Abstract
The salinization of agriculture soils over the globe has become one of the most devastating stresses and is significantly limiting cultivated land area, and crop productivity and quality. It is very imperative to explore both salinity tolerance in plants and insights into approaches (and underlying mechanisms) for effectively controlling salinity impacts. To this end, the role of phytohormone salicylic acid (SA) and plant nutrient sulfur (S) in promoting salinity tolerance has been researched in isolated studies, and SA–S interaction results have been little discussed. Given this, taking into account recent literature on SA, S and soil salinity, this paper aimed to (i) overview of the major impacts of soil salinity on plant health; (ii) highlight the significance of SA and S in improving plant salinity tolerance; (iii) discuss the role and underlying mechanism of SA, S and their interaction in the modulation of plant growth and development under salinity stress; and also to (iv) appraise the discussed literature and enlighten the major prospects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity stress and plant health
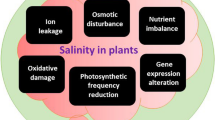
The world agriculture is under serious threat due to the increasing human population, and reduction in the arable land (Shahbaz and Ashraf 2013). Additionally, abiotic stresses as a major contributing factor decrease productivity by more than 50%. One of the factors, salinity stress, is increasing rapidly and is becoming the main concern for reductions in crop productivity and quality (Shahbaz and Ashraf 2013). Notably, the increasing salinization of world cultivable lands at an annual rate of 10% has been estimated to be culminated into more than 50% of the arable land be salinized by the year 2050 (Jamil et al. 2011). The soil salinity may develop as natural or induced by human activity. The long-term natural accumulation of salts (including Cl− of Na+, Ca2+ and Mg2+ and sometimes SO42− and CO32−) in the soil or surface water contributes to the primary or natural salinity. On the other hand, the disruption of the hydrologic balance of the soil between water applied (irrigation or rainfall) and water used by crops (transpiration) as a result of anthropogenic activities cause the secondary soil salinity (Munns 2005; Hasanuzzaman et al. 2011). Soils are regarded as saline when the saturation extracts (ECe) in the root zone exhibits: electrical conductivity (EC) > 4.0 dS m−1 (≈ 40 mM NaCl) at 25 °C and 15% exchangeable Na+ ion. Potential mechanisms underlying salinity impacts in plants include: (i) salinity-mediated impairments in growth and development via water stress; (ii) cytotoxicity due to excessive uptake of ions such as Na+ and Cl−; (iii) impaired nutritional imbalance and metabolism of mineral nutrients; and (iv) oxidative stress due to physiological imbalance between oxidants and antioxidants (Isayenkov and Maathuis 2019). Halophytic group of plants can tolerate up to 1.0 M salt concentration, whereas most crop plants are glycophytes and exhibit significant variations in their tolerance to salinity levels (Srivastava et al. 2015). A salt concentration of the saturation extracts up to 10 g/l can be tolerated by the highly tolerant crops, whereas the moderately tolerant crops can withstand salt concentration up to 5.0 g/l. The sensitive crops cannot tolerate even 2.5 g/l (Shahbaz and Ashraf 2013). Contingent to the concentration and time of exposure of salt, plant genotypes, and environmental factors, salinity impact on plant growth, development, and physiological/biochemical processes greatly vary in plants (Hasanuzzaman et al. 2011).
Salinity impacts every aspect of growth and development of plants. Briefly, seed germination is the first growth stage of the plant’s life cycle and is very salinity-sensitive, and often exhibits high mortality rates. Moreover, plant salt-tolerance at the germination stage has been considered critical for the successful growth of plants in saline conditions. Salinity impact on the germination has been reported in several crop plants including Arabidopsis (Xu et al. 2011), Zea mays (Carpici et al. 2010; Khodarahmpour et al. 2012), and Brassica spp. (Ulfat et al. 2007). The variation in salinity tolerance may occur within species, cultivar, or individual lines at their growth stage (Parihar et al. 2015). Any change in various components of photosynthesis can have profound effects on its overall status in plants under stress. Salinity stress severely impacts all the phases of photosynthesis (Ashraf and Harris 2013; Jahan et al. 2019, 2020; Sehar et al. 2019).
Salinity stress impacts reactive oxygen species (ROS) and the antioxidants-mediated metabolism in plants (Choudhury et al. 2013; Anjum et al. 2015; Sehar et al. 2019; Jahan et al. 2020). ROS such as H2O2, O2•−and •OH are capable of degrading lipids, proteins, and other macromolecules leading to the cellular metabolic arrest. On the other hand, salinity stress can also differentially modulate the major components of the antioxidant defense system (Sehar et al. 2019; Jahan et al. 2020). In turn, the elevation in ROS-metabolizing enzymes and/or metabolites can help salinity-exposed plants to efficiently scavenge varied ROS and also minimize ROS-caused potential consequences. NaCl stress-induced elevation in the activity of catalase and ascorbate peroxidase minimized the toxic effects of salinity in a number of studies (Ren et al. 2018; Jahan et al. 2019; Sehar et al. 2019 (Fig. 1).
Schematic representation of the major enzymatic antioxidants and non-enzymatic antioxidant involved in the metabolism of reactive oxygen species (ROS) in plants (modified after Das and Roychoudhury 2014)
Elevation in soil salinity can impact the uptake and metabolism of several plant nutrients including Ca, K, N, P and S (Nazar et al. 2011; Syeed et al. 2011; Astolfi and Zuchi 2013; Khan et al. 2015). Salinity-accrued disruption in the acquisition of the majority of these nutrients may involve direct competition between ions at various transporters in the root plasma membrane (such as K+-selective ion channels) or by the decreased osmotic potential of solution reducing the mass flow of mineral nutrients in the root (Parihar et al. 2015).
Strategies for minimizing salinity stress impacts in plants
Reclamation of the salinity-affected soils has not always been feasible in minimizing the potential salinity impacts on plants/crops. Additionally, approaches to be adopted for controlling the salinity impacts on plants must be eco-friendly and sustainable. However, sustainable approaches (comprising microbial and molecular tools) to date adopted for the alleviation of salinity impact on plants are not a hundred percent feasible and cost-effective (Shokat and Großkinsky 2019). Hence, the adoption of approaches for modulating the plant’s own physiological and metabolic strategies adopted for counteracting the salinity impact at the cellular and whole-plant levels can be promising.
