Abstract
In April 2018, the national health insurance coverage of MRI-guided vacuum-assisted breast biopsy (VAB) was instituted with the application of the Japan Breast Cancer Society. Although MRI-guided VAB has been considered as a special procedure for a long time, having an access to this procedure should be recommended for facilities performing breast MRI as in Western countries. From now on, relevant societies should make efforts in data collection and quality control of MRI-guided VAB in Japan. We must avoid the following. To delay the early diagnosis of breast cancer in the judgment of an inaccurate indication, perform unnecessary biopsy due to overestimation of diagnosis, and reduce the success rate of MRI-guided VAB with immature techniques. This review explains the current status of MRI-guided VAB in Japan and shares procedure and biopsy data as a future reference from an experienced facility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
MRI-guided vacuum-assisted breast biopsy (VAB) began to be covered by the national health insurance in Japan in April 2018, as a result of an application made by the Japanese Breast Cancer Society alone. Earlier to that, MRI-guided VAB was a special examination not covered by health insurance, and performed at a dedicated special hospital. Therefore, only a limited number of institutions have reported the usefulness of MRI-guided VAB in Japan [1,2,3]. However, at present, with MRI-guided VAB being covered by health insurance, if performing breast MRI, it would also be necessary to be equipped to perform MRI-guided VAB or to cooperate with an institution that can perform MRI-guided VAB, as recommended in the guidelines from the United States [4]. MRI-guided VAB is considered as an indispensable procedure, particularly when conducting MRI surveillance of high-risk women.
Necessity of MRI-guided VAB in Japan
In general, lesions detected by MRI are called MRI-detected lesions. After a lesion is detected by MRI, ultrasonography (US) is performed in the area where the lesion was detected by MRI. This examination is generally called second-look US, but at present, use of the term MRI-targeted US is recommended. If MRI-targeted US detects a lesion consistent with that on MRI, it is desirable to perform US-guided biopsy. On the other hand, if an MRI-detected lesion cannot be visualized by MRI-targeted US (i.e., MR-only visible lesion), MRI-guided biopsy would be indicated.
So far in Japan, if a lesion cannot be visualized by MRI-targeted US, follow-up MRI was performed. If the lesion was malignant, follow-up would have had to be continued until the lesion grew sufficiently as to be detected by US [5]. LaTrenta et al. [6] concluded that although the incidence of cancer is higher in patients whose lesions can be visualized by MRI-targeted US than in those whose lesions cannot be visualized by this method (43% vs. 14%), it would be difficult to assert that patients whose lesions cannot be detected by MRI-targeted US do not require biopsy.
In Japan, a report by Sakamoto et al. [7] provides useful information. The success rate of US-guided VAB for MRI-detected lesions was 91% (144/159) [7], which was clearly inferior to that of MRI-guided needle localization or MRI-guided VAB in overseas studies (95–100%) [8,9,10,11,12,13,14,15,16,17]. In addition, considering that 9.4% (15/159) of the lesions could not be accurately resected using US-guided VAB and that additional MRI is required to determine whether the biopsy procedure is successful or not, MRI-guided VAB is considered as an indispensable procedure in Japan. Furthermore, Sakamoto et al. [18] additionally reported that of the 986 lesions that were examined by US-guided VAB, the initial US-guided VAB could not correctly diagnose breast cancer in 26% (8/31) of the MRI-detected lesions. Namely, there is a risk that 26% of breast cancers may be misdiagnosed as benign and left untreated. Moreover, the problem is that the diagnosis of a benign lesion give a false relief to both the patient and the physician. Therefore, when performing US-guided VAB for MRI-detected lesions, this risk must always be borne in mind.
Recently, several studies have been reported by Japan [19, 20]; however, no comparison with MRI-guided VAB procedures has been made. Comparison with MRI-guided VAB is considered to be essential for the development of new procedures that could replace MRI-guided VAB.
MRI-guided VAB procedure in our hospital
We started to perform MRI-guided VAB at our hospital soon after the procedure began to be covered by health insurance. Currently, the number of cases is increasing and there are 3–4 biopsy cases per month, including post-operative follow-up for hereditary breast and ovarian cancer (HBOC) syndrome and surveillance of BRCA1/2 mutation carriers. As a result, Showa University Hospital has the largest number of MRI-guided VAB cases in Japan after covering by health insurance.
