Abstract
Objective
To investigate the clinical accuracy of magnetic resonance imaging-guided breast vacuum-assisted biopsy (MR-VAB).
Methods
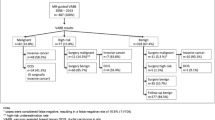
Of 97 scheduled MR-VAB for single MRI lesions (negative second-look sonography) categorised as BI-RADS 4 or 5, 4 were cancelled (undetected lesion = 2, technical problems = 2). Twenty-one patients lost to follow-up were excluded.
Results
Twenty-three patients (median age 51 years) were at high risk (BRCA1 = 11, BRCA2 = 7, familial risk = 5), 23 had a suspected local recurrence of breast cancer. Seventy-two imaged lesions (focus = 1, mass enhancement = 32, non-mass-like enhancement = 39) were targeted with a 10-gauge VAB probe using MRI guidance, with a median of 18 specimens per lesion (median procedural time 72 min, range 50–131 min) followed by clip placement. In the case of benignity, MRI follow-up was performed (19 patients, median 389 days, range 33–1,592) or mammography (3 patients, median 420 days, range 372–1,354). According to histopathology results, 29 lesions were benign, 10 were high-risk (papillary = 2, radial scar = 1, atypical epithelial hyperplasia = 7) and 33 malignant (ductal carcinoma in situ = 8, invasive cancers = 25). Three false negative results and 3 complications occurred (1 malaise, 1 skin defect, 1 infection).
Conclusion
MRI-guided VAB represents an accurate tool for the histological diagnosis of lesions visible only at MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to its high sensitivity, breast MRI is able to detect breast cancers occult to standard imaging or physical examination and has become a useful tool in breast imaging as an adjunct to mammography and sonography [1]. Although the clinical impact of breast MRI, such as in breast cancer staging, remains under debate principally because of a limited specificity of the technique and is still under clinical investigation [2–6], a number of indications have now been validated: equivocal mammogram, ultrasound or physical examination findings for which the diagnosis cannot be confidently established by conventional imaging [7], axillary node metastasis from unknown primary site [8], monitoring response to neoadjuvant chemotherapy [9], and breast cancer screening in high-risk population [10–12].
When breast MRI detects breast lesions that are suspicious or highly suggestive of malignancy, a second-look ultrasound examination is performed. If a sonographic correlate for the MRI-detected lesion is confidently identified, biopsy is usually performed under sonographic guidance [13]. When there is no sonographic correlates or only vague sonographic findings, biopsy has to be performed under MRI guidance [14]. Vacuum-assisted biopsy (VAB) is a minimally invasive procedure that provides large tissue samples. It is a safe and accurate technique routinely performed under stereotactic and sonographic guidance [15, 16]. Several studies have shown promising results of breast VAB under MRI guidance [17–24].
The objective of our study was to estimate the clinical accuracy of MRI-guided VAB of lesions only visible at MRI.
Materials and methods
Population
We retrospectively reviewed the medical records of 97 patients aged from 28 to 79 years (mean age 51 years) consecutively referred to our department for MRI-guided VAB between December 2003 and April 2008.
Lesions included in the study were MRI-detected lesions described according to the Breast Imaging and Reporting Data System (BI-RADS) Breast MRI Lexicon [25] and classified as BI-RADS category 4 or 5. Lesions with mammographic or sonographic correlate were excluded, as well as four lesions for which procedures could not be performed (disappearance of the MRI-detected lesion the day of the biopsy in 2 patients and technical limitations in the other 2 patients). Eighteen patients with benign histological findings and 3 patients with high-risk lesions were lost to follow-up after MR-guided biopsies and were therefore excluded from the study. Thus, our study population comprised 72 patients.
Patients were referred to our institution, which specializes in breast cancer treatment, by their respective physician for a diagnostic issue or for the screening of high-risk patients after oncogenetic counselling: BRCA1/2 mutation carriers, familial history of breast/ovarian cancer(s) that confer a cumulative lifetime breast cancer risk superior to 30% as determined with the Claus model (negative genetic tests) [26]. Patients were also referred from institutions without MRI guidance at their disposal for breast biopsy. The other patients were followed in our institution after a previously treated breast cancer.