Broadly, complex physiological traits, metabolic pathways, and molecular or gene networks are involved in the adaptation or tolerance of plants to salinity stress. However, the major mechanisms underlying the plant salinity tolerance are far from being completely understood. Hence, it is imperative to enlighten the major strategies to be adopted for the minimization of salinity stress impacts in plants. To this end, it has been widely reported that the strategies modulating many processes (including ion homeostasis, compatible solute accumulation, and osmotic adjustments and the regulation of cellular antioxidants) have significantly contributed to plant stress tolerance (Gupta and Huang 2014). To improve salinity tolerance, the majority of the aforesaid processes could be modulated in salinity-exposed plants by supplementing them with different exogenous bioregulators, such as osmoprotectants, phytohormones, signaling molecules, polyamines, antioxidants and various trace elements (Hasanuzzaman et al. 2013).
At the physiological level, osmotic adjustment is an adaptive mechanism involved in salinity tolerance. Osmolytes regulate osmotic adjustment and safeguard the proteins and other cell membranes against various stress factors on cellular metabolism (Sharma et al. 2019). Salinity-exposed plants were reported to exhibit the biosynthesis of osmoprotectants such as proline, glycine betaine, trehalose, sorbitol, glucose, and ectoine, where these molecules were argued to significantly contribute in hyperosmotic stress tolerance generated from salt stress (Hasanuzzaman et al. 2013). Compatible solutes have also been reported to mitigate the damaging effects of salt stress mainly by reducing the impact of stress-induced ROS (Cuin and Shabala 2008). Nitric oxide (NO) is a gaseous, free radical, and redox-signaling molecule with diverse functions, and it has been reported to affect plant responses to various stress factors including soil salinity (Sehar et al. 2019; Jahan et al. 2020). In salt-affected plants, NO can significantly improve the seed vigor and germination, scavenge ROS, alleviate oxidative damage, improve the antioxidant defense mechanism, plant growth, ionic balance (K+:Na+, Mg2+:Na+, and Ca2+:Na+ ratios), water content, and chlorophyll content, and also increase chlorophyll a fluorescence curves (Ahmad et al. 2016; Sehar et al. 2019; Jahan et al. 2020). Another signaling molecule and non-proteinogenic amino acid β-aminobutyric acid was also reported to minimize salinity impacts in Brassica napus (Al-Mahmud et al. 2020). Phytohormones have long been considered as essential endogenous molecules involved in regulating plant development and tolerance or susceptibility of diverse stresses, including salinity (Ryu and Cho 2015). The list of phytohormones involved in salinity tolerance includes abscisic acid (ABA) (Ren et al. 2018), cytokinins (CK) (Wang et al. 2015), BRs (Ahanger et al. 2020), methyl jasmonate (MeJA) (Ryu and Cho 2015), gibberellin (GA) and ethylene (Khan et al. 2012), salicylic acid (SA) (Palma et al. 2013; Khan et al. 2015). On the other, minerals such as S (Nazar et al. 2011; Astolfi and Zuchi 2013), N (Iqbal et al. 2015; Singh et al. 2016), P (Fahad et al. 2016), Si (Coskun et al. 2016), Ca (Parvin et al. 2015), and many more are used for the salinity stress-alleviation in plants.
Prominence of salicylic acid and sulfur in stress acclimation
Salicylic acid has been widely reported to protect plants against abiotic stresses since it regulates important plant physiological processes including photosynthesis, nitrogen metabolism, proline (Pro) metabolism, glycine betaine production, control of antioxidant defense system, and plant–water relations (Khan et al. 2015). Additionally, SA can also induce defense-related genes and stress resistance in biotic stressed plants (Wani et al. 2016). A plethora of studies has confirmed the role of SA in plant tolerance to varied abiotic stresses including metals/metalloids (Khan et al. 2015; Zhang et al. 2015), salinity (Iqbal et al. 2014), ozone (Tamaoki 2008), UV-B radiation (Mohammed and Tarpley 2009), drought (Nazar et al. 2015b) and temperature stress (Siboza et al. 2017). On the other hand, plant stress tolerance can also be achieved by maintaining the status of plant-mineral nutrients such as S (Nazar et al. 2011; Rais et al. 2013; Fatma et al. 2014; Anjum et al. 2015; Hussain et al. 2019; Jahan et al. 2020). Both organic and inorganic forms of S are present in soils. Elementary S or its different oxidation forms (sulfide, sulfate, thiosulfate, etc.) represent the inorganically bound S and contribute only 10–15% of total S. Organically bound S occurs in organic compounds namely amino acids, proteins, polypeptides, and others, and contributes about 75–90% of the total S. The content of S in soils varies widely. In humid climates, S concentration is typically around 0.02–2.0%, moorland soils may contain 1.0% and in marshland, S concentration can be as high as around 3.5%. Therefore, organic matter content, soil parent material, and the amount of S added via fertilizer amendments and atmospheric deposition are responsible for the wide variations in the total S in soils (Scherer 2009). Notably, the role of the major components and end products of S assimilation has been widely reported in plant tolerance to major abiotic stresses including metals/metalloids (Anjum et al. 2008; Khan et al. 2009a; Masood et al. 2012; Asgher et al. 2014), salinity (Khan et al. 2009b; Fatma et al. 2014; Hussain et al. 2020), and chilling (Kopriva et al. 2001). Notably, there occurs a very close link between SA and S in terms of their physiological functions in plants under stress. For example, the interaction-outcomes of SA and S significantly contributed to plant growth, metabolism and stress tolerance; a higher GR activity and elevated S/Cys-GSH content were reported with exogenous SA (Pál et al. 2014); SA was involved in increased cysteine and GSH level as a result of SA-mediated increased activity of ATP-S and serine acetyltransferase (SAT) activity (Nazar et al. 2011, 2015a). Despite these facts, information is scanty in the literature available on approaches minimally impacting the environment, and the potential individual and combined roles of SA and S in eliminating soil salinity impacts on plant health and productivity.
Given these in light of recent literature, this paper aimed first to present a picture of the role and underlying mechanism of SA and S in the modulation of the response of germination, growth, photosynthetic and growth characteristics, oxidative stress markers, and the major components of both antioxidant defense system and S assimilatory pathways to soil salinity. Secondly, this paper appraises the discussed literature and also enlightens major knowledge-gaps on the subject.
