Abstract
Objectives
The objective of this study is to report on the performance of the MRI-guided VABB in our center and to look at the long-term outcome of biopsies with benign histology over a period of 19 years.
Methods
In a single-center retrospective review study, data of 600 VABB procedures performed between September 1999 and March 2017 were evaluated. We collected patient demographics, histopathological diagnosis at MRI-VABB, and basic lesion characteristics (size, location). Data from the Belgian Cancer Registry was cross-referenced with our database to find out which patients with benign MRI-VABB results developed a malignant lesion over time.
Results
These 600 VABB procedures were performed in 558 women with a mean patient age of 51.8 years (range 18–82 years). Our technical success rate was 99.3%. We found 27.67% B5 lesions, 9.82% B3 lesions, and 0.17% B4 lesions. Of 362 benign MRI-guided VABBs, follow-up data was available for a mean follow-up period of 7.6 years (0.8–18.3). Only one (0.3%) biopsy was a false negative lesion after MRI-guided VABB during follow-up. Short-term FU-MRI provided no increase in detection rate.
Conclusion
The accuracy of MRI-guided VABB is high with a very low false negative rate of 0.3% on long-term follow-up. The value of short-term FU-MRI for every case after MRI-guided VABB may be questioned.
Key Points
• MRI-guided vacuum-assisted breast biopsies yield a large portion of clinically relevant lesions (9.82% B3, 0.17% B4, and 27.67% B5 lesions).
• The false negative biopsy rate of MRI-guided VABB in this study with a mean follow-up time of 7.6 years was only 0.3%.
• Performing a short-term follow-up MRI after a benign MRI-guided VABB concordant to the MRI appearance may be questioned.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The diagnostic accuracy of magnetic resonance imaging (MRI) for the diagnosis of lesions in the breast is well studied. It offers high sensitivity but variable specificity with a PPV of 56% and a NPV of 100% [1]. The European guidelines for image-guided breast biopsies state that for suspicious lesions only visible on MRI, MRI-guided VABB is mandatory when second-look/targeted ultrasound fails to reveal these lesions. Second-look ultrasound may reveal MRI detected lesions in 46 to 71% [2, 3]. Therefore, a large number of these cases depend on this MRI-guided biopsy technique for their diagnostic work-up. MRI-guided VABB, lacking real-time control, requires a high level of expertise.
Several previous studies reported on the results of MRI-guided VABB but data on long-term follow-up outcomes of benign MRI-guided VABB concordant to the MRI appearance and the necessity of short-term FU-MRI are limited [5,6,7,8,9,10,11,12,13,14,15]. In this study, we focused on the long-term outcome of benign MRI-guided VABB in a series of 600 consecutive biopsies by cross-referencing our database to the national cancer registry which records all new Belgian breast cancers.
Materials and methods
Study design
This single-center retrospective study of MRI-guided VABBs in the Hospital AZ Sint-Jan AV, Bruges, reviewed all 600 procedures performed by the same experienced radiologist (J.C.), between September 1999 and March 2017. This radiologist has experience in MRI of the breast since 1991 and performed the first MRI-guided VABBs in 1999. The biopsies were performed in accordance to the guidelines from the European Society of Breast Imaging for diagnostic interventional breast procedures [2, 16]. Data of 52 of our patients were partly included in a multicenter study that was published by Heywang-Köbrunner et al in 2001 [17]. These data were also used in the publications by Perlet et al in Eur Radiol. (2002), Hefler et al in Eur Radiol. (2003), and Perlet et al in Cancer (2006) [9, 18, 19]. Our new dataset includes 506 additional patients for a total of 600 biopsies, with a much longer follow-up period. Patients meeting the following inclusion criteria were considered for MRI-guided biopsy: lesions categorized as suspicious (BI-RADS 4 or 5), not visible on a targeted mammogram or ultrasound and visualized on MRI and previous BI-RADS 3 lesions that had become more suspicious on a follow-up MRI, ideally performed in the optimal window of the menstrual cycle (day 5–15) [20]. By exception, patients in the preoperative planning were also considered for an MRI-guided VABB without having to meet all of the abovementioned criteria, so that therapy for the other lesion would not be delayed. The indication for the MRI-guided breast intervention was discussed in an interdisciplinary board for the patients of our hospital. For the externally referred patients, there was contact with the referring physician and a detailed clinical history file was provided before the biopsy was planned.
