Abstract
Objectives
To determine risk factors for transient severe motion (TSM) artifact on arterial phase of gadoxetic acid–enhanced MRI using a large cohort.
Methods
A total of 2230 patients who underwent gadoxetic acid–enhanced MRI was consecutively included. Two readers evaluated respiratory motion artifact on arterial phase images using a 5-point grading scale. Clinical factors including demographic data, underlying disease, laboratory data, presence of ascites and pleural effusion, and previous experience of gadoxetic acid–enhanced MRI were investigated. Univariable and multivariable logistic regression analyses were performed to determine significant risk factors for TSM. Predictive value of TSM was calculated according to the number of significant risk factors.
Results
Overall incidence of TSM was 5.0% (111/2230). In the multivariable analysis, old age (≥ 65 years; odds ratio [OR] = 2.01 [95% CI, 1.31–3.07]), high body mass index (≥ 25 kg/m2; OR = 1.76 [1.18–2.63]), chronic obstructive pulmonary disease (OR = 6.11 [2.32–16.04]), and moderate to severe pleural effusion (OR = 3.55 [1.65–7.65]) were independent significant risk factors for TSM. Presence of hepatitis B (OR = 0.66 [0.43–0.99]) and previous experience of gadoxetic acid–enhanced MRI (OR = 0.52 [0.33–0.83]) were negative risk factors for TSM. When at least one of the significant factors was present, the predictive risk was 5.7% (109/1916), whereas it was 16.3% (17/104) when at least four factors were present.
Conclusion
Knowing risk factors for transient severe motion artifact on gadoxetic acid–enhanced MRI can be clinically useful for providing diagnostic strategies more tailored to individual patients.
Key Points
• Old age, high body mass index, chronic obstructive pulmonary disease, and moderate to severe pleural effusion were independent risk factors for transient severe motion artifact on gadoxetic acid–enhanced MRI.
• Patients with hepatitis B or previous experience of gadoxetic acid–enhanced MRI were less likely to show transient severe motion artifact.
• As the number of risk factors for transient severe motion artifact increased, the predicted risk for it also showed a tendency to increase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gadoxetic acid (Eovist/Primovist; Bayer Healthcare) is a hepatocyte-specific MRI contrast agent. Because it enables both dynamic contrast-enhanced T1-weighted imaging and hepatobiliary phase imaging that provides improved lesion conspicuity and lesion-to-liver contrast, it is useful for the detection and characterization of focal hepatic lesions [1,2,3,4]. Given these advantages of gadoxetic acid over other extracellular contrast agents, the use of gadoxetic acid in liver MRI has been increasing in clinical practice.
Despite the clinical usefulness of gadoxetic acid, several studies have reported that transient severe motion (TSM) artifact, caused by a sudden onset of severe motion due to breath-hold failure during the arterial phase, occurs more frequently on gadoxetic acid–enhanced MRI than on extracellular contrast-enhanced MRI, i.e., 13–18% vs. 2–5% [5,6,7,8]. Because imaging characteristics of focal hepatic lesions on arterial phase play an essential role, particularly in the diagnosis of hepatocellular carcinoma (HCC) [9], poor arterial phase image quality caused by TSM can potentially mitigate the advantages of hepatobiliary phase imaging with gadoxetic acid–enhanced MRI. Although the exact cause of TSM remains unclear, identifying the risk factors associated with TSM is important for developing strategies to minimize its occurrence and to determine which patients have an elevated probability of TSM and should undergo alternative methods (e.g., multiple arterial phase liver MRI using parallel imaging and compressed sensing) [3, 10, 11].
Although several previous studies suggested potential risk factors for TSM occurrence [10,11,12,13,14,15,16,17,18,19] from inherent individual to technical factors, the reported results are inconsistent across the studies. We believed that this was partly attributed to the limited study population size. Therefore, we aimed to determine the risk factors for TSM occurrence using a large study cohort.
Materials and methods
The Institutional Review Board of Asan Medical Center approved this retrospective study, and the need for informed consent was waived.
Study participants
A total of 2602 patients who underwent a gadoxetic acid–enhanced liver MRI examination between January 2017 and June 2017 at a single tertiary institution were retrospectively identified (Figure 1). All eligible adult patients (≥ 18 years old) were consecutively enrolled without any further restrictions for study inclusion (i.e., eligible patients, regardless of the reason for the MRI examination, were included). Of the 2594 eligible patients, 364 patients were excluded because of a lack of laboratory or anthropometric data within 1 month of MRI examination to reflect immediate status of the patients at MRI examination. For patients with multiple MRI examinations during the study period, the earliest MRI examination was selected for the analysis. Finally, 2230 patients with 2230 MRI examinations were included in our study. To validate the study result, 456 patients who underwent gadoxetic acid–enhanced liver MRI between January 2016 and June 2016 at another tertiary institution, whose data served as the test data, were enrolled.
