Abstract
Purpose
To acknowledge the facts of gadoxetate disodium-related events in Japan and to achieve better MR practice by analyzing large cohort data with various MR parameters.
Materials and methods
This prospective multi-institutional study included 1993 patients (1201 men, mean age 66.4 ± 12.8 years), who received dynamic MRI with gadoxetate disodium (gadoxetate group, n = 1646) or extracellular gadolinium-based contrast agents (other-GBCAs group, n = 347) between January and November 2016. Recorded data covered adverse reactions including dyspnea, breath-hold failure during acquisition, respiratory artifacts rated with a four-point scale, and MR parameters. We compared data between the two groups in whole cohort and age-, gender-, and institution-matched subcohort using χ2 test (n = 640). Logistic regression model was used to reveal independent associates of substantial artifacts in arterial phase imaging.
Results
Transient dyspnea rarely occurred in gadoxetate or other-GBCAs group (both < 1%). Gadoxetate group (vs other-GBCAs group) showed higher rates of breath-hold failure (whole cohort, 18.2% vs 7.7%, p < 0.001; subcohort, 17.6% vs 6.3%, p < 0.001) and substantial artifacts in arterial phase (7.2% vs 2.2%, p = 0.001; 7.4% vs 1.7%, p = 0.001). With single arterial phase protocol, substantial artifacts under gadoxetate were independently associated with age (odds ratio [OR] = 1.04, p < 0.001), hearing difficulty (OR = 2.92, p = 0.008), breath-hold practice required (OR = 1.61, p = 0.039), and short acquisition time (OR = 0.43, p = 0.005). Multiple arterial phase acquisition did not reduce the incident rate of substantial artifacts.
Conclusion
Gadoxetate disodium was associated with breath-hold failure and substantial artifacts in arterial phase imaging, but not with dyspnea in Japan. Shorter acquisition time should be used to sustain image quality in gadoxetate disodium-enhanced arterial phase imaging.
Key Points
• Gadoxetate disodium administration leads to breath-hold failure and substantial imaging artifacts in arterial phase MRI in Japan.
• Contrast agent-induced dyspnea in arterial phase and adverse reactions are rare in Japan, without showing differences between gadoxetate disodium or other extracellular gadolinium-based contrast agents.
• Shorter acquisition time significantly reduces gadoxetate-induced imaging artifacts in the arterial phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The visualization and characterization of focal liver lesions in magnetic resonance imaging (MRI) has been greatly improved since the implementation of hepatobiliary contrast agents [1,2,3,4,5]. However, a number of current studies revealed associations between intravenous injection of contrast agents and unfavorable events during image acquisition, such as acute transient dyspnea and transient severe motion (TSM), which are most prominent in the hepatic arterial phase imaging [6, 7]. Contrast-related dyspnea and TSM are exclusively reported for gadoxetate disodium (Primovist®, Eovist®, Bayer-Schering Healthcare), which is widely used in liver MRI. The incidence rates of severe motion artifacts and transient dyspnea following gadoxetate disodium injection are 8–20% and 7–14%, respectively [6,7,8,9,10]. Likewise, recent literature also showed that maximum breath-hold time was shortened by ~ 10 s after gadoxetate disodium injection compared to gadoterate meglumine [11]. Although these phenomena are temporary and self-limited, and therefore not harmful for the patient, they are of high clinical relevance, since arterial phase is essential for the characterization of hepatic lesions [12, 13]. Up to now, most published data are consistent with approving the presence of gadoxetate disodium-related dyspnea/transient severe motions/artifacts. However, the reported incidence rate of these phenomena considerably varies. Interestingly, two studies with Japanese cohorts found remarkably lower rates of self-reported dyspnea of 0.2–2% [8, 14] than that reported with the US cohort.
In spite of these consistent results about gadoxetate disodium-related imaging artifacts in arterial phase imaging, the cause of this phenomenon is still unknown. Previous literatures proposed various methods which may address this issue, e.g., multiple arterial phase acquisition, oxygen inhalation, and modified breath-holding method [7, 15,16,17,18,19,20,21,22,23,24]. Among the various MR protocols available in the clinical scanner, no conclusive strategy has been established to sustain the image quality of gadoxetate disodium-enhanced arterial phase images in a clinical setting.