Biopsies with benign histology were either correlated on a 2-weekly multidisciplinary team meeting (MDT) for non-referred patients or at their referring center for referred patients. A second VABB was considered when the reliability of the initial VABB was uncertain or when the biopsy was discordant with the short-term FU-MRI. Referred patients had this short-term FU-MRI at their referring centers.
All patients with an initially benign VABB result were matched with the Belgian Cancer Registry database to countercheck for subsequent breast cancer during long-term follow-up.
High-risk and malignant lesions were treated according to the national guidelines after MDT [4].
MRI protocol and biopsy
We used the following MR systems: Symphony (1.5 T, Siemens), Achieva (1.5 T, Philips), Achieva Quasar Dual (3 T, Philips), and Ingenia (3 T, Philips). The sequences remained unchanged over time, but the resolution and signal noise ratio improved with the development of newer MR systems. The most recent biopsy protocol consisted of a 2D sagittal T1-weighted sequence for visualization of the markers inside the compression device (flip angle 10; repetition time/echo time (TR/TE) 4.8/2.4 ms; voxel size 1 × 1 × 3 mm), a dynamic 3D T1-weighted THRIVE sequence (flip angle 10; repetition time/echo time (TR/TE) 5.4/2.4 ms; voxel size 0.9 × 0.9 × 0.9 mm) with image acquisition before and at 90 and 180 s after gadolinium administration (0.1 mmol/kg body weight at 3 ml/s), and a 3D T1-weighted sequence (flip angle 15; repetition time/echo time (TR/TE) 5.4/2.4 ms; voxel size 0.9 × 0.9 × 0.9 mm) to check the position of the localizing device. For every patient with a benign result, a short-term FU-MRI was scheduled 2 months post-procedural or recommended to the referring center.
Until 2016, gadobutrol (Magnevist®, Bayer) was used, thereafter gadopentetate dimeglumine (Gadovist®, Bayer).
In the first years (1999–2005), there was no automated software for the localization of the needle. DynaCAD® (Invivo, Philips Healthcare) software became available in 2006 and we used it for motion artifact correction, image subtraction, and calculation of the lesion coordinates and to check the position of the needle.
Material
First in use was the Mammotome® VABB system (Ethicon), together with a prototype breast biopsy coil that was developed by S.H. Heywang-Köbrunner, Siemens, and Epoxonic (patents by S.H. Heywang-Köbrunner al. 1992–1997). The aiming device was manufactured by Fischer. In 2006, we started working with a dedicated movable trolley, which allowed us to take the biopsies outside the MR suite. In 2012, the EnCor® (SenoRX) VABB system became available. The selection of biopsy system in each case was left to the discretion of the operator. The Mammotome® system was preferred for lesions with a more difficult localization. The EnCor® system was preferred for cases where the lesion was more diffuse and easy to target. For each VABB, 12–36 samples were taken with a 7G needle. The number of samples depended on lesion size, breast size, and location of the lesion. Tissue was immediately fixed in buffered formalin. An MRI-compatible clip was inserted into the biopsy cavity.
Data collection and analysis
Before every procedure, a written consent and form specifying date of birth and procedure, indication for the biopsy, lesion type, and lesion size was completed. After the procedure technical difficulties and complications were filled in on the form and the result of the biopsy was added later. In Belgium, registration of all breast biopsy results to the Belgium Cancer Registry is mandatory. The cooperation with this national registry allowed us to match follow-up data of all Belgium patients, regardless whether their biopsy was malignant or benign.
Statistical analysis
We performed descriptive statistics on our database using SPSS 26 (IBM). Qualitative variables were summarized by count and percentage, which included frequencies of diagnosis, evolution of the number of biopsies over time, number of biopsies per patient, and identification of biopsies with a benign histology with a reported breast malignancy to the national cancer registry.
Results
Characteristics of the study population
In the period 1999–2017, 600 MRI-guided VABB procedures were performed in 558 patients. The first decade, the mean number of biopsies was 20 (range 2–34) per year, increasing steadily each year. The second decade, the mean number of biopsies was 49 (range 38–57) per year (Fig. 1).