The clinical setting for breast MRI is detailed in Table 1.
MRI
Diagnostic MRI was performed in our department for patients previously treated and followed in our institution on a 1.5-T unit (Symphony, Siemens, Erlangen, Germany) using a dedicated surface breast coil and a standard protocol including morphological axial T1 and T2-weighted TSE sequences, axial and sagittal dynamic T1-weighted three-dimensional water excitation fast low-angle shot sequences before and after contrast medium injection with subtractions.
For patients referred from other institutions, MRI was performed in other imaging departments and was reinterpreted by the physicians (AT, CEK, FT, CM) of our department all specialised in breast imaging with clinical experience in breast cancer centre of 15, 10, 15 and 2 years respectively.
Mammograms were either performed in our institution or reinterpreted if performed outside our institution. A second-look sonography was performed in our institution and did not show sonographic correlates of the MRI-detected lesion.
Biopsy procedure
MRI-guided biopsies were performed by 4 radiologists specialising in breast imaging (CEK, AT, FT, CM); all had previous experience in breast MRI and percutaneous biopsies under stereotactic and sonographic guidance. The results we present include our learning experience.
The patient was positioned prone in a 1.5-T magnet (Symphony, Siemens, Erlangen, Germany). A dedicated breast surface coil and breast biopsy device were used; biopsies were obtained with a 10-gauge handheld biopsy system (VACORA VAB system, BARD France SAS, Voisins Le Bretonneux, France). The breast was compressed in the mediolateral direction by two compression plates which consist of flexible bars. A fiducial marker was placed in the compression system. An axial T1-weighted three-dimensional water excitation fast low-angle shot sequence (2-mm section thickness, field of view 320 × 320 mm, matrix 512 × 512, acquisition time 25 s) was repeated before and five times after contrast medium injection at a rate of 2 ml/s (0.2 ml/kg, Dotarem, Guerbet, France) using an automated injector (Optistar LE, Mallinckrodt, Hazelwood, Mo). Subtracted sequences were generated. Then, MRI images were reviewed to target the marker (native image) and the lesion (subtracted image). From their respective positions, spatial coordinates were calculated (differences of location between the marker and the lesion) according to the different possible angle approaches of the biopsy device. The patient was then withdrawn from the MRI unit. The breast was cleansed with chlorhexidine gluconate (Hibitane Champ 0.5%). Skin and deep anaesthetisation was performed by injection of 10 ml of lidocaine hydrochloride with epinephrine (Lidocaïne Adrenaline, Aguettant, Lyon, France). After a small incision in the skin, a coaxial introducer system consisting of an outer plastic sheath and an inner titanium stylet was advanced and the tip of the sheath was placed 1 cm in front of the lesion. The inner titanium stylet was removed and an inner plastic stylet was placed. An axial SE T1-weighted sequence (TR/TE 533/14 ms, matrix size 515 × 512, 4-mm section thickness, 150-ms acquisition time) was performed to control the needle placement. Once the position of the coaxial plastic sheath was checked, the patient was moved out the magnet on the patient table, the patient staying motionless with the breast maintained under compression. The biopsy device was then adapted to the aiming device attached to the patient table outside the MRI tunnel and placed into the breast through the coaxial needle The device was fired to advance the needle into the centre of the lesion. Multiple samples were obtained by turning the needle clockwise around its axis. Six dynamic series were repeated after contrast medium injection to control the partial or complete removal of the enhancing lesion. A titanium clip was placed to mark the biopsy site. Afterwards, an axial T1-weighted TSE sequence was performed to assess clip placement and search for post-procedure haematoma. Two orthogonal mammograms were performed after the MRI-guided biopsy to document marker placement and accuracy of the clip placement with regard to the MRI-guided biopsy site was assessed.