Therefore, the purpose of our study was to acknowledge the facts of gadoxetate disodium-related events in Japan to achieve better MR practice by analyzing large cohort data with various MR parameters.
Materials and methods
This prospective non-randomized observational study included eight Japanese medical institutions. Approval was granted by the institutional review boards of all institutions and informed consent of the participants was obtained at each institution. Data were assessed anonymously.
Study population
Between January and November 2016, 2128 subjects were enrolled for this study, among which 1780 patients received gadoxetate disodium and 348 other contrast agents (Fig. 1). From these, 135-s examinations in the same patient were excluded to avoid a possible bias caused by the known association between respiratory motion artifacts and prior episode of arterial phase motion. The total study population included 1993 patients (1201 men, 792 women, mean age 66.4 ± 12.8 years) consisting of 1646 (1021 men, 625 women, mean age 66.7 ± 12.5 years) scanned with gadoxetate disodium and 347 (180 men, 167 women, mean age 64.7 ± 14.0 years) with other gadolinium chelate-based extracellular contrast agents (GBCAs). Examination purpose and underlying disease included liver cirrhosis (n = 1143; 57.4%), metastasis (n = 242; 12.1%), biliary disease (n = 115; 5.8%), pancreatic disease (n = 316; 15.9%), kidney disease (n = 35; 1.8%), and other causes (n = 142; 7.1%) (Suppl. Table 1).
Additionally, a subcohort of pairs was built by matching for age, gender, and institutions. This subcohort consisted of 320 patients (149 men, 171 women, mean age 65.5 ± 12.2 years) with gadoxetate disodium and 320 patients (149 men, 171 women, mean age 65.8 ± 12.5 years) with other GBCAs. The impact of multiple arterial phase acquisition on the prevalence of substantial artifacts was assessed in patients from 3 institutions that performed both single and multiple arterial phase protocols under gadoxetate disodium administration (n = 950). Demographic characteristics were representative of the whole cohort: institution 1 (n = 140; 86 men, 54 women, mean age 65.1 ± 13.5 years), institution 2 (n = 355; 211 men, 144 women, mean age 68.2 ± 11.3 years), institution 3 (n = 455; 296 men, 159 women, mean age 68.1 ± 10.9 years).
Magnetic resonance imaging acquisition
Examinations were performed using 1.5 Tesla or 3.0 Tesla MR scanners. In all institutions, pre-contrast and dynamic phases were acquired during breath-hold. Gadoxetate disodium (Primovist®, Bayer Healthcare Pharmaceuticals) was administered intravenously at a standard dose of 0.025 mmol/kg body weight and a rate of 1 ml/s, followed by saline flush in all institutions. Other GBCAs included gadodiamide (Omniscan, Daiichi-Sankyo Pharmaceutical), gadobutrol (Gadovist, Bayer HealthCare), gadoteridol (ProHance, Eisai Pharmaceutical), and gadoterate meglumine (Magnescope/ Dotarem, Guerbet), which were administered at a standard dose of 0.5 mmol/kg body weight followed by saline flush. Oxygen inhalation was performed in 2 multiple arterial phase protocols in two institutions and 1 single arterial phase protocol in one institution. Detail MR protocols for arterial phase acquisitions are shown in the Appendix (Suppl. Table 2).
Recorded data and image analysis
MR technologists performing the MR acquisition at each institution recorded the data below during and shortly after the MR examination.
Details of MR parameter including acquisition time and multi-arterial phase application.
Breath-hold fidelity via monitoring of respiratory bellows wave form. Hereby, breath-hold success was defined as straight or slightly varying wave during image acquisition (Types 1–3 in Fig. 2). Failure was noted as onset of pronounced oscillations (Types 4–5 in Fig. 2).
Requirement of breath-hold practice.
Hearing difficulty or cognitive disability that may make breath-hold fidelity difficult.
Self-reported adverse reactions by answering the inquiry that MR technologists asked: including dyspnea, nausea or vomiting, warm sensation, abdominal discomfort, allergic-like reactions, e.g., sneezing, itchiness, rash, and throat tightness.