Salicylic acid in mitigation of salinity impacts
Salicylic acid signaling in salinity tolerance
Of the two main pathways for SA biosynthesis (shikimic acid pathway and malonic acid pathway), the shikimic acid pathway is mainly involved in the endogenous synthesis of SA in plants, where phenylalanine, an aromatic amino acid acts as a precursor (Khan et al. 2015). On the other, the phenylalanine-mediated pathway of SA biosynthesis involves phenylalanine ammonia-lyase (PAL) and cinnamate-4-hydroxylase as the key enzymes. PAL converts phenylalanine into trans-cinnamic acid by the elimination of ammonium from phenylalanine, whereas cinnamate-4-hydroxylase converts cinnamic acid into coumaric acid by the hydroxylation at the C4 position of cinnamic acid. Eventually, the oxidation of the side chain of coumaric acid followed by hydroxylation leads to the synthesis of SA (Per et al. 2017).
Once synthesized, SA is being transported in and out of the cells, tissues and organs, where several transporters are involved. Particularly under pathogen infections, only the methylated form (MeSA), considered to be the long-distance signaling molecule travels in plant tissue locally as well as systemically. Among the various forms of SA, Researchers have identified SA induction–deficient (sid1 and sid2) in two Arabidopsis thaliana. Later, sid1 was identified to be allelic to ENHANCED DISEASE SUSCEPTIBILITY 5 (EDS5), a member of the multidrug and toxin extrusion (MATE) transporter family (reviewed by Maruri-López et al. 2019). The process of SA signaling is initiated when the cells, tissues or organs are under (biotic/abiotic) stress. In turn, the binding of SA to some specific receptors has to occur in order to induce defense signaling. SA methyl transferase 1 (SAMT1) and SA-binding protein 2 (SABP2) are the major SA-receptors and control the balance between SA and MeSA (Jayakannan et al. 2015). The list of major SABPs involved in SA signaling network includes SABP1-catalase, SABP2-MeSA Esterase, SABP3-β carbonic anhydrase, non-expressor of pathogenesis-related protein 1–4 (NPR1/2/3/4)-signaling proteins, glutathione S-transferase isoenzymes (GSTF2, GSTF8, GSTF10, GSTF11), thioredoxin-m1 (TRXm1), GH3-acyl acid amido synthetase (GH3.12/PBS3), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; GAPA-1, GAPA-2, GAPC-1, and GAPC-2 (reviewed by Pokotylo et al. 2019). Recognized as an endogenous natural signal molecule SA regulates physiological and biochemical processes and thereby regulates growth and development and is also involved in defense mechanisms (Gunes et al. 2007; Iqbal et al. 2014; Khan et al. 2015). Extensive reports are also available on SA signaling and SA-mediated control of important genes involved in salinity-exposed plants (Csiszár et al. 2014; Jayakannan et al. 2015). A summary of recent studies reported on the aspect has been given in Table 1. Salicylic acid receptor, NPR1 (non-expresser of PR protein 1) is argued as a master regulatory protein of SA-dependent defense responses by being a transcriptional co-activator of PR-gene expression (Jayakannan et al. 2015). NPR1-mediated SA signaling has been argued pivotal for controlling Na+ entry into roots and the subsequent long-distance transport into shoots; enhancing H+-ATPase activity in roots, and increasing K+ concentration in shoots during salt stress (reviewed by Jayakannan et al. 2015). In salinity-exposed Tiricum aestivum, SA (0.5 mM) was reported to bring enhancements in the transcriptomic rate of antioxidant genes such as DHAR, GR, GPX2, MDHAR, GST1, GST2, and GS (Li et al. 2013). Salicylic acid-mediated upregulation of SlGSTZ2, SlGSTL3, and SlGSTF4 of GST gene family has also been reported (Csiszár et al. 2014). Earlier, SA-supply was reported to enhance the expression of salt stress-mediated responsive genes encoding sinapyl alcohol dehydrogenase, cinnamyl alcohol dehydrogenase, cytochrome P450, heat shock proteins, and chaperones (Jumali et al. 2011). Salicylic acid can also regulate the expression of NahG (a bacterial gene encoding SA hydroxylase) for the response to salt stress (Nie et al. 2015). Salicylic acid was also involved in the regulation of the transcript levels of the GA3ox1 gene and contributed to improved seed germination under salinity stress (Lee et al. 2010). Additionally, SA has also been reported to regulate Nudix hydrolases genes and the expression levels of both ClNUDX1 and ClNUDX2 under salt stress (Huang et al. 2012). Salicylic acid-induced accumulation of K+, and soluble sugars but decrease in Ca+ in roots; enhanced synthesis of photosynthetic pigments, and maintained membrane integrity (El-Tayeb 2005); strong inhibition in Na+ and Cl− accumulation and stimulation in accumulation of N, Mg, Fe, Mn, P and Cu (Gunes et al. 2007); and the influence of SA on cation uptake in plants, and eventual modulation of the ratio of Na+ to other cations (Eraslan et al. 2008) were also reported potentially involved in SA-mediated plant salinity tolerance. Salicylic acid can also improve plant salinity tolerance by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel (Jayakannan et al. 2013).
Sulfur in mitigation of salinity impacts
Sulfur assimilation
Among the major plant nutrients, S is a macronutrient for the plant and has been a major constituent in a range of S-amino acids like cysteine and methionine (Met), and S-compounds such as GSH, homo-GSH, and PCs. The assimilatory pathway of S in plants mainly involves: (i) ATP sulfurylase (ATP-S)-mediated activation of sulfate to adenosine 5′-phosphosulfate (APS);(ii) APS reductase (APR)-mediated reduction of APS into sulfite; and (iii) the reduction of sulfite into sulfide, and its incorporation into cysteine (Cys), a direct precursor for the synthesis of GSH (Anjum et al. 2015) (Fig. 2).