MRI acquisition protocols
MRI was acquired using a 1.5-T (Magnetom Avanto, Siemens Healthineers, n = 1169) or 3.0-T (Magnetom Skyra, Siemens Healthineers, n = 774; or Ingenia, Philips Healthcare, n = 287) MRI scanner with a phased-array torso coil. Unenhanced and contrast-enhanced three-dimensional gradient-echo T1-weighted images were obtained after administration of gadoxetic acid (0.1 mL/kg) at a rate of 1 mL/s followed by a 20-mL saline flush. Contrast-enhanced images were acquired in the arterial phase (5 s after peak enhancement of the aorta determined by a test-bolus method), portal phase (50 s after contrast agent injection), transitional phase (3 min after contrast agent injection), and hepatobiliary phase (20 min after contrast agent injection). The breath-hold time for arterial phase image acquisition was 14 s.
Review of clinical data
The following clinical factors potentially associated with TSM were obtained from the electronic database at our institution: (a) age; (b) morphometric data (sex, height, body weight, and body mass index); (c) etiology of liver disease (hepatitis B, hepatitis C, alcoholic liver disease, nonalcoholic fatty liver disease, or autoimmune hepatitis); (d) presence of liver cirrhosis; (e) presence of other chronic disease (hypertension, diabetes mellitus, asthma, or chronic obstructive pulmonary disease [COPD]); (f) laboratory data (albumin, total bilirubin, prothrombin time, creatinine, and the Model for End-stage Liver Disease [MELD] score in patients with liver cirrhosis [20]); and (g) previous treatment of hepatic malignancy (surgery, radiofrequency ablation, transcatheter arterial therapy, or radiation therapy). Previous experience of gadoxetic acid–enhanced MRI and history of MRI contrast allergy were also analyzed. The presence and degree of ascites and pleural effusion were analyzed in the MRI examination. Ascites were classified as none to minimal (thickness less than 5 mm), mild (asymmetric distribution without abdominal distension), or moderate–severe (symmetric distribution with abdominal distension) [21]. Pleural effusion was classified as none to minimal (thickness less than 5 mm), mild (thickness of 5–10 mm), or moderate–severe (thickness greater than 10 mm) [10, 22].
Evaluation and grading of respiratory motion artifacts
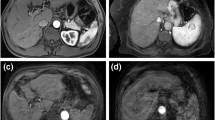
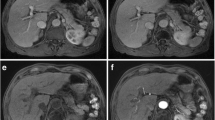
Two board-certified radiologists (D.W.K. and S.H.C. with 7 and 10 years of experience in liver MRI) who were blinded to the clinical data independently analyzed the arterial phase gadoxetic acid–enhanced MRI. If there was any discrepancy, the discordant cases were re-evaluated until a consensus was reached. The following 5-point grading scale system was used to evaluate respiratory motion artifacts on arterial phase imaging [8]: grade 1, no artifact; grade 2, minimal artifact with no effect on diagnostic quality; grade 3, moderate artifact with some but not severe effect on diagnostic quality; grade 4, severe artifact with effect on diagnostic quality but still interpretable; and grade 5, extensive artifact with non-diagnostic image (Figure 2). Consistent with previous studies [10, 22, 23], no particular level of images was designated for the evaluation, and the degree of respiratory motion artifacts was determined after reviewing all the MRI images of the liver. TSM was defined as grade 4 or 5 respiratory motion artifact on arterial phase image and grade 2 or less motion artifact on the unenhanced images and other contrast-enhanced images including portal phase, transitional phase, and hepatobiliary phase.
Scoring system for respiratory motion artifacts on arterial phase imaging. (a) Grade 1, no artifact; (b) grade 2, minimal artifacts with no effect on diagnostic quality; (c) grade 3, moderate artifacts with some non-severe effects on diagnostic quality; (d) grade 4, severe artifacts affecting diagnostic quality but image still interpretable; and (e) grade 5, extensive artifacts with a non-diagnostic image
Statistical analysis
We calculated the incidence of TSM using gadoxetic acid–enhanced MRI in total eligible patients. Subgroup analysis in patients who underwent MRI examinations with extracellular contrast agents within 1 year was performed, and the TSM incidence using the MRI findings with gadoxetic acid and that using the MRI findings with extracellular contrast agent was compared using the McNemar test.