Patient age ranged from 18 to 82 years with a mean patient age of 51.8 years. Four hundred fifty-seven patients (81.9%) were referred from other hospitals (Table 1).
Mean lesion diameter was 12 mm (range 1.4–100 mm). Of all biopsied lesions, 54.3% (326/600) were smaller than 1 cm and 7% (42/600) were smaller than 5 mm.
Biopsy results
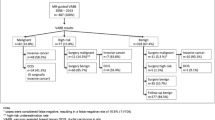
The total number of each histological diagnosis and its B-category is listed in Table 2. Overall we found a 27.7% (166/600) malignancy rate (B5) with an additional 0.2% (1/600) being suspicious for malignancy (B4). Another 9.8% (59/600) biopsies yielded a B3 lesion and 62.3% (374/600) were biopsies with benign histology.
There were 40 patients who had 2 separate biopsies and 1 patient had 3 separate biopsies (Fig. 2).
In 29 of the 40 cases where the patient had 2 biopsies, the second biopsy was done because of an additional suspicious lesion on MRI. In 11 of the 40 cases, the same lesion was biopsied a second time. Technical difficulties during the first procedure caused 3 repeat biopsies, discordant correlation resulted in 2 repeat biopsies, and 5 lesions persisted during follow-up after a benign histology leading to a second VABB. The eleventh repeat biopsy was the only case with a histology suspicious for malignancy. After multidisciplinary team meeting, it was decided to obtain more material for diagnostic purposes.
Two of the abovementioned 11 repeat biopsies resulted in an upgrade to malignancy: one biopsy initially revealed a lesion with uncertain malignant potential (atypical lobular hyperplasia) (B3) and the already mentioned eleventh repeat biopsy was suspicious for malignancy (B4) on the initial VABB.
Nine of the 11 repeat biopsies had an initial biopsy with a benign histology and the additional biopsy remained benign in 7 cases. In 2 cases, both during follow-up, the second biopsy yielded a higher B-category. One case (initial biopsy: usual ductal hyperplasia) had a repeat MRI-guided VABB 1 year later resulting in the diagnosis of an intraductal papilloma without atypia. A second case who underwent a VABB in 2005 of a nodular lesion in the upper outer quadrant of the left breast, which yielded a B2 lesion, developed LCIS of the non-pleomorphic type (B3) at the site of the clip after 7 years (Fig. 3).
Upgrade to a higher B-category on repeat VABB. a Enhancing lesion in the upper outer quadrant of the left breast, diameter 24 mm (pre-MRI-guided VABB). b Enhancing lesion in the same region of the upper outer quadrant of the left breast, diameter 15 mm (post-MRI-guided VABB 5 months later). c, d Second VABB 7 years later of the same enhancing lesion in the upper outer quadrant of the left breast with correct position of the needle, revealing LCIS of the non-pleomorphic type
Only one patient underwent three separate MRI-guided VABBs during 2 sessions. This patient had an MRI during follow-up after invasive carcinoma which showed 3 suspicious lesions: 2 lesions in the right breast and 1 lesion in the left breast (Fig. 2).
The technical success rate was 99.3%: in one case, the biopsy procedure was prematurely stopped due to excessive bleeding. In three other patients, a repeat biopsy was necessary because of a technical inadequate first procedure that resulted in failure to obtain a sample of the targeted lesion. A minor medical complication was prolonged bleeding without need for treatment, which occurred in 44 cases (7.3%). Significant medical complications were severe bleeding and important skin perforation. Excessive bleeding needing long manual pressure occurred in 10 cases (1.7%). As stated above, in one patient (0.2%), the bleeding during the biopsy procedure was so heavy that it had to be stopped prematurely, but sufficient acquired tissue revealed an invasive ductal carcinoma. In one case (0.2%), significant medial skin perforation occurred.
Procedural difficulties comprised technical software and hardware problems, difficult lesion accession, and target lesion alterations. No technical problems occurred in 503 cases (83.8%). The access to the lesion was difficult in 44 cases (7.3%). The target lesion appeared much less enhancing or displaced compared to the initial MR in 34 cases (5.7%). Software and hardware problems occurred in 5 (0.8%) and 12 (2.0%) cases respectively. Patient movement caused significant procedure difficulty in 2 cases (0.3%).