All biopsies were performed by two radiologists (N.T. and M.T.) specializing in breast imaging. The biopsies were performed on a 1.5-T MR scanner (Signa HDxt Ver.16; GE). The VAB units used were a 10-gauge breast biopsy system (EnCor Enspire Breast Biopsy System; BD (C.R. Bard, Inc.), Tempe, AZ) or a 9-gauge breast biopsy system (ATEC® Breast Biopsy System; Hologic, Inc., Marlborough, MA, USA).
The procedure consisted of the following steps.
-
1.
Compression plate and marker placement
The patient was positioned prone on the breast coil, and the affected breast was compressed moderately by two compression plates. A grid-type compression plate was used. Two markers (vitamin E capsule) were placed in the block near the predicted puncture site. A grid and markers were drawn on a transparent sheet, and the sheet was fixed on the monitor of the workstation, referring to a sagittal image. The scale of enlargement was adjusted to make the size of the blocks on the monitor screen the same as that of the blocks on the sheet.
-
2.
Contrast-enhanced MRI
In this position, the patient was moved into the magnet, and MRI was performed before and after intravenous injection of 10 ml of Gd-DOTA from a 10-ml syringe (Magnescope; Guerbet Japan, Tokyo, Japan). A contrast agent was injected to obtain a pre-/post-biopsy dose ratio of about 8:2. Transverse and sagittal three-dimensional (3D)-VIBRANT sequences with fat suppression (TR/TE 5.6/2.7 or 4.0/1.5; flip angle 12°; field of view 20 or 23cm; matrix 320 × 192; slice thickness 1 mm; time of acquisition 60–71s) were obtained.
-
3.
Estimation of the puncture site
The target lesion was confirmed by contrast-enhanced MRI. The puncture site was detected according to the positional relationships among the grid line, markers, and the lesion on the sagittal image. The depth was measured on the transverse image, as the distance from the skin to the lesion.
-
4.
Sterilization and anesthesia
The skin within the block to be punctured was sterilized. After an anesthetic was injected into the subcutaneous tissue and around the lesion, an incision of about 4 mm was made in the skin, and an introducer was inserted.
-
5.
Insertion of the introducer
The introducer was inserted so as to set the lesion at the center of the opening of the VAB needle (Fig. 1). After the introducer was inserted into the target site, the block was fixed moderately, and the introducer was removed (Fig. 2). Then, an obturator was inserted into the introducer cannula (Fig. 3), and images were obtained for confirmation.
Fig. 1 Fig. 2 -
6.
Insertion of the VAB needle: tissue sampling
After confirming that the lesion was set at the appropriate position, the obturator was removed, and the VAB needle was inserted. After obtaining several samples, the obturator was inserted again, and the images were obtained again for confirmation. Additional tissue sampling was performed as needed. After the tissue sampling was completed, in many of the cases, markers (UltraClip Dual Trigger Breast Tissue Marker; BD (C.R. Bard, Inc.), Tempe, AZ, or TriMark® Biopsy Site Marker; Hologic, Inc., Marlborough, MA, USA) were retained in the breast.
Results of MRI-guided VAB in our hospital
Patient selection
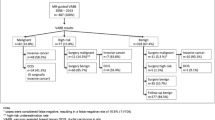
The initial consecutive biopsy data are introduced below. Between April 2018 and July 2019, a total of 31 patients (32 lesions) with a mean age of 52 years (range, 29–77 years) were judged as being suitable candidates for MRI-guided VAB.
-
1.
High-risk surveillance (n = 5; 3 carriers of BRCA1 mutation and 2 carriers of BRCA2 mutation): Among the 5 patients, MRI was performed for surveillance of the contralateral breast in 3 patients who already had breast cancer. In one of the patients, who had newly diagnosed breast cancer, preoperative MRI suggested a lesion in the contralateral breast. The fifth patient had never previously been diagnosed as having breast cancer; the MRI, performed for surveillance, suggested the presence of a lesion in this patient.
-
2.
Preoperative staging (n = 14)
-
3.