Scheme representing the assimilation of sulfur (S), its involvement in the glutathione (GSH) synthesis, and GSH-mediated control of oxidants (reactive oxygen species, ROS) generated due to salinity in plants. GR, glutathione reductase; GSSH, oxidized glutathione; AsA, reduced ascorbate; MDHA, monodehydroascorbate; DHAR, dehydroascorbate reductase; MDHAR, monodehydroascorbate reductase; APX, ascorbate peroxidase; CAT, catalase; SOD, superoxide dismutase. O2−, superoxide; H2O2, hydrogen peroxide
Sulfur-mediated salinity tolerance
In several studies, the assimilation of the applied S yielded the cellular Cys and GSH contents as a result of increased activity of ATP-S and thereby improved overall growth (Hussain et al. 2019; Nazar et al. 2011; Fatma et al. 2014, 2016). The supplied S-mediated improvements in the gas exchange parameters and photosynthetic pigments were also argued as a major mechanism underlying S-induced increases in intrinsic water-use efficiency (WUE) and ribulose 1,5 bisphosphate carboxylase/oxygenase (Rubisco) activity (Astolfi and Zuchi 2013; Khan et al. 2014b; Hussain et al. 2019) (Table 2). In several instances, the supplied S-led increases in the cellular S-containing compounds (such as GSH, methionine, thioredoxins, vitamins, and coenzyme A) and GSH-associated antioxidant enzymes were reported to strengthen antioxidant defense system in salinity-exposed plants (Nazar et al. 2011, 2014b; Astolfi and Zuchi 2013; Fatma et al. 2013, 2014; Hussain et al. 2019) (Fig. 3). Notably, among S-compounds, GSH, h-GSH, and PCs are known for their involvement in plant tolerance to varied abiotic stresses. Cys is a major component of GSH, h-GSH, and PCs, whereas several key stress-metabolites such as ethylene, are controlled by Met through its first metabolite SAM (Anjum et al. 2015). Salinity has been reported to significantly increase the transcript levels of both ATP-S and APR genes in plants (Sharma et al. 2015). These authors also observed a higher expression of APR genes in B. juncea seedlings even in the absence of stress with the expression of genes of both APR and ATP-S, much higher in tolerant cultivar compared to sensitive one. Earlier, the occurrence of a complex signaling network in the regulation of APR was reported in salinity-exposed Arabidopsis roots (Koprivova et al. 2008). Active uptake of sulfate uptake at the root plasma membrane is facilitated by the Group 1 sulfate transporters (Smith et al. 1995). Studies have reported Sultr1;2 as the primary sulfate transporter expressed in the root, but Sultr1;1 exhibited very low expression therein (Koralewska et al. 2008, 2009). The expression of APR in plants is regulated largely by reduced sulfur compounds (Koralewska et al. 2009; Davidian and Kopriva 2010). Notably, considered for a long time as a toxic by-product of cell metabolism, H2S, used as an S-source, has been reported to contribute in regulating Na+/K+ balance (Mostofa et al. 2015; Jiang et al. 2019). H2S-mediated maintenance of the Na+/K+ balance was evidenced to involve H2S-induced regulation of the expression of plasma membrane H+-ATPase (PM H+-ATPase), salt overly sensitive 1 (SOS1), a plasma membrane Na+/H+ antiporter and SKOR, an outward rectifying K+ channel (Jiang et al. 2019). Notably, PM H+-ATPase is known to sustain an H+ gradient by promoting Na+ efflux and H+ influx to drive Na+/H+ antiport across the plasma membrane (Jiang et al. 2019). Earlier, H2S-induced increase in Na+ extrusion and decrease in Na+ uptake and thereby plant salinity tolerance were argued to involve H2S-mediated regulation of the membrane-bound translocation proteins of the SOS1 pathways (Deng et al. 2016). Additionally, H2S-mediated regulation of the expression of SOS pathway genes was found to keep the balance of Na+ and K+ in the test plant (Christou et al. 2013).
Schematic representation of the potential mechanisms underlying the role of salicylic acid (SA), sulfur (S), and the result of the interaction between SA, S, and gaseous phytohormone ethylene in salinity impact-mitigation in plants. AVG, 2-aminoethoxyvinylglycine (ethylene biosynthesis inhibitor); ACC, 1-aminocyclopropane-1-carboxylate (ethylene precursor); ACS, 1-aminocyclopropane-1-carboxylic acid synthase; S-AdoMet, S-adenosyl methionine (ethylene production-precursor); APS, adenosine 5′-phosphosulfate; TBARS, thiobarbituric acid reactive substances; GSH, reduced glutathione
Salicylic acid and S interaction with other phytohormones
In addition to interacting with plant nutrient S, SA has been reported to interact with several phytohormones, polyamines, nitric oxide, and also with other plant nutrients. In several reports, S co-coordinately worked with different phytohormones including auxins, gibberellins, cytokinins, abscisic acid, brassinosteroids, ethylene, nitric oxide, and salicylic acid in different plants (Pál et al. 2014; Khan et al. 2010, 2014a, b, 2015; Hasanuzzaman et al. 2018; Jahan et al. 2019). The results of the interaction of SA with nutrient S and other phytohormones significantly contributed to plant growth, metabolism, and stress tolerance (Hasanuzzaman et al. 2018). Exogenous SA can elevate S/Cys-GSH content, and also result in a higher GR activity (Pál et al. 2014). SA-mediated increase in GSH contents was argued as the result of the SA-mediated increase in activity of ATP-S and serine acetyltransferase (SAT), and the contents of S and Cys (Nazar et al. 2011, 2014a; Hussain et al. 2020). Notably, a sulfotransferase (SOT12) has been reported to regulate SA homeostasis via sulfation (Baek et al. 2010); and the S-nitrosylation was involved in SA signaling (Mikkelsen et al. 2003). SA-induced enhancement in the N and S assimilation hence inducing salinity tolerance in plants were reported to involve SA-mediated upregulation of the activity of enzymatic and non-enzymatic antioxidant systems (Nazar et al. 2011). The application of SA accelerated the uptake of P, N, K, Ca, Mg, and efflux of Na+ ions, and thereby increased the growth of salt-stressed Cucumis sativus and protected this crop against salinity impacts (Yildirim et al. 2008). In another instance, exogenously applied SA (0.5 mM) improved the growth, yield, and gas exchange in salt-stressed Z. mays by regulating the high ratio of K+/Na+ and Ca+/Na+ (Tufail et al. 2013). SA-mediated reversal of the salt-mediated alteration in the contents of Na, K, Ca, and Mg content was found in T. aestivum (Al-Hakimi and Hamada 2001). In combination with selenium, SA was found to reduce the temperature-accrued increased ROS by enhancing antioxidant metabolism and significantly reducing Na+/K+ ratio in NaCl exposed plants (Abdel-Salam 2016). SA-mediated reduction in the concentration of K and P was also reported in the shoot and root of salinity stressed Z. mays (Gunes et al. 2007). However, SA-supply was also reported to inhibit K+ uptake and accelerate Na+ in S. lycopersicum supplied with SA (Szepesi et al. 2009). Yoshida and Noguchi (2009) found that SA improved the S uptake and regulated GSH biosynthesis during ozone stress in Arabidopsis.