Long-term outcome of biopsies with benign histological findings
Follow-up data was available for 573 of 600 biopsies from 533 of 558 patients (95.5%). Twenty-five patients (27 biopsies) had no follow-up data in the Belgium Cancer Registry as they were foreigners. These were therefore excluded from the follow-up analysis (Fig. 4). Of the 374 biopsies with benign histological findings, 362 (96.7%) had follow-up data.
The mean follow-up time of our cases was 7.6 years (range 10 months–18 years 3 months). For the biopsies with benign histology, a follow-up of 2 years was available in 90% of cases and a follow-up of 3 years was available in 84%.
In 16 of the 362 MRI-guided biopsies with benign histology (4.4%), a breast cancer was reported to the Belgium Cancer Registry. Six of these were biopsies to rule out multicentricity/multifocality (registered primary malignancies and a benign MR-guided VABB for a 2nd separate suspicious lesion) and three cases occurred in the contralateral breast. Seven cases had a reported malignancy developing in the ipsilateral breast over time. (Table 3) (Figs. 5 and 6).
Patient 5 from Table 3, false negative result. a–d Images of the enhancing lesion on MRI with a correct localization of the needle in the left breast (c, d). e Post-biopsy control MRI 5 months later shows a persisting enhancing lesion next to the clip. f Resection specimen of the tumor with the tumor next to the clip
Patient 4 from Table 3, true negative result. a Coronal, axial, and sagittal image of a small enhancing nodule of 6 mm at 9 o’clock in the anterior third of the left breast. b MRI-guided VABB showed a correct positioning of the needle at 9 o’clock (arrow). c Coronal, axial, and sagittal image on a follow-up MRI 6 months later showed a new enhancing nodule of 7 mm at 10 o’clock in the posterior third of the same breast. d Second-look ultrasound showed a nodular lesion at 10 o’clock prepectoral in the left breast at a distance of 3 cm from the residual small hematoma/fibrous tissue after MRI-guided VABB. Ultrasound-guided core needle biopsy of this new nodule revealed an invasive lobular cancer
In 6 of these 7 cases (Table 3, patient 1, 2, 3, 4, 6, and 7), a malignant lesion developed in the same breast in which the initial benign VABB was taken but in a different region. As shown in Table 3, patient 1, 2, 3, 6, and 7 developed a malignant lesion in a different quadrant of the same breast. Patient 4 developed a malignancy in the same quadrant of the same breast but in a clearly different region (Fig. 6). Hence, in these cases, we were dealing with true negative biopsy results. One patient (0.3%) (Table 3, patient 5) with a nodular lesion in the upper outer quadrant of the left breast developed a malignancy 2 years later at the site where the clip of the VABB was left. This was a false negative result, even though the images at the time of the VABB showed a correct localization of the needle in the region of the suspected lesion (Fig. 5). The initial histology showed fibrosis and periductal inflammation. FU-MRI was performed in this case on which the lesion was still visible, but it was smaller.
Together with the abovementioned two cases from the repeat biopsies with a higher B-category on repeat biopsy, a total 3 of the 362 biopsies (0.8%) with initial benign histology received a final B3 or B5 diagnosis during follow-up at the site of the clip.
Discussion
Our goal was to report on the results of our centers’ experience and analyze the long-term outcome of MRI-guided VABB with benign histology.
The results of our 600 procedures underline the diagnostic importance of MRI-guided VABBs of often subcentimetric lesions only visible on MRI, yielding an overall malignancy rate of 27.7% which is in line with the 22–33% malignancy rate previously reported in literature [6, 12,13,14, 21].