The study subjects were divided into two groups: a TSM group (respiratory motion artifact grade of 4 or 5) and a non-TSM group (respiratory motion artifact grade of 0 to 3). Univariable and multivariable logistic regression analyses were performed to determine the independent significant risk factors for TSM. Variables with p < .05 in the univariable analysis were included in the multivariable analysis. Odds ratios (OR) with 95% confidence intervals (CI) were calculated for each variable. p < .05 was considered statistically significant. The predicted risk of TSM occurrence was calculated for combinations of the significant risk factors using the number of significant risk factors as cutoff points. To validate our study result, we evaluated whether the significant factors in our study were also significant in the test cohort and calculated the predictive risk of the TSM occurrence according to the number of these significant factors.
Inter-reader reliability for the grading of respiratory motion artifact was evaluated using the overall percentage of agreement and weighted kappa (κ) statistics. All statistical analysis was performed using R version 4.0.3 (R Foundation for Statistical Computing).
Results
Patient characteristics
Table 1 summarizes the clinical characteristics of the patients. The mean age of the 2230 patients was 60.5 years and 1686 were men. Hepatitis B was the most common etiology of liver disease (n = 1419 [63.6%]), followed by alcoholic liver disease (n = 278 [12.5%]) and hepatitis C (n = 199 [8.9%]). Liver cirrhosis was present in 1461 patients (65.5%). A total of 1314 patients (58.9%) had a history of previous treatment for hepatic malignancies, with these including surgery (n = 498 [22.3%]), radiofrequency ablation (n = 307 [13.8%]), transcatheter arterial therapy (n = 328 [14.7%]), and radiation therapy (n = 181 [8.1%]). One thousand and eighty-eight (48.8%) patients had previous experience of gadoxetic acid–enhanced MRI examination, and 35 (1.6%) had a history of MRI contrast allergy. Moderate–severe ascites and pleural effusion were found in 94 (4.2%) and 16 (0.7%) patients, respectively.
Evaluation of respiratory motion artifacts
Table 2 summarizes MRI techniques of contrast-enhanced T1-weighted images. Using 2230 MRI examinations with gadoxetic acid, on the basis of the 5-point grading system, 866 (38.8%), 1022 (45.8%), 231 (10.4%), 102 (4.6%), and 9 (0.4%) patients were considered to have respiratory motion artifact of grade 1, grade 2, grade 3, grade 4, or grade 5, respectively. Therefore, there was no respiratory motion artifact (grade 1) in 38.8% of MRI examinations, whereas TSM (grade 4 or 5) was detected in 5.0% of MRI examinations. Of these 2230 patients, 138 underwent MRI examinations with an extracellular contrast agent within 1 year. The incidence of TSM was found to be significantly lower using MRI with an extracellular contrast agent than that using MRI with gadoxetic acid (2.2% vs. 9.5%, p = .006).
The overall percentage of agreement and weighted κ for the grading of respiratory motion artifacts between the two readers were 79.9% and 0.78, respectively.
Risk factors for TSM
The results of the univariable and multivariable logistic regression analyses are summarized in Table 3. In the univariable analysis, age (≥ 65 years; OR = 2.53, p < .001), BMI (≥ 25 kg/m2; OR = 1.74, p = .005), hepatitis B (OR = 0.43, p < .001), hypertension (OR = 1.88, p = .001), COPD (OR = 7.06, p < .001), albumin (OR = 1.87, p = .001), history of surgery (OR = 0.57, p = .042), previous experience of gadoxetic acid–enhanced MRI examination (OR = 0.55, p = .004), and moderate to severe pleural effusion (OR = 4.27, p < .001) were significantly associated with TSM occurrence.
Multivariable analysis revealed that old age (≥ 65 years; OR = 2.01, p = .001), high BMI (≥ 25 kg/m2; OR = 1.76, p = .005), COPD (OR = 6.11, p <. 001), and moderate to severe pleural effusion (OR = 3.55, p = .001) were independent risk factors for TSM occurrence (Table 3). By contrast, previous experience of gadoxetic acid–enhanced MRI (OR = 0.52, p = .006) was an independent negative risk factor for TSM occurrence. Hepatitis B was also found to be a negative risk factor for TSM occurrence with borderline statistical significance (OR = 0.66, p = .048). Of note, the proportion of patients who had experience of gadoxetic acid–enhanced MRI before the study period was significantly higher in those patients with hepatitis B than in those without hepatitis B (57.9% [821/1419] vs. 32.9% [267/811]; p < .01).