Postoperative follow-up (n = 2; one patient had bilateral lesions, and bilateral MRI-guided VAB was performed on separate days.)
-
4.
Diagnostic (n = 9; mammographic calcification, 3 patients; mammographic mass, 1 patient; bloody nipple discharge, 5 patients)
-
5.
Follow-up after neoadjuvant chemotherapy (n = 1; for placing a clip before the breast cancer lesions disappeared)
In this study, the data of 30 patients (31 lesions) (mean age 53 years; range 29–77 years), excluding the case mentioned above in category 5, were analyzed. Both screening US and MRI-targeted US failed to visualize the lesion in all of the 30 patients.
Frequency of cancelation on the day of the scheduled biopsy
In two (6.7%) of the 30 patients, the target lesion failed to be visualized on the MRI performed on the day of the scheduled biopsy in our study, and the biopsy was postponed. Both of these patients were BRCA1 mutation carriers. One of these two patients underwent preoperative MRI for newly diagnosed breast cancer, and the other, who had never previously been diagnosed as having breast cancer, underwent MRI for the purpose of surveillance.
The frequency of cancelation in our study was lower than that in a multicenter study conducted in Europe (12%;80/649) [17]. However, Tozaki et al. [3] reported that the frequency of cancelation is only 1% (1/102 cases). The probable reason for cancelation in our cases was background parenchymal enhancement (BPE) due to the menstrual cycle mimicking a suspicious non-mass lesion rather than the lesion itself, and another follow-up MRI might have been necessary before an MRI-guided biopsy was scheduled. Both the patients in whom the biopsy was canceled were BRCA1 mutation carriers and in their 30s. Both data from Japan and overseas have shown a relatively high likelihood of occurrence of triple-negative breast cancer in BRCA1 mutation-positive women [21, 22]. Therefore, MRI-guided biopsy was scheduled without performing follow-up MRI in these patients.
At our hospital, when performing MRI-guided biopsy in cases where BPE is possible, we do not open the biopsy kit until the target lesion is identified on contrast-enhanced MRI. If the lesion is not confirmed on the day of the scheduled biopsy, we cancel the MRI-guided biopsy, and claim insurance only for the contrast-enhanced MRI. The above information is given verbally to the patient just before the biopsy. However, it is considered that the frequency of cancelation on the day of the scheduled biopsy should be reduced by having a precise understanding of the patient's menstrual cycle status, and more accurate differentiation between BPE and significant lesions.
Histopathological characteristics
According to Tozaki et al., based on studies conducted in Japan, the rate of malignancy was 35% (36/102) in a series of 102 cases [3] and 38% (115/301) in a series of 301 cases (Fig. 4) [23]. Our results were the similar frequency. The histopathological examination of the biopsy specimens revealed 11 (38%) malignant lesions, 5 (17%) high-risk lesions, and 13 (45%) benign lesions in our study. The malignant lesions comprised 3 cases of invasive ductal carcinoma, 1 case of invasive lobular carcinoma, and 7 cases of ductal carcinoma in situ (DCIS). In addition, the high-risk lesions comprised 4 cases of papilloma and 1 case of intraductal proliferative lesion. The benign lesions comprised 3 cases of fibroadenoma, 4 cases of mammary ductal epithelial hyperplastic changes, 1 case of adenosis, 1 case of screlosing adenosis, 1 case of capillary hemangioma, and 3 cases of other lesions.
After surgery following the biopsy, 2 cases were upgraded from DCIS to invasive ductal carcinoma. The patients with the high-risk lesions were followed up without surgery performed after the biopsy. The underestimation rate was 6.9% (2/29). Finally, 11 (38%) of the 29 biopsied lesions were malignant: in situ carcinoma, n = 5; invasive carcinoma, n = 6. All DCIS was classified as low grade (n = 5).
The reported frequency in a review article of high-risk lesions was 3%–21% [24], and these frequencies are similar to that in our study (17%; 5/29). Thus, we believe that our indications for MRI-guided VAB were appropriate, and these data are considered to be very important reference values for facilities commencing MRI-guided VAB.