Generally, a FU-MRI is recommended at 6 months after a benign concordant biopsy to check on satisfactory targeting and/or lesion stability, although some national guidelines advocate a faster follow-up [15, 22]. Unlike stereotactic VABB and ultrasound-guided VABB, the MRI-guided VABB lacks real-time confirmation of lesion removal [12, 14]. Moreover, MRI of the biopsy cavity immediately after MRI-guided VABB may sometimes not reliably exclude a residual lesion because of leakage of gadolinium in combination with post-procedural bleeding. Therefore, we do not perform an immediate post-procedural control MRI sequence on routine basis in favor of a short-term FU-MRI at 2 months. This allows us to perform a new gadolinium-enhanced T1-weighted sequence, which we find more performant in subcentimetric lesions. Also, when part of lesion persists, this allows us to perform an additional follow-up at 8 months post-biopsy to assess lesion stability.
When the remaining lesions remain stable, a follow-up period of 2 years can be regarded as adequate for follow-up in consideration with the individual risk factors of the patient [3, 13, 23, 24]. Ninety percent of our patients with a benign MRI-guided VABB had follow-up data of minimum 2 years. To the best of our knowledge, there are no studies with a mean follow-up period of 7.6 years or longer, which is a strength of this study. Our data shows that only 1 biopsy (0.3%) was a false negative result with a malignant breast lesion developing at the site of the clip from the initial benign VABB 2 years prior. Long-term FU data in literature are rare, with a relative short follow-up period, varying from 7 to 52 months [8, 9, 12,13,14,15, 21, 25]. Pinkney et al found an overall false negative rate with short-term FU-MRI of 1.4% (range 0.8–2.4%) in the literature [12]. Krug et al reported a false negative findings range of 0–11.7% [21]. In our false negative case, a short-term FU-MRI was performed on which the lesion was still visible but became smaller. If in the referring center, appropriate response would have been given to the short-term FU-MRI findings (repeat biopsy) then the long-term false negative rate would probably have been 0% instead of 0.3%. This case shows similarity to the report of Huang et al where a decrease in size of the lesion with a histology of inflammation led to the presumption that the biopsy was adequate [14]. Thus, when short-term FU-MRI is performed, repeat biopsy should be considered depending on the proportion of the lesion removed, the level of initial suspicion, and critical assessment of radiological-pathological correlation.
Imaging follow-up in patients with a biopsy with benign histology resulted in repeat biopsies in 11 cases, and in 5 of them, a second VABB was performed because the lesion persisted on the short-term FU-MRI. In 2 of these 5 cases, a lesion with uncertain malignant potential was found. However, the case with LCIS at the site of the clip had a 7-year interval between the 2 biopsies with the second enhancing lesion being smaller than the first targeted lesion, which makes it very unlikely that this was the same lesion as 7 years before.
The necessity of short-term FU-MRI has been questioned in a few recent reports [12, 14, 15]. However, intravenous gadolinium administration is possible in the short-term FU-MRI which allows the detection of an enhancing residual lesion that might then require either a repeat biopsy or at least a second follow-up MRI. In our patients, short-term FU-MRI did not provide an increased detection rate of malignant breast lesions. Based on our results, short-term follow-up by MRI could be omitted for unequivocally representative B2 lesions with compatible imaging findings. However, depending on the initial degree of suspicion, imaging findings before and during biopsy, and the type of histology, short-term follow-up might still be useful to consider in the remaining cases. This approach has already been advocated by Shaylor et al [15]. Investigation of the reproducibility of these results for different teams and for different techniques (e.g., removal of smaller volumes of tissue) remains necessary.
One of the limitations of our study is the high referral rate of patients which made it not possible to retrieve all clinical and imaging follow-up information. This was also experienced by other large referral centers [26]. This referral gap in data was partly resolved by our collaboration with the Belgium Cancer Registry, to obtain long-term follow-up data of most of our patients (95.5%).
The use of different console biopsy systems might be regarded as a limitation, but to the best of our knowledge, no biopsy device is proven to be superior. A recent study comparing three different biopsy devices found no difference in false negative or underestimation rates [5].
In conclusion, our results prove that MR-VABB is a highly reliable technique with a very low long-term false negative ratio. The need for short-term follow-up MRI may be reduced to cases without unequivocal correlation. When follow-up is performed, critical multidisciplinary assessment is warranted and a second follow-up MRI or repeat biopsy should be considered for persisting lesions.