When at least one of the six significant risk factors (age ≥ 65 years, BMI ≥ 25 kg/m2, COPD, moderate to severe pleural effusion, no hepatitis B, and no previous experience of gadoxetic acid–enhanced MRI) was present, the predictive risk for TSM was 5.7% (109/1916; Table 4). When at least two, three, or four of these six risk factors were present, the predictive risks of TSM were 7.3% (87/1191), 12.1% (59/486), and 16.3% (17/104), respectively.
In the test cohort, the incidence of TSM was 6.6% (30/456). Five of these six significant risk factors, including age ≥ 65 years, BMI ≥ 25 kg/m2, COPD, moderate to severe pleural effusion, and the absence of hepatitis B, were also found significant in the test cohort (p ≤ .038) (Supplementary Table S1). Previous experience using gadoxetic acid–enhanced MRI showed a borderline statistical significance in the test cohort (p = .057). The predictive risk for the TSM occurrence according to the number of significant risk factors in the test cohort is summarized in Supplementary Table S2.
Discussion
In our study, transient severe motion artifact occurred in 5.0% of 2230 gadoxetic acid–enhanced MRI examinations. Our study found that old age (≥ 65 years), high body mass index (≥ 25 kg/m2), chronic obstructive pulmonary disease, and moderate to severe pleural effusion were significant independent risk factors for occurrence of transient severe motion artifact, whereas the presence of hepatitis B and previous experience of gadoxetic acid–enhanced MRI were significant negative risk factors. As the number of risk factors for transient severe motion artifact increased, the predictive risk of transient severe motion artifact also increased. In addition, because these factors were also significant for the TSM occurrence in the test cohort, our study result might be useful for general application in clinical practice.
Although previous studies have investigated the risk factors associated with TSM (Table 5), the results were inconsistent across the studies. For example, some studies [10,11,12, 15, 17, 19] have revealed a significant association of the risk factors with TSM, whereas other studies have not identified any risk factors [13, 14, 16, 18]. Furthermore, most studies have reported risk factors of TSM using univariable analysis, and only a few studies [12, 15] have reported contrast dose, COPD, breath-hold failure, male, and high BMI, as independent risk factors of TSM using multivariable analysis. However, the reported risk factors were variable across the studies. Such inconsistency may be in part attributed to the relatively small study population. Recently, a meta-analysis has reported a higher TSM incidence in studies with a Western population or those using a 5-point scale for the TSM determination; however, these studies are limited because of substantial study heterogeneity. Moreover, the investigation was performed on a per-study basis without obtaining individual patient data of eligible studies [8]. In this regard, our study has strength as it comprehensively evaluated the TSM-related risk factors using individual patient data of a large study population (> 2000), and had sufficient statistical power.
Consistent with previous studies [11, 12, 15, 18, 24], predisposing factors, including old age (OR = 2.01), high BMI (OR = 1.76), COPD (OR = 6.11), and moderate to severe pleural effusion (OR = 3.55), for diminished general breath-hold capacity might have contributed to TSM. In COPD patients in particular, the response to tachypnea induced by gadoxetic acid during the arterial phase (i.e., dynamic hyperinflation) may exacerbate motion artifact [12, 25]. Furthermore, in patients with a high BMI, the higher weight-adjusted contrast dose (0.1 mL/kg) administered may also contribute to a higher likelihood of TSM [12]. Considering that previously reported risk factors are inconsistent and not unanimously validated (e.g., Kim et al and Bashir et al suggested previous TSM as a risk factor for TSM occurrence, whereas Hayashi et al suggested high BMI and gadolinium exposure [10, 11, 19]), our study results should be clinically useful for improving the understanding of TSM, because we comprehensively analyzed the potential risk factors for TSM occurrence. In addition, because we found that the predicted risk of TSM increased as the number of risk factors for TSM increased, and that the incidence of TSM was significantly lower using MRI with an extracellular contrast agent than that using MRI with gadoxetic acid (2.2% vs. 9.5%), our study could be helpful to select patients who need other diagnostic tools such as extracellular contrast-enhanced MRI or multiple arterial-phase liver MRI.
