrast, only a 12% decrease was observed for Shanyou63 (Fig. 4A). Similar patterns occurred also for GAs (Fig. 4B). The average contents of IAA and GAs decreased by 48.9% and 55.9% in Teyou559 and 20.4% and 30.2% in Shanyou63, respectively, as compared to the control plants. Interestingly, after heat treatments for 3 days or 5 days, the anther IAA content was maintained at the similar level between these two cultivars (Fig. 4A) but the GAs levels remained much higher in Shanyou63 than in Teyou559 (Fig. 4B).
In contrast to other parameters measured in this study, high temperature treatments resulted in the increase in anther ABA content for both cultivars (Fig. 4C), suggesting ABA as a stress hormone. Interestingly, the heat susceptible cultivar Teyou559 showed a higher increase than the heat tolerant Shanyou63.
Discussion
Heading and flowering time, the most crucial stage for fertility, is very sensitive to stressful environments. High temperature stress at this stage can induce sterility and thus limit grain yield (Satake and Yoshida 1978; Tan et al. 1985; Tang et al. 2006). In present study, we have demonstrated that high temperature exposure to 39°C for as little as 4 h could drastically decrease fertility as well as pollen activity and germination of indica hybrid rice (Figs. 1 and 2). This kind of decrease is positively correlated to treatment duration. The longer the treatment is applied, the more severe the effects. Our study supports the notion that fertility decrease is a phenotypic behavior of rice under heat stress while decrease in pollen activity and germination might be the main physiological reason for sterility.
Damage to pollen under high temperature differs in various cultivars. Pollen activity as well as fertility of the high-temperature tolerant cultivar Shanyou63 decreased much less than those of the susceptible cultivar Teyou559 (Fig. 2), which confirmed the observed agronomic traits for these two cultivars. Hence, this variability among cultivars could be utilized in breeding programs for production of high temperature tolerant cultivars. Adoption and breeding high-temperature tolerant rice cultivars would be one of the most effective countermeasures to maintain high productivity and stability of rice under heat stress.
Amounts of endogenous hormones in anthers of both Shanyou63 and Teyou559 changed significantly under high temperature stress (Fig. 4). The contents of IAA and GAs decreased while ABA increased obviously. The longer the treatment performed, the more severe the decrease of IAA and GAs were observed. Decrease of IAA and GAs and increase of ABA in the high-temperature tolerant cultivar were less drastic than the susceptible cultivar. Previous studies showed that hormones play an important role in the development of male fertility. The maintenance of certain contents of IAA and GAs is necessary to normal development of anthers. Lack of sufficient active IAA and GAs would cause pollen abortion, which is a common reason for male sterility (Nakajima et al. 1991; Shimizu and Kuno 1967; Tang et al. 1996; Yang et al. 1990). ABA is a key factor to prevent premature anther development and, when necessary, trigger pollen abortion (Tang et al. 1996; Yang et al. 1990). Relatively low level of ABA in anthers is required for normal anther/pollen development. Therefore, we consider that deficiency in IAA and GAs and abnormal increase in ABA level induced by high temperature may be a main physiological mechanism for floret sterility and lower fecundity.
On the other hand, free proline and soluble proteins in anthers provide energy, nutrition and osmosis environment necessary for pollen germination and elongation (Plif 1981; Song et al. 1999). In addition, free proline and soluble proteins can regulate cell infiltration and stabilize cell membrane structure, which contributes to maintain cell structure and function under abiotic stresses (Jiang et al. 1997; Smirnoff 1993). Our results show that contents of free proline and soluble proteins in anthers of Shanyou63 and Teyou559 decreased significantly during heat treatments (Fig. 3). The decrease in the high-temperature susceptible cultivar was more rapid and profound than that in the tolerant cultivar. It appears that contents of free proline and soluble proteins in anthers are closely correlated with pollen activity. The decrease of free proline and soluble protein induced by high temperatures may be another major stress indicator, along with hormonal changes, for decrease in pollen activity and floret sterility in rice plants.