The gaseous hormone ethylene has been reported to exhibit myriad roles in plants and modulation of growth and photosynthesis phenomena under optimal as well as stressful conditions (Khan and Khan 2014). Ethylene can also control photosynthesis through regulating stomatal movement and thereby allowing more influx of CO2 for carboxylation under optimal and stressful environments (Iqbal et al. 2011, 2012; Masood et al. 2012; Khan et al. 2013). In another instance, ethylene-supply was reported to increase the stomatal aperture and thereby increased the diffusion rate of CO2 from the atmosphere to the intercellular spaces (Acharya and Assmann 2009). There occurs a close relation between SA and 1-aminocyclopropane-1-carboxylic acid synthase (ACS) under stressful conditions, where SA can inhibit ethylene biosynthesis by restricting the conversion of 1-aminocyclopropane-1-carboxylate to ethylene, and protect plants against stress impacts (Khan et al. 2012, 2015). A close relation also exists between S, ethylene, and SA, where S is known to influence ethylene biosynthesis through SAM, ethylene production-precursor; and SA has been reported to inhibit ethylene (Masood et al. 2012; Khan et al. 2014a, b; Nazar et al. 2015b). The use of 2-aminoethoxyvinyl glycine (AVG), ethylene biosynthesis inhibitor) in the experiment blocked ethylene synthesis; however, it produced similar effects to SA and SA-analog, INA, on the contents of S-containing compounds (Hussain et al. 2019; Khan et al. 2014a, b). Also, AVG- and SA-mediated inhibition in the activity of ACS was reported in earlier studies (Khan et al. 2012, 2015). Thus, the interplay between SO42−, SA and ethylene can occur which may play a major role in the modulation of salinity-exposed plants supplied with nutrients such as S and/or phytohormone such as SA (Fig. 3).
Appraisal and prospects
The literature reviewed herein evidenced that soil-salinization is a major threat to agriculture. It has been the major stress factor for severely impacting crop health and reducing yield. There occurs a host of approaches for mitigating salinity stress impacts in plants. The strategy of using plant nutrient S and phytohormone SA has emerged as a potential approach for modulating several processes including ion homeostasis, compatible solute accumulation, and osmotic adjustments and the regulation of cellular antioxidants and eventually contributing to plant stress tolerance. It is noteworthy to mention here that through involvement in S-containing compounds, such as GSH and SAM, S contributes to salinity tolerance via directly or indirectly. On the other hand, the appraisal of the literature related to SA, it is suggested that exogenous SA could ameliorate the salinity-toxicity symptoms in plants by improving photosynthetic capacity, enhanced antioxidant protection through modulating antioxidant defense system components, ROS-metabolism, and increasing accumulation of soluble carbohydrates. The role of S (or its source) is very clear in the regulation of Na+/K+ balance, the expression of plasma membrane H+ ATPase, SOS1, and SKOR in salinity stressed plants. However, the mechanism underlying the role of SA in the maintenance of ionic homeostasis under salinity stress is poorly understood (Jayakannan et al. 2015). To this end, on the one hand, the supplied SA decreased K+ and P in shoot and root tissues of salinity-exposed maize (Gunes et al. 2007) and barley shoots (El-Tayeb 2005). On the other hand, SA-supply failed to affect Na+ and Cl− concentrations in salinized spinach roots and shoots (Eraslan et al. 2008); and also, SA-application inhibited K+ uptake and increased Na+ uptake in tomato plants (Szepesi et al. 2009). Additionally, the use of SA or S in salinity-exposed plants has been done either in isolated studies or SA and S have been cross-talked without convincing outcomes in context with plant salinity tolerance. The exploitation of S and SA as a stress management tool could be a subject of interest as this mineral nutrient and phytohormone combination can impart tolerance to many crops against the salinity. It is very imperative to unravel the insights into the major or minor regulatory point in S assimilation; SA-mediated regulated processes and the potential involvement of other phytohormones (such as ethylene) in SA + S-mediated salinity tolerance.
References
Abdel-Salam MM (2016) Effect of foliar application of salicylic acid and micronutrients on the berries quality of “Bez El Naka” local grape cultivar. Science 6:178–188
Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol 69:451–462
Ahanger MA, Mir RA, Alyemeni MN, Ahmad P (2020) Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol Biochem 147:31–42
Ahmad P, Latef AAA, Hashem A, Abd Allah EF, Gucel S, Tran LSP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Ahmad F, Singh A, Kamal A (2017) Ameliorative effect of salicylic acid in salinity stressed Pisum sativum by improving growth parameters, activating photosynthesis and enhancing antioxidant defense system. Biosci Biotechnol Res Commun 10:481–489
Al-Hakimi AMA, Hamada AM (2001) Counteraction of salinity stress on wheat plants by grain soaking in ascorbic acid, thiamin or sodium salicylate. Biol Plant 44:253–261
Al-Mahmud J, Al Hasanuzzaman M, Khan MIR, Nahar K, Fujita M (2020) β-Aminobutyric acid pretreatment confers salt stress tolerance in Brassica napus L. by modulating reactive oxygen species metabolism and methylglyoxal detoxification. Plants. https://doi.org/10.3390/plants9020241
Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA (2008) Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione: Sulphur protects mustard from cadmium toxicity. Plant Growth Regul 54:271–279
Anjum NA, Gill R, Kaushik M, Hasanuzzaman M, Pereira E, Ahmad I, Tuteja N, Gill SS (2015) ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front Plant Sci 6:210
Asgher M, Khan NA, Khan MIR, Fatma M, Masood A (2014) Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol Environ Saf 106:54–61
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190
Astolfi S, Zuchi S (2013) Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiol Plant 35:175–181
Aziz A, Ashraf M, Sikandar S, Asif M, Akhtar N, Shahzad SM, Wasaya A, Raza A, Babar BH (2019) Optimizing sulfur for improving salt tolerance of sunflower (Helianthus annuus L.). Soil Environ 38:222–233