Our study also found that hepatitis B and previous experience of gadoxetic acid–enhanced MRI were significant negative risk factors for TSM occurrence. In respect to these, it seems logical to focus on previous experience of gadoxetic acid–enhanced MRI as a negative risk factor for TSM, rather than hepatitis B. As hepatitis B virus infection is associated with development of HCC, even in the absence of liver cirrhosis due to DNA integration of the hepatitis B virus [9], repetitive imaging evaluation including MRI is often underway in hepatitis B virus carriers (to detect HCC before progression to advanced disease), whereas in other chronic liver diseases, the presence of liver cirrhosis is a prerequisite for surveillance for HCC [26]. Therefore, although the exact mechanism for the reduced risk of TSM in patients with hepatitis B is not fully understood, it may be associated with the more frequent experience of MRI compared with patients without hepatitis B (57.9% [821/1419] vs. 32.9% [267/811]; p < .01). Although there were conflicting results about whether previous exposure of gadoxetic acid was significantly associated with TSM occurrence or not, our results imply that the experience of gadoxetic acid–enhanced MRI may decrease the TSM occurrence, which was in line with previous studies reporting the usefulness of pre-scan breath-hold training [10, 11, 18, 19, 27]. However, our result should be cautiously interpreted because the lower risk of TSM in the experienced patients may be partially attributed to a selection bias, with patients who have shown previous substantial motion artifact being selected to undergo alternative imaging modalities such as computed tomography or extracellular contrast-enhanced MRI.
Our study has several limitations. First, selection bias due to the retrospective study design may be an inevitable limitation. However, we tried to minimize this limitation by including a large number of patients (more than 2000) to evaluate the various factors associated with TSM. Second, we obtained MRI examinations performed in institutions of Korea, a country in which hepatitis B virus is endemic. The effects of ethnicity and the etiology of chronic liver disease on TSM need to be further evaluated in multinational multicenter studies. Third, the incidence (5.0%) of TSM was relatively low compared with previous meta-analysis-reported incidence (pooled incidence, 13.0%) [8]. Considering that a substantial portion (63.6%) of the study population consisted of patients with hepatitis B or who (48.8%) underwent gadoxetic acid–enhanced MRI, which were significant negative risk factors of TSM in our study, these factors might have attributed to a relatively low incidence of TSM. Furthermore, when we compared it with Asian studies using a 5-point grading system, the incidence of TSM in our study was similar to previously reported values (4.8–8.2%) [14, 19, 28, 29] and also comparable to a previous study using similar MRI techniques [30]. Last, the number of positive cases was inherently small for some of the potential risk factors (e.g., asthma, COPD, and pleural effusion).
In conclusion, old age, high BMI, COPD, and pleural effusion were independently associated with the high risk of TSM occurrence, whereas hepatitis B and previous experience of gadoxetic acid–enhanced MRI were associated with a lower risk of TSM occurrence. As the number of these significant risk factors increased, the predictive risk of transient severe motion artifact also increased (5.7% in the presence of ≥ one significant factor and 16.3% in the presence of ≥ four significant factors). Therefore, knowing such risk factors for TSM can be clinically useful as it allows for the provision of more patient-tailored diagnostic strategies aiming at qualified diagnostic imaging.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- OR:
-
Odds ratio
- TSM:
-
Transient severe motion
References
Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY (2010) Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 255:459–466
Sano K, Ichikawa T, Motosugi U et al (2011) Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology 261:834–844
Grazioli L, Bondioni MP, Haradome H et al (2012) Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology 262:520–529
Motosugi U, Ichikawa T, Morisaka H et al (2011) Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology 260:446–453
Davenport MS, Viglianti BL, Al-Hawary MM et al (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266:452–461
Song JS, Choi EJ, Park EH, Lee JH (2018) Comparison of transient severe motion in gadoxetate disodium and gadopentetate dimeglumine-enhanced MRI: effect of modified breath-holding method. Eur Radiol 28:1132–1139
Rimola J, Darnell A, Belmonte E et al (2020) Does transient arterial-phase respiratory-motion-related artifact impact on diagnostic performance? An intra-patient comparison of extracellular gadolinium versus gadoxetic acid. Eur Radiol 30:6694–6701