If the patient failed their breath-hold in the pre-contrast scan, the MR technologists were instructed to make patients perform one or more breath-holds on the table without MR scanning as a practice of breath-hold scan. The MR technologists were also instructed to ask patients if they had any adverse reactions after the contrast injection, for which a standardized inquiry (“Did you feel anything different after the contrast injection?”) was used in all institutions. Hereby, biasing the patient towards reporting a possible occurrence of dyspnea was avoided by asking openly for any adverse reaction. Prior to the MR examination, all patients received a standardized education about the purpose, procedure, contraindications, and possible adverse reactions (as part of gaining informed consent), which also addressed the possibility of adverse reactions to the contrast medium including breath-hold failure. MR technologists also recorded if the patient had hearing difficulty or cognitive disability, which are realized during the conversation before the scan.
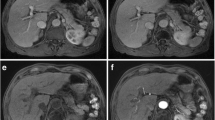
Image analyses of T1-weighted pre-contrast as well as dynamic post-contrast arterial phase and portal venous phase (PVP) were performed by on-site board-certified radiologists with experience in abdominal MR imaging of more than 6 years. Respiratory imaging artifacts were graded with a 4-point scoring system: G1 = no artifacts, G2 = mild artifacts, no effect on diagnostic quality, G3 = moderate artifacts, impeded diagnostic quality, G4 = severe artifacts, non-diagnostic. For further analyses, grades 3 and 4 were defined as substantial artifacts, grade 4 as severe artifact (Fig. 3). The readers were blinded regarding patients’ breath-hold failure and subjective dyspnea. All data collection was carried out using a standardized questionnaire. To standardize the grading of artifacts by the 8 radiologists, sample images for G1–G4 were placed on the questionnaire to let them grade the images by referring the sample side-by-side.
Inter-observer agreement
Kappa values among the readers from all 8 institutions were calculated from 80 randomly selected cases (10 from each institution) containing all four grades of the scoring system. A value below 0.20 defined disagreement, 0.20–0.40 poor agreement, 0.41–0.60 moderate agreement, 0.61–0.80 good agreement, and over 0.80 excellent agreement.
Statistics
All descriptive data are described as absolute numbers and percentages (categorical variables) or as means and standard deviation (continuous variables). Comparative analyses between gadoxetate disodium and other GBCAs were performed by using Wilcoxon and χ2 test for the whole study population as well as the matched-pair group. In order to reveal the effects solely related to contrast agent administration, a subanalysis was performed with those patients who successfully held their breath in the pre-contrast scan. All the above analyses were undertaken in the whole cohort and age-, gender-, and institution-matched subcohort.
For the identification of factors influencing image quality, univariate and multivariate logistic analysis was performed in those patients who underwent single arterial phase MR protocol with gadoxetate disodium (n = 1171) by using substantial artifacts as dependent variable. The independent variables included age, gender, pleural effusion, ascites, hearing difficulty, cognitive impairment, and acquisition time. For the variable “age,” patients were sorted into groups covering a decade (0–10 years, 11–20 years, 21–30 years, the same applies hereafter). The acquisition time was categorized into 3 groups (long [> 20 s], standard [> 13 s and ≦ 20 s], short [≤ 13 s]). The effect of multiple arterial phase acquisition on the prevalence of substantial artifacts was assessed with odds ratio (OR) for 3 institutions that performed both single and multiple arterial phase protocols and combined OR which was calculated by variance-based method.
A p value of < 0.05 was considered statistically significant. All analyses but ORs were performed using JMP version 13 (SAS Institute Inc). The calculation of combined OR was performed by R version 3.5.0.
Results
Demographics and recorded adverse reactions of the whole study population as well as the matched-pair subcohort are depicted in Table 1. Kappa values between the readers of all participating institutions showed moderate to excellent agreement (Suppl. Table 3).
Adverse reactions and self-reported dyspnea
In the whole cohort, adverse reactions in general were reported from 3.0% (50/1646) of patients receiving gadoxetate disodium and 2.3% (8/347) of patients receiving other GBCAs (p = 0.461). In both contrast agent groups, self-reported dyspnea occurred in < 1% of the patients (0.5% [9/1646] after gadoxetate disodium and 0.3% [1/347] after other GBCAs, p = 0.535). No significant difference in the rate of adverse reaction was observed between the two groups. These results were the same in the matched-pair subcohort (Table 1).