Incidence of malignancies in various groups
The incidences of malignancies in relation to the indications were as follows: 67% (2/3) in BRCA1/2 mutation-positive cases, 67% (2/3) in cases that underwent biopsy as postoperative examination, 36% (5/14) in cases that underwent biopsy for preoperative staging, and 22% (2/9) in cases that underwent biopsy for other indications. This trend toward decreasing incidence of malignancies was consistent with the results of other studies [3, 17].
However, the very important differences between our study and the study reported in Japan previously [3] were the candidates for biopsy including high-risk patients who were BRCA1/2 mutation carriers (17%; 5/30). As described previously, the biopsy was canceled in 2 patients. Two patients had invasive ductal carcinoma measuring 5 mm in diameter (Fig. 5), and the remaining one case is benign result (Fig. 6). Therefore, the importance of MRI-guided VAB in high-risk patients with BRCA1/2 mutations should be recognized, even though the number of subjects was limited.
A 52-year-old female with a BRCA1 mutation after the right mastectomy. a Sagittal contrast-enhanced T1-weighted MR image before VAB shows a 5-mm enhancing mass (arrow) of the left breast. b Sagittal contrast-enhanced T1-weighted MR image after VAB shows complete removal of the enhancing lesion. Histologic evaluation of the VAB specimens revealed invasive ductal carcinoma, triple-negative
A 50-year-old female with a BRCA2 mutation after the left mastectomy. a Sagittal contrast-enhanced T1-weighted MR image during follow-up after surgery shows a 3-mm enhancing focus (arrow) of the right breast. b Transverse contrast-enhanced T1-weighted MR image before VAB shows an enhancing focus (arrow) of the right breast. Histopathological examination of the biopsy specimens revealed the fibroadenoma
Conclusion
An application by the Japanese Breast Cancer Society led to MRI-guided VAB being covered by the national health insurance in Japan, and medical services covered by health insurance have to be provided by institutions related to the society. In the future, data from the whole country have to be scientifically examined and quality control needs to be properly performed, mainly by the Japanese Breast Cancer Society. Delayed diagnosis of breast cancer due to inaccurate determination of the indications of MRI-guided VAB in high-risk surveillance, unnecessary biopsy due to overestimation of diagnosis, and a reduction in the success rate of MRI-guided VAB due to inexperience in performing the procedure must be avoided as much as possible. Sharing experience of MRI-guided VAB including detailed procedure and biopsy results will be helpful to those involved in breast MRI and breast cancer management in the era of high-risk screening.
References
Tozaki M, Yamashiro N, Fukuma E. MR-guided vacuum-assisted breast biopsy using a non-titanium needle. Magn Reson Med Sci. 2007;6(4):259–64.
Tozaki M, Yamashiro N, Suzuki T, Kawano N, Ozaki S, Sakamoto N, et al. MR-guided vacuum-assisted breast biopsy: is it an essential technique? Breast Cancer. 2009;16(2):121–5.
Tozaki M, Yamashiro N, Sakamoto M, Sakamoto N, Mizuuchi N, Fukuma E. MR-guided vacuum-assisted breast biopsy: results in 100 Japanese women. Jpn J Radiol. 2010;28(7):527–33.
ACR practice parameter for the performance of magnetic resonance imaging-guided breast interventional procedures. American College of Radiology; 2016. (https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Guided-Breast.pdf#search=%27ACR%2C+MRguided+biopsy%27).
Suzuki T, Tozaki M, Yamashiro N, Sakamoto N, Abe S, Hoshi K, et al. A case of breast cancer in which MRI detected an abnormality two years before diagnosis (in Japanese with English abstract). Jpn J Breast Cancer. 2008;23:557–60.
LaTrenta LR, Menell JH, Morris EA, Abramson AF, Dershaw DD, Liberman L. Breast lesions detected with MR imaging: utility and histopathologic importance of identification with US. Radiology. 2003;227:856–61.
Sakamoto N, Tozaki M, Fukuma E, Higa K, Tsunoda Y, Abe S, et al. The role of ultrasound-guided vacuum-assisted biopsy in the management of MRI-detected lesions (in Japanese with English abstract). Jpn J Breast Cancer. 2007;22:275–9.
