Abstract
Aims
To evaluate motion artifacts, breath-hold failure, acute transient dyspnea, and clinical parameters during hepatic arterial phase of gadoxetate disodium-enhanced magnetic resonance (MR) imaging.
Methods
This was an institutional review board-approved observational prospective study (written informed consent acquired) performed in 250 consecutive patients, who underwent liver MR with a multiarterial phase technique. Oxygen saturation (SatO2) and heart rate (HR) were monitored, while patients reported subjective symptoms. Breath-holds were assessed using prospective acquisition correction technique (PACE) monitors. Three readers independently analyzed all images to establish the presence of motion artifacts. Nonparametric statistical testing and Fleiss’ kappa were used.
Results
No statistical differences in SatO2 and HR values were observed during the entire length of MR examination. The PACE graphs showed an altered breath-hold in 16/250 patients (6.4%), however only 6 patients self-reported symptoms during the procedure, and among these 6 subjects, only 2 suffered from acute transient dyspnea (0.8%). Motion-related artifacts increased mostly in the third arterial phase of gadoxetate disodium acquisition (p < 0.0001): The artifacts incidence was 2.9% in the first phase; 4.0% in the second; and 19.5% in the third. This increase was mainly due to patients’ inability to hold their breath for the entire duration of the examination. However, at least one gadoxetate disodium arterial phase without motion artifacts and adequate for acquisition timing, was acquired in all MR examinations.
Conclusion
The incidence of breath-hold failure and acute transient dyspnea after gadoxetate disodium administration increased during the third arterial phase only. Our protocol allowed the acquisition of at least one arterial phase not compromised by motion artifacts and adequate for acquisition timing, in all patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to current guidelines, the diagnosis of hepatocellular carcinoma in patients with chronic liver disease is based on the arterial phase enhancement of liver lesions followed by rapid washout in the portal and late venous phases [1]. A multiphase dynamic imaging is also fundamental for establishing the diagnosis of other hepatic lesions [2]. Multiarterial phase imaging has been shown able to improve the detection and differential diagnosis of hypervascular focal liver lesions [3].
Gadoxetate disodium and gadobenate dimeglumine are widely used in liver MR for their added value in hepatobiliary phase imaging [2]. The key advantage of gadoxetate disodium is the acquisition of hepatobiliary phase within 20 min after intravenous administration, compared to the time − 90 to 120 min necessary for hepatobiliary phase imaging after gadobenate dimeglumine administration [4].
On the other hand, the clinical practice and some observational studies reported an increased incidence of artifacts in the arterial phase due to breathing difficulties after gadoxetate disodium administration. Davenport et al. [5] firstly reported an association between gadoxetate disodium and an “acute self-limiting dyspnea that can have a deleterious effect on arterial phase MR image quality”; other authors defined that phenomenon “transient severe motion” [6] or “breathlessness due to long breath-time” [7]. Irrespective of its definition, this event can even lead to severely degraded gadoxetate disodium arterial phase images in 18% of MR examinations, resulting in some cases in nondiagnostic imaging [5]. Many studies investigated the causes of this phenomenon [8,9,10,11], and several approaches have been applied to reduce motion artifacts: (a) lowering the contrast injection rate [12], (b) using multiple arterial phase technique [6], (c) shortening the scanning time [7, 13], (d) using a modified breathing command [14], and (e) diluting contrast medium [15, 16]. Thanks to these strategies severe motion artifacts even decreased to 2.4% [7]—an incidence similar to that found using other contrast media. However, McClellan et al. [17] recently reported a reduced arterial phase breath-holding duration in healthy volunteers administered with gadoxetate disodium. Based on these contradictory data and on the fundamental importance of gadoxetate disodium as contrast agent in liver MR, we designed an observational prospective interindividual study to evaluate the incidence of motion artifacts, breath-hold failure and acute transient dyspnea, and the variation of clinical parameters [oxygen saturation (SatO2) and heart rate (HR)], in patients undergoing liver MR with the multiarterial phase (controlled aliasing in parallel imaging results in higher acceleration—CAIPIRINHA algorithm) technique.
Materials and methods
This observational prospective single-site study was approved by the institutional review board, and it was compliant with the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all subjects prior to study initiation.
Subjects
Data were collected from 250 consecutive patients who underwent gadoxetate disodium-enhanced liver MR, according to current medical practice and inclusion criteria, from July 2014 to March 2015. In total, 128 of them were cirrhotic patients, 73 were patients who developed liver lesions during the follow-up for oncologic diseases, and 49 were patients with incidental hepatic lesions. Patients (mean age 61.2 ± 13.8 years; age range 22–87 years) consisted of 109 women (mean age 60.3 ± 14.1 years; age range 24–87 years) and 141 men (mean age 62.1 ± 13.4 years; age range 21–82 years).