Transient severe motion artifacts and breath-hold failure
Substantial imaging artifacts were significantly higher in the arterial phase after administration of gadoxetate disodium compared to other GBCAs in the whole cohort (8.3% vs 3.2%, p = 0.001) as well as in the matched-pair subcohort (8.8% vs 2.5%, p < 0.001). Gadoxetate disodium led to a higher rate of severe artifacts than other GBCAs, which was significant for the whole cohort (1.4% vs 0.0%, p = 0.023), but not for the matched patients (0.6% vs 0.0%, p = 0.542). No significant differences of artifact occurrence could be seen in the PVP or the pre-contrast phase for either study cohort (Table 2). Breath-hold failure was recorded significantly more often after gadoxetate injection than after other GBCAs in the arterial phase (whole cohort, 23.0% vs 14.4%, p < 0.001; subcohort, 25.9% vs 13.1%, p < 0.001), as well as for the matched subcohort in the PVP (whole cohort, 10.6% vs 7.5%, p = 0.096; subcohort, 14.1% vs 7.2%, p = 0.005) (Table 2).
Even in the subanalysis for the subpopulation with successful breath-hold in the pre-contrast scan, the incidence of substantial and severe contrast-induced artifacts, as well as breath-hold failure rate in arterial phase was significantly higher after gadoxetate disodium than after other GBCAs (Table 3).
Factors associated with imaging artifacts after gadoxetate disodium administration
Multivariate analysis revealed a significant association between substantial imaging artifacts in arterial phase and age group (OR = 1.04, p < 0.001), hearing difficulty (OR = 2.92, p = 0.008), the patients for whom breath-hold practice was required (OR = 1.61, p = 0.039), and shorter acquisition time (≤ 13 s) versus standard acquisition time (OR = 0.43, p = 0.009), but not other factors, such as gender (p = 0.06), pleural effusion (p = 0.985), ascites (p = 0.169), and cognitive impairment (p = 0.130) (Table 4). In addition, Cochrane Armitage Trend Test showed substantial imaging artifacts can be less frequent in short acquisition protocols (Fig. 4).
Rates of substantial artifacts according to acquisition time in patients, who underwent single arterial phase MR protocol with gadoxetate disodium (n = 1171). Substantial imaging artifacts after gadoxetate disodium administration occurred less frequent in the arterial phase with examination times of ≤ 13 s compared to standard and longer acquisition time
Multiple arterial phase acquisition
The application of multiple (≥ 3) arterial phase acquisition had no significant effect on the rate of gadoxetate-induced substantial imaging artifacts in the 3 institutions that performed both single and multiple arterial phase MR protocol (OR = 0.75–0.98) (Fig. 5). The combined odds ratio was 0.89 (95% confidence interval, 0.47–1.67).
Substantial artifacts: multiple (≥ 3) arterial phase vs single arterial phase acquisition. Odds ratio of multiple (≥ 3) arterial phase to single arterial phase acquisition for the incident of substantial artifacts in the institutions, which performed both single- and multiple arterial phase MR protocols (n = 950). Combined odds ratio was calculated by variance-based method and shown at the bottom using fixed effect model (FE model). Multiple (≥ 3) arterial phase acquisition did not significantly reduce the rate of substantial imaging artifacts in the arterial phase after gadoxetate disodium injection
Discussion
In our prospective multi-institutional study on a large Japanese patient cohort, we showed that substantial motion artifacts in the arterial phase of abdominal MR imaging occurred significantly more frequently in patients receiving gadoxetate disodium compared with patients receiving other gadolinium-based contrast agents. Likewise, breath-hold failure in the arterial and portal venous phase acquisition was also more frequently observed in the patients receiving gadoxetate disodium. However, the incidence of adverse reactions in general and self-reported dyspnea in particular was not significantly higher after gadoxetate disodium compared to other GBCAs. Shorter acquisition protocol would be beneficial to avoid imaging artifact in gadoxetate disodium-enhanced arterial phase imaging.