Histopathological work-up and imaging-histological correlation
A median of 18 specimens per lesion was obtained (range 6–48). Specimens were fixed in neutral buffered formalin. The tissue sampled was entirely embedded and examined. At least one step section was acquired and analysed from each specimen of the VAB at the beginning of our experience. From April 2005, three step sections per specimen were analysed.
For all the patients, results of MRI-guided biopsies were reviewed during a multidisciplinary session to assess radio-pathological concordance. Images of the post-procedural MRI were reviewed to assess the quality of the lesion removal. Pre-procedural images were reviewed in comparison with histology results to assess the radio-histological concordance. When histological findings could not explain the MRI findings, surgical biopsy was recommended. In cases of benign histological findings concordant with the MRI pattern and if a representative removal could be assumed from post-interventional MRI images, MRI follow-up was recommended at 4–6 months. If the lesions persisted unchanged at MRI follow-up, excisional biopsy was recommended.
Data collection and statistical analysis
The study was conducted according to our institution’s ethical guidelines and was approved by the institutional breast group. Data collected included MRI lesion size and type, complications, number of specimens sampled per lesion, MRI-guided histological findings, follow-up, final surgical histology, and complications. The time of the biopsy was determined by calculating the interval between the initial MRI localizing sequence and the final post-procedural MRI sequence.
Data were entered into a computerized spreadsheet (Excel, Microsoft, Redmond, WA) for analysis.
Results
Technical feasibility
Two lesions could not be sampled because of their deep location close to the pectoral muscle.
The rate of technically feasible MRI-guided biopsies was 98%.
The median procedural time was 72 min (range 50–131 min).
MRI
The median maximal axis of lesions sampled was 12 mm (size range 4–70 mm), 10 mm for mass enhancements (range 4–26 mm) and 16 mm for non-mass-like enhancements (range 5–70 mm).
According to the BI-RADS lexicon, the 72 sampled lesions corresponded to a mass enhancement in 32 lesions (44%) and a non-mass-like enhancement in 39 (54%). Among the non-mass-like enhancements, there were 6 enhancements with a focal distribution (15%), 3 with a linear distribution (8%), 23 with a ductal distribution (59%), 7 with a segmental distribution (18%) and none with a regional distribution (0%). One focus was also sampled because of the clinical context: in a woman with a metastatic axillary lymph node ipsilateral to a previously treated breast cancer the unique finding identified was a focus contralateral to the axillary metastasis.
Pathology results
Of the 72 lesions sampled, 33 were malignant (46%), 10 were high-risk (14%) and 29 were benign (40%) (Table 2).
Malignant histological subtypes are detailed in Table 2. According to the Elston–Ellis modification of the Scarff–Bloom–Richardson grading system [27], 8 invasive tumours were classified as grade 1, 9 as grade 2, 3 as grade 3, and 4 could not be assessed because the size of the invasive component was too small. Except for one patient who decided to be treated elsewhere, all patients with malignant results where operated on in our institution. Histology results for the operative specimen were concordant with the preoperative diagnosis in 17 invasive carcinomas and 5 ductal carcinoma in situ (DCIS). After surgery, 2 DCIS (25%) were upgraded to invasive ductal carcinoma (IDC). One DCIS exhibited a microinvasion; 7 DCIS had a high or intermediate grade. While preoperative histological diagnosis was invasive carcinoma, surgical histology yielded in situ carcinoma in 3 (DCIS = 2, lobular carcinoma in situ (LCIS) = 1). At final surgical histology, a biopsy scar without residual detectable carcinoma was found in 4 patients with a preoperative diagnosis of IDC and 1 patient with DCIS.
In the high-risk lesion group, 8 patients underwent surgery (atypical ductal hyperplasia (ADH) = 1, LCIS = 1, atypical columnar cell hyperplasia = 2, indeterminate vascular lesion = 1, papilloma = 2, radial scar = 1); only one lesion (ADH) was upgraded to DCIS at surgery. Two cases with very small foci of lobular atypical hyperplasia were discussed during a multidisciplinary consensus and considered to have been successfully and completely sampled. Surgery was not recommended and MRI follow-up at 17 and 22 months provided no suspicious findings.