Morris EA, Liberman L, Dershaw DD, Kaplan JB, LaTrenta LR, Abramson AF, et al. Preoperative MR imaging-guided needle localization of breast lesions. AJR Am J Roentgenol. 2002;178:1211–20.
Fischer U, Vosshenrich R, Keating D, Bruhn H, Döler W, Oestmann JW, et al. MR-guided biopsy of suspect breast lesions with a simple stereotaxic add-on device for surface coils. Radiology. 1994;192:272–3.
Fischer U, Vosshenrich R, Döler W, Hamadeh A, Oestmann JW, Grabbe E. MR imaging-guided breast intervention: experience with two systems. Radiology. 1995;195:533–8.
Kuhl CK, Elevelt A, Leutner CC, Gieseke J, Pakos E, Schild HH. Interventional breast MR imaging: clinical use of a stereotactic localization and biopsy device. Radiology. 1997;204:667–75.
Kuhl CK, Morakkabati N, Leutner CC, Schmiedel A, Wardelmann E, Schild HH. MR imaging-guided large-core (14-gauge) needle biopsy of small lesions visible at breast MR imaging alone. Radiology. 2001;220:31–9.
Heywang-Köbrunner SH, Heinig A, Schaumlöffel U, Viehweg P, Buchmann J, Lampe D, et al. MR-guided percutaneous excisional and incisional biopsy of breast lesions. Eur Radiol. 1999;9:1656–65.
Perlet C, Heinig A, Prat X, Casselman J, Baath L, Sittek H, et al. Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur Radiol. 2002;12:1463–70.
Prat X, Sittek H, Grosse A, Baath L, Perlet C, Alberich T, et al. European quadricentric evaluation of a breast MR biopsy and localization device: technical improvements based on phase-I evaluation. Eur Radiol. 2002;12:1720–7.
Viehweg P, Heinig A, Amaya B, Alberich T, Laniado M, Heywang-Köbrunner SH. MR-guided interventional breast procedures considering vacuum biopsy in particular. Eur J Radiol. 2002;42:32–9.
Perlet C, Heywang-Kobrunner SH, Heinig A, Sittek H, Casselman J, Anderson I, et al. Magnetic resonance-guided, vacuum-assisted breast biopsy: Results from a European multicenter study of 538 lesions. Cancer. 2006;106(5):982–90.
Sakamoto N, Tozaki M, Higa K, Abe S, Ozaki S, Fukuma E. False-negative ultrasound-guided vacuum-assisted biopsy of the breast: difference with US-detected and MRI-detected lesions. Breast Cancer. 2010;17(2):110–7.
Uematsu T, Takahashi K, Nishimura S, Watanabe J, Yamasaki S, Sugino T, et al. Real-time virtual sonography examination and biopsy for suspicious breast lesions identified on MRI alone. Eur Radiol. 2016;26(4):1064–72.
Izumori A, Kokubu Y, Sato K, Gomi N, Morizono H, Sakai T, et al. Usefulness of second-look ultrasonography using anatomical breast structures as indicators for magnetic resonance imaging-detected breast abnormalities. Breast Cancer. 2020;27(1):129–39.
Nakamura S, Takahashi M, Tozaki M, Nakayama T, Nomizu T, Miki Y, et al. Prevalence and differentiation of hereditary breast and ovarian cancers in Japan. Breast Cancer. 2015;22(5):462–8.
Murakami W, Tozaki M, Nakamura S, Ide Y, Inuzuka M, Hirota Y, et al. The clinical impact of MRI screening for BRCA mutation carriers: the first report in Japan. Breast Cancer. 2019;26(5):552–61.
Tozaki M. Current status of MRI-guided biopsy and construction of training system (in Japanese with English abstract). Jpn J Breast Cancer. 2019;34(4):311–4.
Heller SL, Moy L. Imaging features and management of high-risk lesions on contrast-enhanced dynamic breast MRI. AJR Am J Roentgenol. 2012;198(2):249–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Takahama, N., Tozaki, M. & Ohgiya, Y. Current status of MRI-guided vacuum-assisted breast biopsy in Japan. Breast Cancer 28, 1188–1194 (2021). https://doi.org/10.1007/s12282-020-01107-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01107-x