The rate of self-reported dyspnea (0.5%) after gadoxetate administration in our study locates on the lowest range of reported rates of dyspnea (7–14%) from western countries, mostly the USA [6,7,8,9,10]. The two previous studies originated in Japan found rates of self-reported dyspnea of 0.2–2% [8, 14], which were consistent with our results. In a Korean cohort, the incident rate of dyspnea was 6.5%, ranking between Japan and the USA [25]. Although we cannot explain the reason of this discrepancy, racial difference might be one cause. Another possible reason for this discrepancy might be different doses of contrast agent commonly used depending on the geographic region. Whereas the Japanese institutions presented in this study applied a standard dose of 0.025 ml/kg body weight, the USA institutions frequently use doses up to twice as high, often at a fixed regime of 10 mL [6,7,8]. A higher contrast agent dose is but a risk factor for respiratory motion-related artifacts [26, 27]. Contrast media was injected at a slower rate of 1 ml/s compared to other studies using a flow of 2 ml/s, which may have resulted in reduced patient’s agitation and consequently lower rate of dyspnea respectively artifacts. Furthermore, at all participating institutions of our study patients’ ability to hold their breath was evaluated during the pre-contrast scan. In case of breath-hold failure, MR technologists were instructed to make patients practice one or more breath-holds on the table without MR scanning.
Imaging artifacts after gadoxetate administration in the arterial phase have been shown to be predominantly associated with breath-hold failure [8, 28]. We were able to relate the breath-hold patterns with imaging artifacts by monitoring the respiratory waveforms on the monitors of MR scanners. From this result, we can aim to get patients hold their breath during the acquisition for the sake of avoiding artifacts. Up to now, many methods have been proposed to address transient severe motion and sustain image quality of gadoxetate disodium-enhanced arterial phase images: the administration of contrast agent in 50% dilution to minimize artifacts [16, 29]; informing the patients about possible dyspnea and performing breath-hold training before the scan [17]. In addition to the above solutions, our results suggest shortening the acquisition time would be a simple and effective way to reduce artifacts in arterial phase imaging. In our study, about 13% (159/1171) of the patients showed oscillations of the respiratory waveform at the end of the breath-hold duration, which can be perceived as imaging artifacts. Shorter acquisition time, therefore, would be an effective strategy to avoid respiratory artifacts in those patients. However, accurate timing of the late arterial phase poses a challenge in MR imaging. Obtaining a well-timed late arterial phase is but essential for the detection and characterizations of hypervascular liver lesions, such as HCC. For appropriate arterial phase timing, current literature recommends bolus tracking instead of a fixed delay, as the latter is prone to timing errors caused by individual factors and the injection protocol. Another strategy is the performance of single-breath-hold multi-arterial phase acquisition to obtain adequate well-timed late hepatic arterial phase images even in patients with transient severe motion [7, 30, 31]. Pietryga et al demonstrated that fast multi-arterial phase imaging in a single breath-hold with three image sets provides adequate images in 98% of the cases [7]. Additionally, this method allows the assessment of the evolution of lesion enhancement over time, which is beneficial for detailed characterization.
In order to improve the quality of arterial phase images, other techniques also have been shown to be conceivable. The clinically established key sequence for liver dynamic MRI is a fat-suppressed 3D T1-weighted spoiled gradient echo sequence, as it provides adequate SNR, spatial, and temporal resolution and can be sufficiently performed during breath-hold. A widely accepted method to reduce acquisition time or improve spatial resolution is parallel imaging technique. CAIPIRINHA (controlled aliasing in parallel imaging results in higher acceleration) VIBE and radial k-space sampling 3D GRE sequence (Radial-VIBE) likewise constitute promising approaches. Breath-hold-free dynamic MR protocols using state-of-the art MR techniques [19,20,21,22] allow for examinations without impairment of image quality. We believe that the latter could be the only solution for those 93 out of 1171 patients of our study, who could not hold their breath from the beginning of the acquisition, which means that artifact-free arterial phase cannot be always expected even with a substantially shortened acquisition time, e.g., a few seconds.
A variety of studies have reported on possible risk factors for transient severe motion, including chronic obstructive pulmonary disease (COPD), volume and injection rate of gadoxetate administration, body mass index (BMI), male sex, and prior episode of arterial phase motion in MR examination [6,7,8, 26, 27, 32,33,34]. In addition to those, our study suggested that hearing difficulty reported by MR technologists can be an independent risk of substantial artifact. In those cases, the command of breath-hold should be modified to well communicate to the patients in the scanner [18].