Twenty-nine lesions (40%) yielding benign results are presented in Table 2. Pathology results were considered to be concordant with the BI-RADS category in 26 cases.
Nineteen patients with benign pathology results at MRI-guided biopsies underwent 1–4 MRI examinations, with a median follow-up of 12 months (median 389 days, range 33–1,592 days), without detrimental findings. In another 3 patients, MRI follow-up was not performed but mammography and clinical follow-up were negative for a median of 420 days (range 372–1,354 days). One patient with benign histological findings underwent mastectomy for ipsilateral breast cancer, distinct from the lesion sampled.
Despite benign histology results, 5 lesions were subsequently excised following the MRI-guided biopsy.
Two lesions were persistent at MRI follow-up despite a satisfactory post-procedural evaluation raising questions about the representativity of the biopsy specimens (Fig. 1) and 1 non-mass-like ductal clumped enhancement was judged discordant with the histological finding of fibrous mastopathy. All appeared to be benign at surgical histology.
A 60-year-old woman was suspected of local recurrence 3 years after breast-conserving therapy of the left breast for ductal invasive carcinoma. This patient had no other risk factor. MRI showed a round, ill-defined and homogenous mass enhancement categorised BI-RADS 4 in the upper quadrants of the treated breast. a Axial T1-weighted subtracted dynamic sequence showed a mass enhancement, measuring 6 mm. b On the post-procedural axial T1-weighted subtracted dynamic image, no residual enhancement was seen after biopsy; contrast medium leakage appeared at the periphery of the biopsy cavity. c On MRI follow-up, most of the mass enhancement targeted was still visible behind the marker on axial T1-weighted subtracted dynamic sequence. d Surgical excision was performed after MRI-guided wire localisation. Like MRI-guided biopsy, surgical histology showed fibrous mastopathy and adenosis. Axial T1-weighted control image showed the wire behind the marker, centred on the residual enhancement targeted
Two other lesions were found to be malignant: one was related to an error in targeting and the biopsy of the other one was interrupted because of a vasovagal reaction. In the latter, a second look at the initial MRI-guided biopsy samples showed a small component of invasive ductal carcinoma in the periphery of the specimens on additional levels acquired. Initially, only one section had been explored for histological analysis.
Finally, a 12-mm micronodular non-mass-like enhancement in a BRCA2-carrier patient appeared to be a false negative. It was judged correctly sampled on post-biopsy control images (Fig. 2). Histology results showing fibrocystic changes were deemed concordant during a multidisciplinary meeting. One year later, irregular microcalcifications with a ductal orientation categorised BI-RADS 5 appeared in the area of the former MRI-guided biopsy, corresponding to a high-grade ductal carcinoma in situ at stereotactic VAB.
A 40-year-old BRCA2 mutation carrier with ductal non-mass-like enhancement of the left breast categorised BI-RADS 4. a Axial T1-weighted subtracted dynamic image of the breast positioned for MRI-guided VAB. A 12-mm ductal non-mass-like enhancement was seen in the upper-outer quadrant. b Post-procedure axial T1-weighted image. The biopsy cavity appeared correctly centred compared with the lesion targeted. Histological findings were fibrocystic mastopathy with columnar cell change without atypia. c Persistent residual enhancement on MRI follow-up performed after a 1-year interval. d On follow-up mammogram images (image zooming from craniocaudal mammography of the left breast), appearance of microcalcifications matching the MRI enhancement, corresponding to high-grade DCIS with necrosis at stereotactic VAB
Positive predictive values (PPV) of MRI enhancement features
PPV of breast MRI features were 0% for foci (0/1), 52% (17/33) for mass enhancements and 41% for non-mass-like enhancements (16/39). In this last type of enhancement and according to the spatial distribution of enhancements, PPV were 50% for focal distribution (3/6), 33% for linear distribution (1/3), 35% for ductal distribution (8/23), 50% for segmental distribution (4/7) and 0% for regional distribution (0/0).