Abbreviations
- IAA:
-
Indole-3-acetic acid
- GAs:
-
Gibberellic acids
- ABA:
-
Abscisic acid
References
He ZP (eds) (1993) Experimental instruction for crop chemical controls. Peking Agriculture University Publishing House, Peking
Jiang MY, Guo SC, Zhang XM (1997) Proline accumulation in rice seedling exposed to oxidative stress in relation to antioxdation. Acta Phytophysiol Sin 23:347–352 (in Chinese)
Li XZ, Liang MZ, Zhou GQ (2002) Effect of environment condition on pollen activity and seed set during flowing time of rice. Acta Agron Sin 28:417–420 (in Chinese)
Mackill DJ, Coffman WR, Rutger JN (1982) Pollen shedding and combining ability for high temperature tolerance in rice. Crop Sci 22:730–733
Matsui T, Omasa K (2002) Rice (Oryza sativa L.) Cultivars Tolerant to High Temperature at Flowering: Anther Characteristics. Ann Bot 89:683–687
Matsui T, Omasa K, Horie T (2000) High temperatures at flowering inhibit swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.) Plant Prod Sci 3:430–434
Matsui T, Omasa K, Horie T (2001) The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Prod Sci 4:90–93
Matsushima S, Ikewada H, Maeda A et al (1982) Studies on rice cultivation in the tropics. 1. Yielding and ripening responses of the rice plant to the extremely hot and dry climate in Sudan. Jpn J Trop Agric 26:19–25
Nakajima M, Yamaguchi I, Kizawa S (1991) Semi-quantification of GA1 and GA4 in male sterile anthers of rice by radioimmunoassay. Plant Cell Physiol 32:511–513
Plif G (1981) The proline content and fertility of the pollen in breed maize lines. Acta Bet Acad Sci 27:179–181
Satake T, Yoshida S (1978) High temperature-induced sterility in indica rice at flowering. Jpn J Crop Sci 47:6–10
Shimizu M, Kuno K (1967) Some cyto-histo logical observations on the morphogenetically abnormal rice spikelets caused by a low temperature. Proc Crop Sci Soc Jpn 36:489–502
Smirnoff N (1993) The role of active oxygen in the response of plant to water deficit and desiccation. New Phytol 125:27–32
Song J, Nada K, Tachibana S (1999) Ameliorative effect of polyamines on the high temperature inhibition of in vivo pollen germination in tomato (Lycopersicon esculentum Mil.) Sci Hortic 80:203–212
Tan ZH, Lan TY, Ren CF (1985) Studies on high temperature injury on hybrid rice at flowering time and the strategy to avoid high temperature damage. Acta Agronom Sin 11:103–108 (in Chinese)
Tang RS, Mei CS, Zhang JY (1996) Relationship between rice male sterility induction by T03 and level of endogenous hormones. Jiangsu J Agric Sci 12:6–10 (in Chinese)
Tang RS, Zheng JC, Zhang DD (2006) The effects of high temperatures on pollen vitality and seed setting of different rice varieties. Jiangsu J Agric Sci 22:369–373 (in Chinese)
Weiler EW, Jordan PS, Conrad W (1981) Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta 153:561–571
Yang DC, Zhu YG, Tang LJ (1990) The content of four endogenous hormones in leaves and fertility transformation of HPGMR. J Huazhong Agric Univ 9:394–399 (in Chinese)
Zhang B, Tang XH (1992) Studies on rice embryo proteins with developmental stage specificity. Acta Pytophysiol Sin 18:85–92 (in Chinese)
Acknowledgements
This work was financially supported by Natural Science Foundation of Jiangsu Province of P. R. China (Grant No. BK2004002) and National Natural Science Foundation of P. R. China (Grant No. 30470332).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, RS., Zheng, JC., Jin, ZQ. et al. Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54, 37–43 (2008). https://doi.org/10.1007/s10725-007-9225-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-007-9225-8