Our study has several limitations. First, since this was an observational multi-institutional study, several different MR scanners from multiple vendors were used non-randomly according to the clinical purposes. However, by including multiple institutions and various MR parameters, we were able to perform multiple analyses to reveal potential risks/solutions without intervention to the clinical management for better practice in gadoxetate disodium-enhanced MRI. Second, MR technologists were not blinded to the type of contrast agent administered. However, we regard this bias to be negligible, since a standardized questionnaire was used in order to assess all relevant data. Third, the on-site reading was performed by the radiologists participating in this study, which might lead to rater-bias. However, as we performed the preparatory reading session and used reference samples, the effect size of this bias could be small. Also, we tried to standardize this effect by using matched-pair analyses and intra-institutional comparisons. Lastly, in our study population, the rate of cirrhotic patients was higher in the gadoxetic disodium group compared to the group receiving other GBCAs. However, from our data, we could not conclude whether the underlying disease lead to some bias or affected the results in terms of breath-hold ability.
In conclusion, gadoxetate disodium was associated with breath-hold failure and substantial artifacts in arterial phase imaging, but not with dyspnea in Japan. Shorter acquisition time should be used to sustain image quality in gadoxetate disodium-enhanced arterial phase imaging.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- GBCA:
-
Gadolinium-based contrast agent
- MRI:
-
Magnetic resonance imaging
- OR:
-
Odds ratio
- PVP:
-
Portal venous phase
- SD:
-
Standard deviation
- TSM:
-
Transient severe motion
References
Chung YE, Kim MJ, Kim YE, Park MS, Choi JY, Kim KW (2013) Characterization of incidental liver lesions: comparison of multidetector CT versus Gd-EOB-DTPA-enhanced MR imaging. PLoS One 8:e66141
Mohajer K, Frydrychowicz A, Robbins JB, Loeffler AG, Reed TD, Reeder SB (2012) Characterization of hepatic adenoma and focal nodular hyperplasia with gadoxetic acid. J Magn Reson Imaging 36:686–696
Chen L, Zhang J, Zhang L et al (2012) Meta-analysis of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging for the detection of liver metastases. PLoS One 7:e48681
Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO (2007) MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci 6:43–52
Frydrychowicz A, Lubner MG, Brown JJ et al (2012) Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging 35:492–511
Davenport MS, Viglianti BL, Al-Hawary MM et al (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266:452–461
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271:426–434
Motosugi U, Bannas P, Bookwalter CA, Sano K, Reeder SB (2016) An investigation of transient severe motion related to gadoxetic acid-enhanced MR imaging. Radiology 279:93–102
Gutzeit A, Matoori S, Froehlich JM et al (2016) Reduction in respiratory motion artefacts on gadoxetate-enhanced MRI after training technicians to apply a simple and more patient-adapted breathing command. Eur Radiol 26:2714–2722
Kim SY, Park SH, Wu EH et al (2015) Transient respiratory motion artifact during arterial phase MRI with gadoxetate disodium: risk factor analyses. AJR Am J Roentgenol 204:1220–1227
McClellan TR, Motosugi U, Middleton MS et al (2017) Intravenous gadoxetate disodium administration reduces breath-holding capacity in the hepatic arterial phase: a multi-center randomized placebo-controlled trial. Radiology 282:361–368
Bruix J, Sherman M, American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Motosugi U, Ichikawa T, Araki T (2013) Rules, roles, and room for discussion in gadoxetic acid-enhanced magnetic resonance liver imaging: current knowledge and future challenges. Magn Reson Med Sci 12:161–175
Hayashi T, Saitoh S, Tsuji Y et al (2015) Influence of gadoxetate disodium on oxygen saturation and heart rate during dynamic contrast-enhanced MR imaging. Radiology 276:756–765
Namimoto T, Shimizu K, Nakagawa M et al (2018) Reducing artifacts of gadoxetate disodium-enhanced MRI with oxygen inhalation in patients with prior episode of arterial phase motion: intra-individual comparison. Clin Imaging 52:11–15