Learning experience
Three cancers missed by MRI-guided VAB occurred in the beginning of our experience. They were respectively the second, third and seventeenth biopsies, performed by each of the three physicians, respectively.
Three other lesions which appeared questionably sampled were performed in thirty-seventh, twenty-sixth and second position, respectively, and were benign at final surgical histology.
Complications
Three complications were noted in this series. A case of vasovagal reaction, previously described, complicated one MRI-guided biopsy procedure. In another case, the biopsy probe pierced the skin at the opposite side of the breast. This complication occurred while an internal lesion was approached from the lateral skin surface. Sterile strips were applied. No surgical management was needed. One infection of the biopsy site occurred after MRI-guided biopsy and resolved under antibiotic therapy. No bleeding or infectious complications required surgical management.
Clip placement
Marker placement was not assessed in our institution for 2 patients (3%). The marker was considered accurately placed at mammography in 52 patients (72%). For 14 markers (19%), post-biopsy mammogram showed clip displacement, evaluated between 10 and 45 mm from the biopsy site. In 4 patients, the radiologist decided not to place a marker after the biopsy.
Discussion
Technical feasibility
MRI-guided VAB has been performed for several years with success rates ranging from 95 to 100%. Our 98% rate of technically feasible biopsies correlates with these results [17–24].
Procedure
Compared with using the 9-gauge ATEC Suros device employed by Liberman et al., the duration of our procedure was longer, with an estimated median of 72 min, versus a mean time of 33–38 min with the 9-gauge ATEC Suros device [19–21]. In a previous study using the same 10-gauge VACORA device as ours, the mean duration of the procedure was comparable, an estimated 65 min, ranging from 52 to 87 min [28]. Using the 11-gauge Mammotome device, the duration of the procedure was estimated to be approximately 60–70 min [17, 18, 23]. This lengthy procedure is associated with a higher number of core biopsies sampled compared with previous studies: a mean of 18 specimens were sampled per lesion. A mean of 8–14 specimens were collected with the same 10-gauge device [24, 28]. A median of 8–12 specimens were reported when using the 9-gauge ATEC Suros device [19, 21]. In an interdisciplinary consensus on the uses and technique of MRI-guided VAB, European specialists in breast imaging recommended the acquisition of at least 24 cores for an 11-gauge probe (or an equivalent volume if a larger probe is used) [14]. Before this European consensus, we acquired this number of specimens because of our learning experience, the small size of the lesions targeted and of the difficulty inherent to MRI guidance.
Pathology results
Histological analysis indicated a high proportion of malignant lesions, reflecting the numerous women at high risk which, apart from the suspicion of local recurrence and initial staging, represented 32% of our cohort. One study performed as an initial experience showed a high cancer rate of 61% [22] which declined to 29% in subsequent series after a larger number of biopsies had been done [29].
DCIS cases which could only be sampled using MRI guidance showed high and intermediate grades. As shown by Kuhl et al. in a study dedicated to MRI in diagnosis of pure DCIS, breast MRI in a high-risk setting mainly detects high- and intermediate-grade DCIS [30].
We observed in our study two DCIS underestimations among 8 DCIS with surgical excision. Our 25% rate is to be interpreted with care in this small cohort of DCIS, but appears equivalent to results previously published. In a study dedicated to DCIS underestimation, 17% of all pure DCIS were underestimated at 9-gauge MRI-guided biopsy [31]. This study showed that DCIS underestimation was associated with lesion size, underestimation being significantly more likely in lesions measuring 6 cm or more. No other factor correlated with DCIS underestimation. In our series, the two underestimated lesions measured 38 and 30 mm, respectively, on MRI.
The unique case of microinvasion was confirmed as being microinvasive at surgical biopsy. Concerning high-risk lesions, the only atypical ductal hyperplasia was upgraded to DCIS. Among 8 resected high-risk lesions, only this case was malignant at surgical excision (13%).