Baek D, Pathange P, Chung JS, Jiang J, Gao L, Oikawa A, Hirai MY, Saito K, Pare PW, Shi HA (2010) Stress-inducible sulphotransferase sulphonates salicylic acid and confers pathogen resistance in Arabidopsis. Plant Cell Environ 33:1383–1392
Batista VCV, Pereira IMC, de Oliveira P-MS, Canuto KM, Pereira RDCA, Rodrigues THS, de Carvalho HH (2019) Salicylic acid modulates primary and volatile metabolites to alleviate salt stress-induced photosynthesis impairment on medicinal plant Egletes viscose. Environ Exp Bot 167:103870
Carpici EB, Celik N, Bayram G, Asik BB (2010) The effects of salt stress on the growth, biochemical parameter and mineral element content of some maize (Zea mays L.) cultivars. Afr J Biotechnol 9:6937–6942
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:e23681
Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64:1953–1966
Coskun D, Britto DT, Huynh WQ, Kronzucker HJ (2016) The role of silicon in higher plants under salinity and drought stress. Front Plant Sci 7:1072
Csiszár J, Horváth E, Váry Z, Gallé Á, Bela K, Brunner S, Tari I (2014) Glutathione transferase supergene family in tomato: salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol Biochem 78:15–26
Cuin TA, Shabala S (2008) Compatible solutes mitigate damaging effects of salt stress by reducing the impact of stress-induced reactive oxygen species. Plant Signal Behav 3:207–208
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53
Davidian JC, Kopriva S (2010) Regulation of sulfate uptake and assimilation – the same or not the same? Mol Plant 3:314–325
de Souza Freitas WE, de Oliveira AB, Mesquita RO, de Carvalho HH, Prisco JT, Gomes-Filho E (2019) Sulfur-induced salinity tolerance in lettuce is due to a better P and K uptake, lower Na/K ratio and an efficient antioxidative defense system. Sci Hortic 257:108764
Deng YQ, Bao J, Yuan F, Liang X, Feng ZT, Wang BS (2016) Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul 79:391–399
El-Tayeb MA (2005) Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 45:215–224
Eraslan F, Inal A, Pilbeam DJ, Gunes A (2008) Interactive effects of salicylic acid and silicon on oxidative damage and antioxidant activity in spinach (Spinacia oleracea L. cv. Matador) grown under boron toxicity and salinity. Plant Growth Regul 55:207
Faghih S, Ghobadi C, Zarei A (2017) Response of strawberry plant cv.‘Camarosa’ to salicylic acid and methyl jasmonate application under salt stress condition. J Plant Growth Regul 36:651–659
Fahad S, Hussain S, Saud S, Hassan S, Tanveer M, Ihsan MZ, Shah AN, Ullah A, Nasrullah Khan F et al (2016) A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol Biochem 103:191–198
Farahbakhsh H, Pasandi Pour A, Reiahi N (2017) Physiological response of henna (Lawsonia inermis L.) to salicylic acid and salinity. Plant Prot Sci 20:237–247
Farhangi-Abriz S, Ghassemi-Golezani K (2018) How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol Environ Saf 147:1010–1016
Farheen J, Mansoor S, Abideen Z (2018) Exogenously applied salicylic acid improved growth, photosynthetic pigments and oxidative stability in mungbean seedlings (Vigna radiata L.) at salt stress. Pak J Bot 50:901–912
Fatma M, Khan RH, Masood A, Khan NA (2013) Coordinate changes in assimilatory sulfate reduction are correlated to salinity tolerance: involvement of phytohormones. Annu Res Rev Biol 10:267–925
Fatma M, Asgher M, Masood A, Khan NA (2014) Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ Exp Bot 107:55–63
Fatma M, Masood A, Per TS, Khan NA (2016) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front Plant Sci 7:521
Gharbi E, Martínez JP, Benahmed H, Dailly H, Quinet M, Lutts S (2017) The salicylic acid analog 2, 6-dichloroisonicotinic acid has specific impact on the response of the halophyte plant species Solanum chilense to salinity. Plant Growth Regul 82:517–525
Gharbi E, Lutts S, Dailly H, Quinet M (2018) Comparison between the impacts of two different modes of salicylic acid application on tomato (Solanum lycopersicum) responses to salinity. Plant Signal Behav 13:e1469361
Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci EG, Cicek N (2007) Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol 164:728–736
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. https://doi.org/10.1155/2014/701596
Hasanuzzaman M, Hossain MA, Fujita M (2011) Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep 5:353–365
Hasanuzzaman M, Nahar K, Fujita M (2013) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad P, Azooz MM, Prasad MNV (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 25–87
Hasanuzzaman M, Bhuyan MHMB, Mahmud JA, Nahar K, Mohsin SM, Parvin K, Fujita M (2018) Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal Behav 13:e1477905
Huang H, Cao H, Niu Y, Dai S (2012) Expression analysis of nudix hydrolase genes in Chrysanthemum lavandulifolium. Plant Mol Biol Report 30:973–982
Husen A, Iqbal M, Sohrab SS, Ansari MKA (2018) Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata A. Br.). Agric Food Secur 7:44
Hussain SJ, Masood A, Anjum NA, Khan NA (2019) Sulfur-mediated control of salinity impact on photosynthesis and growth in mungbean cultivars screened for salt tolerance involves glutathione and proline metabolism, and glucose sensitivity. Acta Physiol Plant 41:1–13
Hussain SJ, Khan NA, Anjum NA, Masood A, Khan MIR (2020) Mechanistic elucidation of salicylic acid and sulphur-induced defence systems, nitrogen metabolism, photosynthetic, and growth potential of mungbean (Vigna radiata) under salt stress. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10159-4
Iqbal N, Nazar R, Syeed S, Masood A, Khan NA (2011) Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J Exp Bot 62:4955–4963
Iqbal N, Khan NA, Nazar R, Silva JA (2012) Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur assimilation in mustard types that differ in photosynthetic capacity. Environ Exp Bot 78:84–90
Iqbal N, Umar S, Khan NA, Khan MIR (2014) A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ Exp Bot 100:34–42
Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 178:84–91
Isayenkov SV, Maathuis FJM (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80
Jahan B, Sehar Z, Masood A, Anjum NA, Khan MIR, Khan NA (2019) Sulfur availability potentiates phytohormones-mediated action in plants. In: Khan MIR, Reddy S, Ferrante A, Khan NA (eds) Plant signaling molecules: role and regulation under stressful environments. Woodhead Publishing, Elsevier, pp 287–301