Abbreviations
- BI-RADS:
-
Breast imaging-reporting and data system
- CNB:
-
Core needle biopsy
- DCIS:
-
Ductal carcinoma in situ
- EUSOMA:
-
European Society of Breast Cancer Specialists
- FU-MRI:
-
Follow-up MRI
- IDA:
-
Invasive ductal adenocarcinoma
- ILA:
-
Invasive lobular adenocarcinoma
- LCIS:
-
Lobular carcinoma in situ
- LIQ:
-
Lower inner quadrant
- LOQ:
-
Lower outer quadrant
- MX:
-
Mammography
- NHSBSP:
-
National Health Service Breast Cancer Screening Program
- UIQ:
-
Upper inner quadrant
- UOQ:
-
Upper outer quadrant
- US:
-
Ultrasound
- VABB:
-
Vacuum-assisted biopsy of the breast
References
Bennani-Baiti B, Bennani-Baiti N, Baltzer PA (2016) Diagnostic performance of breast magnetic resonance imaging in non-calcified equivocal breast findings: results from a systematic review and meta-analysis. PLoS One 11:99–100
Bick U, Trimboli RM, Athanasiou A et al (2020) Image-guided breast biopsy and localisation: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging 11. https://doi.org/10.1186/s13244-019-0803-x
Morris EA, Comstock CE, Lee CH et al (2013) ACR BI-RADS Magnetic Resonance Imaging. In: D'Orsi CJ, Sickles EA, Mendelson EB et al. ACR BIRADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: Amercian College of Radiology
Wildiers H, Stordeur S, Vlayen J et al (2013) Breast cancer in women: diagnosis, treatment and follow-up. In: KCE Rep. 143 – 3rd Ed. Brussels, Belgium, Belgian Health Care Knowledge Centre (KCE)
Spick C, Schernthaner M, Pinker K et al (2016) MR-guided vacuum-assisted breast biopsy of MRI-only lesions: a single center experience. Eur Radiol 26:3908–3916. https://doi.org/10.1007/s00330-016-4267-9
Imschweiler T, Haueisen H, Kampmann G et al (2014) MRI-guided vacuum-assisted breast biopsy: comparison with stereotactically guided and ultrasound-guided techniques. Eur Radiol 24:128–135. https://doi.org/10.1007/s00330-013-2989-5
Li J, Dershaw DD, Lee CH, Kaplan J, Morris EA (2009) MRI follow-up after concordant, histologically benign diagnosis of breast lesions sampled by MRI-guided biopsy. AJR Am J Roentgenol 193:850–855. https://doi.org/10.2214/AJR.08.2226
Rauch GM, Dogan BE, Smith TB, Liu P, Yang WT (2012) Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. AJR Am J Roentgenol 198:292–299. https://doi.org/10.2214/AJR.11.7594
Perlet C, Heywang-Kobrunner SH, Heinig A et al (2006) Magnetic resonance-guided, vacuum-assisted breast biopsy: results from a European multicenter study of 538 lesions. Cancer 106:982–990. https://doi.org/10.1002/cncr.21720
Malhaire C, El Khoury C, Thibault F et al (2010) Vacuum-assisted biopsies under MR guidance: results of 72 procedures. Eur Radiol 20:1554–1562. https://doi.org/10.1007/s00330-009-1707-9
Meeuwis C, Veltman J, Van Hall HN et al (2012) MR-guided breast biopsy at 3T: diagnostic yield of large core needle biopsy compared with vacuum-assisted biopsy. Eur Radiol 22:341–349. https://doi.org/10.1007/s00330-011-2272-6
Pinkney D, Chikarmane S, Giess C (2019) Do benign-concordant breast MRI biopsy results require short interval follow-up imaging? Report of longitudinal study and review of the literature. Clin Imaging 57:50–55. https://doi.org/10.1016/j.clinimag.2019.05.007
Hayward J, Ray K, Wisner D, Joe B (2016) Follow-up outcomes after benign concordant MRI-guided breast biopsy. Clin Imaging 40:1034–1039. https://doi.org/10.1016/j.clinimag.2016.06.005
Huang M, Speer M, Dogan B et al (2017) Imaging-concordant benign MRI-guided vacuum-assisted breast biopsy may not warrant MRI follow-up. AJR Am J Roentgenol 208:916–922. https://doi.org/10.2214/AJR.16.16576
Shaylor SD, Heller SL, Melsaether AN et al (2014) Short interval follow-up after a benign concordant MR-guided vacuum assisted breast biopsy - is it worthwhile? Eur Radiol 24:1176–1185. https://doi.org/10.1007/s00330-014-3125-x