Discordances
Our 4% rate of discordances approaches the 7% discordance rate reported by Lee et al. at MRI-guided 9-gauge VAB [32]. In this series of 24 discordant lesions from a total of 342 9-gauge MRI-guided biopsies, surgical excision revealed cancer in 30% [32]. In our study, surgical excision revealed 2 invasive cancers in these discordant lesions. Both were encountered after technical obstacles with MRI-guided biopsy. In the first case, we could retrospectively prove that MRI-guided biopsy actually sampled the lesion despite the difficulty of this procedure being interrupted because of a vasovagal reaction. Histological analysis initially failed to identify a small component of invasive ductal carcinoma because only one step section had been carried out. Following this incident, three levels of histological analysis were routinely performed for all MRI-guided biopsy specimens. According to European guidelines, at least 4 hematoxylin and eosin (H&E) step sections should be examined, each with at least one H&E stain [14]. The second malignant case was immediately deemed discordant thanks to the systematic checking of the placement of the probe which immediately appeared inaccurate. Surgical excision could then be rapidly performed, without prejudicial delay. This case illustrates the need to carefully review the probe placement when checking the radio-histological correlation of the biopsy, as error in targeting may not be immediately identified during the procedure.
In the false negative case presented in Fig. 2, targeting of the lesion was initially thought to be accurate and the radiology and histology results were considered concordant. During follow-up, the appearance of pleomorphic calcifications with ductal orientation on conventional mammograms in the biopsy site subsequently showed that the MRI-guided biopsy was actually falsely negative. This case illustrates the limitation of MRI-guided biopsy that lies in the difficulty in controlling the targeting accuracy during biopsy. As shown in this case, even if the radio-histological correlation is checked, it can be deemed concordant and the error in lesion sampling remains undetected.
Learning experience
The three cancers missed by MRI-guided VAB occurred in the beginning of our experience. For two of them, the radiologists had performed three or less MRI-guided VAB. This finding may hence suggest an impact of the learning curve on accuracy of MRI-guided VAB, as has already been shown for stereotactic VAB [33].
Positive predictive values of MRI enhancement features
MRI-guided VAB yielded cancer in 46% of lesions. This 46% PPV of MRI-guided biopsy is higher than the 5–31% PPV previously reported when using the 10-gauge Vacora device [24, 28, 34]. With the 9-gauge ATEC Suros device, PPV ranged from 22 to 61% while with the 11-gauge Mammotome probe, PPV ranged from 26 to 27% [17–19, 22, 23]. The high PPV we report may reflect the high risk for breast cancer in our study population and is also related to the exclusion of the patients lost to follow-up after benign biopsy findings.
PPV of breast MRI features, except for mass enhancements and ductal non-mass-like enhancements, were estimated on limited series of less than 10 lesions, and therefore may be of limited value. Of 33 mass enhancements, 17 (52%) were malignant at histological analysis. This rate exceeds the PPV of 25% reported in a previous study by Liberman et al. for mass enhancements [35]. Ductal non-mass-like enhancements were malignant in 8 of the 23 lesions (35%). This rate exceeds the 26% rate of malignancy reported in ductal enhancement by Liberman et al. [19]. Segmental enhancement corresponded to a malignant lesion in 50% of the cases.
Complications
The rate of 3 complications per 72 biopsies (4%) is equivalent to the complication rate reported in previous studies [17–24]. To prevent contralateral piercing of the skin, we developed a dedicated long arm to allow internal access to internally located lesions and no additional cutaneous complication was recorded when using this device.
Limits
Our study has several limitations. We examined a particularly high-risk population. We performed numerous MRI-guided biopsies for departments that did not have MRI-guided biopsy devices at their disposal, mainly at the beginning of the study. Despite a recommendation for MRI follow-up, some patients did not present for follow-up or delayed in doing so. We could therefore not estimate the negative predictive value of MRI-guided biopsy.