Jahan B, AlAjmi MF, Rehman MT, Khan NA (2020) Treatment of nitric oxide supplemented with nitrogen and sulfur regulates photosynthetic performance and stomatal behavior in mustard under salt stress. Physiol Plant 168:490–510
Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene expression profiling of plants under salt stress. Crit Rev Plant Sci 30:435–458
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2013) Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot 64:2255–2268
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2015) Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul 76:25–40
Jiang JL, Tian Y, Li L, Yu M, Hou RP, Ren XM (2019) H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front Plant Sci 10:678
Jini D, Joseph B (2017) Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci 24:97–108
Jumali SS, Said IM, Ismail I, Zainal Z (2011) Genes induced by high concentration of salicylic acid in Mitragyna speciosa. Aust J Crop Sci 5:296
Khan MIR, Khan NA (2014) Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 251:1007–1019
Khan NA, Anjum NA, Nazar R, Iqbal N (2009a) Increased activity of atp-sulfurylase and increased contents of cysteine and glutathione reduce high cadmium-induced oxidative stress in mustard cultivar with high photosynthetic potential. Russ J Plant Physiol 56:670–677
Khan NA, Nazar R, Anjum NA (2009b) Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in ATP-sulfurylase activity under salinity stress. Sci Hortic 122:455–460
Khan NA, Syeed S, Masood A, Nazar R, Iqbal N (2010) Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int J Plant Biol 1:1–8
Khan NA, Nazar R, Iqbal N, Anjum NA (2012) Phytohormones and abiotic stress tolerance in plants. Springer-Verlag, Berlin/Heidelberg
Khan MIR, Iqbal N, Masood A, Per TS, Khan NA (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav 8:e26374
Khan MIR, Asgher M, Khan NA (2014a) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74
Khan NA, Khan MIR, Asghar M, Fatma M, Masood A, Syeed S (2014b) Salinity tolerance in plants, revisiting the role of sulfur metabolites. J Plant Biochem Physiol 2:1–8
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462
Khodarahmpour Z, Ifar M, Motamedi M (2012) Effects of NaCl salinity on maize (Zea mays L.) at germination and early seedling stage. Afr J Biotechnol 11:298–304
Kim Y, Mun BG, Khan AL, Waqas M, Kim HH, Shahzad R, Lee IJ (2018) Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS One 13:e0192650
Kopriva S, Jones S, Koprivova A, Suter M, Von Ballmoos P, Brander K, Flückiger J, Brunold C (2001) Influence of chilling stress on the intercellular distribution of assimilatory sulfate reduction and thiols in Zea mays. Plant Biol 3:24–31
Koprivova A, North KA, Kopriva S (2008) Complex signaling network in regulation of adenosine 5′-phosphosulfate reductase by salt stress in arabidopsis roots. Plant Physiol 146:1408–1420
Koralewska A, Stuiver CEE, Posthumus FS, Kopriva S, Hawkesford MJ, De Kok LJ (2008) Regulation of sulfate uptake, expression of the sulfate transporters Sultr1;1 and Sultr1;2, and APS reductase in Chinese cabbage (Brassica pekinensis) as affected by atmospheric H2S nutrition and sulfate deprivation. Funct Plant Biol 35:318–327
Koralewska A, Buchner P, Stuiver CEE, Posthumus FS, Kopriva S, Hawkesford MJ, De Kok LJ (2009) Expression and activity of sulfate transporters and APS reductase in curly kale in response to sulfate deprivation and re-supply. J Plant Physiol 166:168–179
Lee S, Kim SG, Park CM (2010) Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol 188:626–637
Li G, Peng X, Wei L, Kang G (2013) Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene 529:321–325
Maruri-López I, Aviles-Baltazar NY, Buchala A, Serrano M (2019) Intra and extracellular journey of the phytohormone salicylic acid. Front Plant Sci 10:423
Masood A, Iqbal N, Khan NA (2012) Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ 35:524–533
Mikkelsen MD, Petersen BL, Glawischnig E, Jensen AB, Andreasson E, Halkier BA (2003) Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol 131:298–308
Mohammed AR, Tarpley L (2009) Effects of elevated ultraviolet-B radiation on productive tillers, spikelet sterility and grain characteristics of southern US rice (Oryza sativa L.) cultivars. J Agron Crop Sci 195:292–300
Mostofa MG, Fujita M, Tran LS (2015) Nitric oxide mediates hydrogen peroxide-and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 77:265–277
Mukhtar I, Shahid MA, Khan MW, Balal RM, Iqbal MM, Naz T, Zubair M, Ali HH (2016) Improving salinity tolerance in chili by exogenous application of calcium and sulphur. Soil Environ 35:56–64
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Nazar R, Iqbal N, Masood A, Syeed S, Khan NA (2011) Understanding the significance of sulfur in improving salinity tolerance in plants. Environ Exp Bot 70:80–87
Nazar R, Umar S, Khan NA (2014a) Involvement of salicylic acid in sulfur induced salinity tolerance: a role of glutathione. Annu Res Rev Biol 4:3875–3893
Nazar R, Khan MI, Iqbal N, Masood A, Khan NA (2014b) Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol Plant 152:331–344
Nazar R, Umar S, Khan NA (2015a) Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal Behav 10:e1003751
Nazar R, Umar S, Khan NA, Sareer O (2015b) Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S Afr J Bot 98:84–94
Nie S, Yue H, Zhou J, Xing D (2015) Mitochondrial-derived reactive oxygen species play a vital role in the salicylic acid signaling pathway in Arabidopsis thaliana. PLoS One 10:e0119853
Nie W, Gong B, Chen Y, Wang J, Wei M, Shi Q (2018) Photosynthetic capacity, ion homeostasis and reactive oxygen metabolism were involved in exogenous salicylic acid increasing cucumber seedlings tolerance to alkaline stress. Sci Hortic 235:413–423
Oraghi Ardebili N, Iranbakhsh A, Oraghi Ardebili Z (2019) Efficiency of selenium and salicylic acid protection against salinity in soybean. Plant Physiol 9:2727–2738
Osman AS, Rady MM (2012) Ameliorative effects of sulphur and humic acid on the growth, anti-oxidant levels, and yields of pea (Pisum sativum L.) plants grown in reclaimed saline soil. J Hortic Sci Biotechnol 87:626–632