Wallis M, Tarvidon A, Helbich T, Schreer I (2007) Guidelines from the European Society of Breast Imaging for diagnostic interventional breast procedures. Eur Radiol 17:581–588. https://doi.org/10.1007/s00330-006-0408-x
Heywang-Köbrunner SH, Bick U, Bradley WG et al (2001) International investigation of breast MRI: results of a multicentre study ( 11 sites ) concerning diagnostic parameters for contrast-enhanced MRI based on 519 histopathologically correlated lesions. Eur Radiol 11:531–546
Perlet C, Heinig A, Prat X et al (2002) Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur Radiol 12:1463–1470. https://doi.org/10.1007/s00330-002-1376-4
Hefler L, Casselman J, Amaya B et al (2003) Follow-up of breast lesions detected by MRI not biopsied due to absent enhancement of contrast medium. Eur Radiol 13:344–346. https://doi.org/10.1007/s00330-002-1713-7
Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L (2005) Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 11:236–241. https://doi.org/10.1111/j.1075-122X.2005.21499.x
Krug B, Hellmich M, Ulhaas A et al (2016) Vacuum-assisted breast biopsies (VAB) carried out on an open 1.0T MR imager: influence of patient and target characteristics on the procedural and clinical results. Eur J Radiol 85:1157–1166. https://doi.org/10.1016/j.ejrad.2016.02.030
Merle N, Despeyroux S, Lombry Y, et al (2011) HTA Summary report: MRI-guided vacuum-assisted breast biopsy (VABB). Available via https://www.has-sante.fr/upload/docs/application/pdf/2012-02/hta_summary_report_mriguided_vacuum-assisted_breast_biopsy__vabb_.pdf
Shin S, Schneider HB, Cole FJ, Laronga C (2006) Follow-up recommendations for benign breast biopsies. Breast J 12:413–417. https://doi.org/10.1111/j.1075-122X.2006.00302.x
Mann RM, Balleyguier C, Baltzer PA et al (2015) Breast MRI: EUSOBI recommendations for women’ s information. Eur Radiol 25:3669–3678. https://doi.org/10.1007/s00330-015-3807-z
Taskin F, Soyder A, Tanyeri A, Öztürk VS, Ünsal A (2017) Lesion characteristics, histopathologic results, and follow-up of breast lesions after MRI-guided biopsy. Diagn Interv Radiol 23:333–338. https://doi.org/10.5152/dir.2017.17004
Ferré R, Ianculescu V, Ciolovan L et al (2016) Diagnostic performance of MR-guided vacuum-assisted breast biopsy: 8 years of experience. Breast J 22:83–89. https://doi.org/10.1111/tbj.12519
Acknowledgments
We want to thank the Belgian Cancer Registry for providing us the long-term follow-up data of the patients who underwent a MRI-guided VABB in our hospital and hence making this study possible.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is J.W. Casselman.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
J.W. Casselman provides presentations for Philips and receives clients for Philips. Also, he organizes workshops for Leica-Devicor Mammotome. None of the other authors have any conflict of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
A minor subgroup of our study (52 patients) was already included in a prior multicenter study by Heywang-Köbrunner SH et al, Eur Radiol (2001) 11:531-546. Also, data of the VABB procedures of these 52 patients was used in the three following publications: Perlet et al, Eur Radiol. (2002) 12(6):1463–70; Hefler et al, Eur Radiol (2003) 13(2):344–6 and Perlet et al, Cancer (2006) 106(5):982–90. Our new dataset includes 507 additional patients for a total of 600 biopsies, now in a single-center setting, with a much more extensive follow-up available for analysis of the false negative rate.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lambert, J., Steelandt, T., Heywang-Köbrunner, S.H. et al. Long-term MRI-guided vacuum-assisted breast biopsy results of 600 single-center procedures. Eur Radiol 31, 4886–4897 (2021). https://doi.org/10.1007/s00330-020-07392-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07392-6