Although the study included 72 lesions, some subgroups contained few lesions, limiting conclusions that could be drawn about these features. Longer-term follow-up of patients with benign histological findings after MRI-guided VAB as well as larger cohorts would be required to truly assess the negative predictive value of MRI-guided VAB. Eighteen patients with benign histological findings at MRI-guided biopsy were secondarily lost to follow-up. The cancer rate appeared particularly high after excluding these patients with benign results who did not present for follow-up in our cancer centre.
Conclusion
MRI-guided VAB represents an accurate tool for the histological diagnosis of lesions visible only at MRI. Three false negatives of MRI-guided biopsy were identified during the study. MRI-guided biopsy is still a lengthy procedure requiring precise evaluation of the biopsy site during the procedure. It is a reliable technique which, in our high-risk cohort, identified malignancy in 46% of the biopsies performed, of which about 73% were invasive.
References
Kuhl CK (2007) Current status of breast imaging. Part 1. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 244:356–378
Morrow M (2008) Magnetic resonance imaging in the breast cancer patient: curb your enthusiasm. J Clin Oncol 26:352–353
Morrow M (2004) Magnetic resonance imaging in the preoperative evaluation of breast cancer: primum non nocere. J Am Coll Surg 198:240–241
Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM, Irwing L (2008) Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 26:3248–3258
Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD (2008) Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol 26:386–391
Fischer U, Zachariae O, Baum F, von Heyden D, Funke M, Liersch T (2004) The influence of preoperative MRI of the breasts on recurrence rate in patients with breast cancer. Eur Radiol 14:1725–1731
Lee CH, Smith RC, Levine JA, Troiano RN, Tocino I (1999) Clinical usefulness of MR imaging of the breast in the evaluation of the problematic mammogram. AJR Am J Roentgenol 173:1323–1329
Orel SG, Weinstein SP, Schnall MD, Reynolds CA, Schuchter LM, Fraker DL, Solin LJ (1999) Breast MR imaging in patients with axillary node metastases and unknown primary site. Radiology 212:543–549
Kuhl CK (2007) Current status of breast MR imaging. Part 2. Clinical applications. Radiology 244:672–691
Lehman CD, Isaacs C, Schnall MD, Pisano ED, Asher SM, Weatherall PT, Bluemke DA, Bowen DJ, Marcom PK, Armstrong DK, Domchek SM, Tomlinson G, Skates SJ, Gatsonis C (2007) Cancer yield of mammography, MR and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology 244:381–388
Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H, Tilanus-Linthorst MM, Muller SH, Meijer S, Oosterwijk JC, Beex LV, Tollenaar RA, de Koning HJ, Rutgers EJ, Klijn JG (2004) Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 351:427–437
Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, Gilbert FJ, Griebsch I, Hoff RJ, Kessar P, Lakhani SR, Moss SM, Nerurkar A, Padhani AR, Pointon LJ, Thompson D, Warren RM, MARIBS study group (2005) Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 365:1769–1778
DeMartini WB, Eby PR, Peacock S, Lehman CD (2009) Utility of targeted sonography for breast lesions that were suspicious on MRI. AJR Am J Roentgenol 192:1128–1134
Heywang-Kobrunner SH, Sinnatamby R, Lebeau A, Lebrecht A, Britton PD, Schreer I, consensus group (2008) Interdisciplinary consensus on the uses and technique of MRI-guided vacuum assisted breast biopsy (VAB): results of a consensus meeting. Eur J Radiol. doi:10.1016/j.ejrad.2008.07.010
Jackman RJ, Marzoni FA, Rosenberg J (2009) False-negative diagnoses at stereotactic vacuum-assisted needle breast biopsy: long-term follow-up of 1280 lesions and review of the literature. AJR Am J Roentgenol 192:341–351