Pál M, Kovács V, Szalai G, Soós V, Ma X, Liu H, Mei H, Janda T (2014) Salicylic acid and abiotic stress responses in rice. J Agron Crop Sci 200:1–11
Palma F, López-Gómez M, Tejera NA, Lluch C (2013) Salicylic acid improves the salinity tolerance of Medicago sativa in symbiosis with Sinorhizobium meliloti by preventing nitrogen fixation inhibition. Plant Sci 208:75–82
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Parvin K, Ahamed KU, Islam MM, Haque MN (2015) Response of tomato plant under salt stress: role of exogenous calcium. J Plant Sci 10:222–233
Per TS, Fatma M, Asgher M, Javied S, Khan NA (2017) Salicylic acid and nutrients interplay in abiotic stress tolerance. In: Nazar R, Iqbal N, Khan NA (eds) Salicylic acid: a multifaceted hormone. Springer, Singapore, pp 221–237
Perveen S, Iqbal N, Saeed M et al (2018) Role of foliar application of sulfur-containing compounds on maize (Zea mays L. var. Malka and hybrid DTC) under salt stress. Braz J Bot 41:805–815
Pokotylo I, Kravets V, Ruelland E (2019) Salicylic acid binding proteins (SABPs): the hidden forefront of salicylic acid signalling. Int J Mol Sci 20:4377
Rais L, Masood A, Inam A, Khan NA (2013) Sulfur and nitrogen co-ordinately improve photosynthetic efficiency, growth and proline accumulation in two cultivars of mustard under salt stress. J Plant Biochem Physiol 1:1
Reich M, Aghajanzadeh T, Helm J, Parmar S, Hawkesford MJ, De Kok LJ (2017) Chloride and sulfate salinity differently affect biomass, mineral nutrient composition and expression of sulfate transport and assimilation genes in Brassica rapa. Plant Soil 411:319–332
Ren CG, Kong CC, Xie ZH (2018) Role of abscisic acid in strigolactone-induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol 18:74
Ryu H, Cho YG (2015) Plant hormones in salt stress tolerance. J Plant Biol 58:147–155
Samadi S, Habibi G, Vaziri A (2019) Effects of exogenous salicylic acid on antioxidative responses, phenolic metabolism and photochemical activity of strawberry under salt stress. Plant Physiol 9:2685–2694
Scherer HW (2009) Sulfur in soils. J Plant Nutr Soil Sci 172:326–335
Sehar Z, Masood A, Khan NA (2019) Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ Exp Bot 161:277–289
Shahbaz M, Ashraf M (2013) Improving salinity tolerance in cereals. Crit Rev Plant Sci 32:37–249
Shan C, Liu H, Zhao L, Wang X (2014) Effects of exogenous hydrogen sulfide on the redox states of ascorbate and glutathione in maize leaves under salt stress. Biol Plant 58:169–173
Sharma R, Mishra M, Gupta B, Parsania C, Singla-Pareek SL, Pareek A (2015) De novo assembly and characterization of stress transcriptome in a salinity-tolerant variety CS52 of Brassica juncea. PLoS One 10:e0126783
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B (2019) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9:285
Shokat S, Großkinsky DK (2019) Tackling salinity in sustainable agriculture-what developing countries may learn from approaches of the developed world. Sustainability 11:4558
Siboza XI, Bertling I, Odindo AO (2017) Enzymatic antioxidants in response to methyl jasmonate and salicylic acid and their effect on chilling tolerance in lemon fruit [Citrus limon (L.) Burm. F.]. Sci Hortic 225:659–567
Singh M, Singh VP, Prasad SM (2016) Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol Biochem 109:72–83
Smith FW, Ealing PM, Hawkesford MJ, Clarkson DT (1995) Plant members of a family of sulfate transporters reveal functional subtypes. Proc Natl Acad Sci U S A 92:9373–9377
Srivastava AK, Srivastava S, Lokhande VH, D’Souza SF, Suprasanna P (2015) Salt stress reveals differential antioxidant and energetics responses in glycophyte (Brassica juncea L.) and halophyte (Sesuvium portulacastrum L.). Front Environ Sci 3:19
Syeed S, Anjum NA, Nazar R, Iqbal N, Masood A, Khan NA (2011) Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol Plant 33:877–886
Szepesi Á, Csiszár J, Gémes K, Horváth E, Horváth F, Simon ML, Tari I (2009) Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L. J Plant Physiol 166:914–925
Tahjib-Ul-Arif M, Siddiqui MN, Sohag AA, Sakil MA, Rahman MM, Polash MA, Mostofa MG, Tran LS (2018) Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J Plant Growth Regul 37:1318–1330
Tamaoki M (2008) The role of phytohormone signaling in ozone-induced cell death in plants. Plant Signal Behav 3:166–174
Torun H (2019) Time-course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol Plant 165:169–182
Tufail A, Arfan M, Gurmani AR, Khan A, Bano A (2013) Salicylic acid induced salinity tolerance in maize (Zea mays). Pak J Bot 45:75–82
Ulfat M, Athar HUR, Ashraf M, Akram NA, Jamil A (2007) Appraisal of physiological and biochemical selection criteria for evaluation of salt tolerance in canola (Brassica napus L.). Pak J Bot 39:1593–1608
Wang Y, Shen W, Chan Z, Wu Y (2015) Endogenous cytokinin overproduction modulates ROS homeostasis and decreases salt stress resistance in Arabidopsis thaliana. Front Plant Sci 6:1004
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176
Xu GY, Rocha PSCF, Wang ML, Xu ML, Cui YC, Li LY, Zhu YX, Xia X (2011) A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234:47–59
Yan Y, Pan C, Du Y, Li D, Liu W (2018) Exogenous salicylic acid regulates reactive oxygen species metabolism and ascorbate–glutathione cycle in Nitraria tangutorum Bobr. under salinity stress. Physiol Mol Biol Plants 24:577–589
Yildirim E, Turan M, Guvenc I (2008) Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J Plant Nutr 31:593–612
Yoshida K, Noguchi K (2009) Differential gene expression profiles of the mitochondrial respiratory components in illuminated Arabidopsis leaves. Plant Cell Physiol 50:1449–1462
Zhang Y, Xu S, Yang S, Chen Y (2015) Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma 252:911–924
Zheng X, Tan DX, Allan AC, Zuo B, Zhao Y, Reiter RJ, Shan D (2017) Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci Rep 7:41236
Author information
Authors and Affiliations
Contributions
Conceptualization, NAK and NAA writing—original draft preparation, FR and NAA writing—review and editing, NAK, NAA and AS supervision, NAK All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rasheed, F., Anjum, N.A., Masood, A. et al. The key roles of salicylic acid and sulfur in plant salinity stress tolerance. J Plant Growth Regul 41, 1891–1904 (2022). https://doi.org/10.1007/s00344-020-10257-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10257-3