Kim DW, Choi SH, Park T, Kim SY, Lee SS, Byun JH (2021) Transient severe motion artifact on arterial phase in gadoxetic acid-enhanced liver magnetic resonance imaging: a systematic review and meta-analysis. Invest Radiol. https://doi.org/10.1097/rli.0000000000000806
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
Kim SY, Park SH, Wu EH et al (2015) Transient respiratory motion artifact during arterial phase MRI with gadoxetate disodium: risk factor analyses. AJR Am J Roentgenol 204:1220–1227
Bashir MR, Castelli P, Davenport MS et al (2015) Respiratory motion artifact affecting hepatic arterial phase MR imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology 274:141–148
Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK (2014) Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol 203:796–802
Furlan A, Close ON, Borhani AA, Wu YH, Heller MT (2017) Respiratory-motion artefacts in liver MRI following injection of gadoxetate disodium and gadobenate dimeglumine: an intra-individual comparative study in cirrhotic patients. Clin Radiol 72:93.e91–93.e96
Kanki A, Tamada T, Abe T, Ikenaga H, Yoshida K, Ito K (2018) Relationship between transient severe motion of the liver in gadoxetic acid or iodinated contrast agent-enhanced imaging and arterial oxygen saturation and heart rate changes. Magn Reson Imaging 53:77–81
Motosugi U, Bannas P, Bookwalter CA, Sano K, Reeder SB (2016) An investigation of transient severe motion related to gadoxetic acid-enhanced MR imaging. Radiology 279:93–102
Ringe KI, von Falck C, Raatschen HJ, Wacker F, Hinrichs J (2018) Evaluation of transient respiratory motion artifact at gadoxetate disodium-enhanced MRI-influence of different contrast agent application protocols. PLoS One 13:e0200887
Vietti Violi N, Argiriadi P, Rosen A et al (2020) Gadoxetate disodium-enhanced MRI: assessment of arterial phase artifacts and hepatobiliary uptake in a large series. Eur J Radiol 132:109313
Well L, Rausch VH, Adam G, Henes FO, Bannas P (2017) Transient severe motion artifact related to gadoxetate disodium-enhanced liver MRI: frequency and risk evaluation at a German institution. Rofo 189:651–660
Hayashi T, Saitoh S, Tsuji Y et al (2015) Influence of gadoxetate disodium on oxygen saturation and heart rate during dynamic contrast-enhanced MR imaging. Radiology 276:756–765
Wiesner R, Edwards E, Freeman R et al (2003) Model for End-stage Liver Disease (MELD) and allocation of donor livers. Gastroenterology 124:91–96
Moore KP, Wong F, Gines P et al (2003) The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 38:258–266
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271:426–434
Davenport MS, Caoili EM, Kaza RK, Hussain HK (2014) Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology 272:123–131
Shah MR, Flusberg M, Paroder V, Rozenblit AM, Chernyak V (2017) Transient arterial phase respiratory motion-related artifact in MR imaging of the liver: an analysis of four different gadolinium-based contrast agents. Clin Imaging 41:23–27
O’Donnell DE, Revill SM, Webb KA (2001) Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164:770–777
Kanwal F, Singal AG (2019) Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology 157:54–64
Gutzeit A, Matoori S, Froehlich JM et al (2016) Reduction in respiratory motion artefacts on gadoxetate-enhanced MRI after training technicians to apply a simple and more patient-adapted breathing command. Eur Radiol 26:2714–2722
Min JH, Kim YK, Kang TW et al (2018) Artifacts during the arterial phase of gadoxetate disodium-enhanced MRI: multiple arterial phases using view-sharing from two different vendors versus single arterial phase imaging. Eur Radiol 28:3335–3346
Ikeno H, Kobayashi S, Kozaka K et al (2020) Relationship between the degree of abdominal wall movement and the image quality of contrast-enhanced MRI: semi-quantitative study especially focused on the occurrence of transient severe motion artifact. Jpn J Radiol 38:165–177
Luetkens JA, Kupczyk PA, Doerner J et al (2015) Respiratory motion artefacts in dynamic liver MRI: a comparison using gadoxetate disodium and gadobutrol. Eur Radiol 25:3207–3213
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant number: NRF-2019R1G1A1099743) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C2383).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Sang Hyun Choi.
Conflict of interest
Sang Hyun Choi declares relationships with the following companies: Bayer Healthcare. The other authors of this manuscript have no conflicts of interest to declare.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
-
Retrospective
-
Diagnostic or prognostic study
-
Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Jang, E.B., Kim, D.W., Choi, S.H. et al. Transient severe motion artifacts on gadoxetic acid–enhanced MRI: risk factor analysis in 2230 patients. Eur Radiol 32, 8629–8638 (2022). https://doi.org/10.1007/s00330-022-08885-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08885-2