Philpotts LE, Hooley RJ, Lee CH (2003) Comparison of automated versus vacuum-assisted biopsy methods for sonographically guided core biopsy of the breast. AJR Am J Roentgenol 180:347–351
Heywang-Kobrunner SH, Heinig A, Schaumloffel U, Viehweg P, Buchmann J, Lampe D, Spielmann RP (1999) MR-guided percutaneous excisional and incisional biopsy of breast lesions. Eur Radiol 9:1656–1665
Perlet C, Heinig A, Prat X, Casselman J, Baath L, Sittek H, Stets C, Lamarque J, Anderson I, Schneider P, Taourel P, Reiser M, Heywang-Kobrunner SH (2002) Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur Radiol 12:1463–1470
Liberman L, Morris EA, Dershaw DD, Thornton CM, Van Zee KJ, Tan LK (2003) Fast MR-guided vacuum-assisted breast biopsy: initial experience. AJR Am J Roentgenol 181:1283–1293
Lehman CD, DePeri ER, Peacock S, McDonough MD, DeMartini WB, Shook J (2005) Clinical experience with MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol 184:1782–1787
Liberman L, Bracero N, Morris E, Thornton C, Dershaw DD (2005) MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. AJR Am J Roentgenol 185:183–193
Orel SG, Rosen M, Mies C, Schnall MD (2006) MR imaging-guided 9-gauge vacuum-assisted core-needle breast biopsy: initial experience. Radiology 238:54–61
Perlet C, Heywang-Kobrunner SH, Heinig A, Sittek H, Casselman J, Anderson I, Taourel P (2006) Magnetic resonance-guided, vacuum-assisted breast biopsy. Results from a European multicenter study of 538 lesions. Cancer 106:982–990
Ghate SV, Rosen EL, Soo MSC, Baker JA (2006) MRI-guided vacuum-assisted breast biopsy with a handheld portable biopsy system. AJR Am J Roentgenol 186:1733–1736
American College of Radiology (2003) Magnetic resonance imaging. In: American College of Radiology. Breast imaging reporting and data system: breast imaging atlas (BI-RADS), 4th edn. American College of Radiology, Reston
Claus EB, Risch N, Thompson WD (1991) Genetic analysis of breast cancer in the Cancer and Steroid Hormone Study. Am J Hum Genet 48:232–242
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. Part 1. The value of histological grade in breast cancer: experience from a large study with long term follow-up. Histopathology 19:403–410
Hauth EA, Jaeger HJ, Lubnau J, Maderwald S, Otterbach F, Kimmig R, Forsting M (2008) MR-guided vacuum-assisted breast biopsy with a handheld biopsy system: clinical experience and results in postinterventional MR mammography after 24 h. Eur Radiol 18:168–176
Han BK, Schnall MD, Orel SG, Rosen M (2008) Outcome of MRI-guided breast biopsy. AJR Am J Roentgenol 191:1798–1804
Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, Kuhn W, Schild HH (2007) MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 11:485–492
Lee J, Kaplan JB, Murray MP, Mazur-Grbec M, Tadic T, Stimac D, Liberman L (2007) Underestimation of DCIS at MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol 189:468–474
Lee J, Kaplan JB, Murray MP, Bartella L, Morris EA, Joo S, Dershaw DD, Liberman L (2007) Imaging histologic discordance at MRI-guided 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol 189:852–859
Liberman L, Benton CL, Dershaw DD, Abramson AF, LaTrenta LR, Morris EA (2001) Learning curve for stereotactic breast biopsy: how many cases are enough? AJR Am J Roentgenol 176:721–727
Gebauer B, Bostanjoglo M, Moesta KT, Schneider W, Schlag PM, Felix R (2006) Magnetic resonance-guided biopsy of suspicious breast lesions with a handheld vacuum biopsy device. Acta Radiol 47:903–913
Liberman L, Morris EA, Lee MJ, Kaplan JB, LaTrenta LR, Menell JH, Abramson AF, Dashnaw SM, Ballon DJ, Dershaw DD (2002) Breast lesions detected on MR imaging: features and positive predictive value. AJR Am J Roentgenol 179:171–178
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malhaire, C., El Khoury, C., Thibault, F. et al. Vacuum-assisted biopsies under MR guidance: results of 72 procedures. Eur Radiol 20, 1554–1562 (2010). https://doi.org/10.1007/s00330-009-1707-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1707-9