Polanec SH, Bickel H, Baltzer PAT et al (2017) Respiratory motion artifacts during arterial phase imaging with gadoxetic acid: can the injection protocol minimize this drawback? J Magn Reson Imaging 46:1107–1114
Song JS, Choi EJ, Park EH, Lee JH (2018) Comparison of transient severe motion in gadoxetate disodium and gadopentetate dimeglumine-enhanced MRI: effect of modified breath-holding method. Eur Radiol 28:1132–1139
Gutzeit A, Matoori S, Froehlich J, Koh D (2016) Reduction in respiratory motion artifacts on gadoxetate acid-enhanced MR images after training technicians. Radiology 279:981–982
Weiss J, Notohamiprodjo M, Taron J et al (2018) Continuous hepatic arterial multiphase magnetic resonance imaging during free-breathing. Invest Radiol 53:596–601
Kaltenbach B, Bucher AM, Wichmann JL et al (2017) Dynamic liver magnetic resonance imaging in free-breathing: feasibility of a Cartesian T1-weighted acquisition technique with compressed sensing and additional self-navigation signal for hard-gated and motion-resolved reconstruction. Invest Radiol 52:708–714
Yoon JH, Yu MH, Chang W et al (2017) Clinical feasibility of free-breathing dynamic T1-weighted imaging with Gadoxetic acid-enhanced liver magnetic resonance imaging using a combination of variable density sampling and compressed sensing. Invest Radiol 52:596–604
Chandarana H, Feng L, Ream J et al (2015) Respiratory motion-resolved compressed sensing reconstruction of free-breathing radial acquisition for dynamic liver magnetic resonance imaging. Invest Radiol 50:749–756
Min JH, Kim YK, Kang TW et al (2018) Artifacts during the arterial phase of gadoxetate disodium-enhanced MRI: multiple arterial phases using view-sharing from two different vendors versus single arterial phase imaging. Eur Radiol 28:3335–3346
Li H, Xiao Y, Wang S et al (2017) TWIST-VIBE five-arterial-phase technology decreases transient severe motion after bolus injection of Gd-EOB-DTPA. Clin Radiol 72:800 e801–800 e806
Park YS, Lee CH, Yoo JL et al (2016) Hepatic arterial phase in gadoxetic acid-enhanced liver magnetic resonance imaging: analysis of respiratory patterns and their effect on image quality. Invest Radiol 51:127–133
Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK (2014) Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol 203:796–802
Morisaka H, Motosugi U, Ichikawa S, Onishi H (2018) Dose-dependence of transient respiratory motion artifacts on gadoxetic acid-enhanced arterial phase MR images. J Magn Reson Imaging 47:433–438
Davenport MS, Malyarenko DI, Pang Y, Hussain HK, Chenevert TL (2017) Effect of Gadoxetate disodium on arterial phase respiratory waveforms using a quantitative fast Fourier transformation-based analysis. AJR Am J Roentgenol 208:328–336
Kim YK, Lin WC, Sung K et al (2017) Reducing artifacts during arterial phase of gadoxetate disodium-enhanced MR imaging: dilution method versus reduced injection rate. Radiology 283:429–437
Grazioli L, Faletti R, Frittoli B et al (2018) Evaluation of incidence of acute transient dyspnea and related artifacts after administration of gadoxetate disodium: a prospective observational study. Radiol Med 123:910–917
Gruber L, Rainer V, Plaikner M, Kremser C, Jaschke W, Henninger B (2018) CAIPIRINHA-Dixon-TWIST (CDT)-VIBE MR imaging of the liver at 3.0T with gadoxetate disodium: a solution for transient arterial-phase respiratory motion-related artifacts? Eur Radiol 28:2013–2021
Bashir MR, Gupta RT, Davenport MS et al (2013) Hepatocellular carcinoma in a North American population: does hepatobiliary MR imaging with Gd-EOB-DTPA improve sensitivity and confidence for diagnosis? J Magn Reson Imaging 37:398–406
Bashir MR, Castelli P, Davenport MS et al (2015) Respiratory motion artifact affecting hepatic arterial phase MR imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology 274:141–148
Haradome H, Grazioli L, Tsunoo M et al (2010) Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging 32:334–340
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Utaroh Motosugi, MD PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because of the retrospective study design.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Kromrey, ML., Hori, M., Goshima, S. et al. Gadoxetate disodium-related event during image acquisition: a prospective multi-institutional study for better MR practice. Eur Radiol 30, 281–290 (2020). https://doi.org/10.1007/s00330-019-06358-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06358-7