MR protocol
Before MR examination, patients’ breath-holding ability and weight were assessed to calibrate the protocol (i.e., number of arterial phases and amount of contrast medium).
All examinations were performed using a 1.5-T scanner (Aera, Siemens Medical Systems) with a 45 mT/m gradient strength (slew rate of 200 mT/m/ms), an 18-element surface phased-array coil, and an 18-element spine coil. Pre-contrast and dynamic phases were acquired in one breath-hold. A multiarterial phase (CAIPIRINHA algorithm) was acquired using a 3D-enhanced T1-weighted high resolution, with linear-filling k-space in a specific acquisition–keyhole sequence (keyhole, acceleration factor PE: 2; acceleration factor 3D: 2; reordering shift: 1); TR/TE, 3.9/1.9; FOV, 40 × 30 cm; image matrix, 320 × 182; parallel imaging factor, 4; flip angle, 10°; bandwidth, 450 Hz; slice thickness: 3 mm. The average acquisition time for the triple arterial phase imaging consisted in a 16–18-s breath-holding period, yielding 80 slices. The multiarterial phase image acquisition has been started 8–10 s after the visual detection of contrast material at the celiac artery with the real-time bolus display method (CARE Bolus; Siemens Healthcare): The celiac artery was visualized about 15 s after injection; then, the first, the second, and the third arterial phases were acquired 23–28, 29–34, and 35–39 s after injection, respectively, anyhow defining an appropriate arterial phase acquisition. During saline injection, the “arterial” phase acquisition has been started 15 s after the start of the injection.
For each patient, the examination included a saline “arterial” phase followed by a gadoxetate disodium arterial phase. Saline (0.1 mL/kg + 30 mL) was injected at 1 mL/s, and after 2 min, gadoxetate disodium (Primovist, Bayer, 0.1 mL/kg) was injected at 1 mL/s, followed by a 30-mL saline flush injected at 1 mL/s.
In our Radiology Department, the MR examinations include the administration of a fixed amount of saline before acquisition of a dynamic multiarterial phase. The saline injection is usually performed prior to contrast agent injection in order to evaluate patient’s vein patency and breath-hold ability. In the present study, we took advantage of this procedure by also collecting the subjective feelings and analyzing the image quality of the saline “arterial” phase, in order to evaluate whether the injection procedure itself can lead to a discomfort and/or motion artifacts. In this way, we were able to evaluate the effect of gadoxetate disodium on occurrence of adverse events (in particular breathing difficulties), motion-related artifacts, and image quality in comparison with saline injection in an observational prospective interindividual study.
Clinical parameters and self-evaluation questionnaire
SatO2 and HR were measured by using a peripheral pulse oximeter prior, during, and after MR examination. For each imaging phase, the lowest SatO2 and the highest HR were recorded.
Breath-hold during arterial phase acquisition was assessed by using the prospective acquisition correction technique (PACE) monitors. Graphs were evaluated in a blinded fashion by a radiologist not involved in the analysis of images artifacts. The presence of sudden pronounced oscillations was considered indicator of poor breath-hold (PACE+), whereas graphs having a straight or slowly varying trend were considered physiological (PACE−).
After each injection (saline and contrast medium), the radiologist asked to the patients whether they experienced any difficulties during the procedure and, if so, to briefly describe them. Then, the radiologist reported the responses in the questionnaire. Only self-reported adverse events were recorded.
Evaluation of motion artifacts
Images were independently assessed in a randomized, blinded fashion by three radiologists (G.B., R.F., and B.F., with 1, 5, and 10 years of experience in abdominal imaging, respectively). In case of disagreement, the highest motion score recorded for any of the three arterial phases was considered to be the overall arterial score. Respiratory motion artifacts were classified by using the following 5-point scale, as previously described [5, 9]: 1, no artifact; 2, minimal artifact with no effect on diagnostic quality; 3, moderate artifact with some effect but no severe effect on diagnostic quality; 4, severe artifact but images were still interpretable; and 5, extensive artifact and images nondiagnostic. We considered images with artifacts from 1 to 3 as good or degraded but still interpretable and images with artifacts from 4 to 5 as nondiagnostic images [5].
Statistical analysis
Continuous variables were tested for normality with the Shapiro–Wilks test: Normal variables were expressed as mean ± standard deviation; not normal variables were expressed as median plus interquartile range (IQR 25th and 75th percentile). For homogeneity, all variables were analyzed with nonparametric tests. Correlated variables were compared with the Wilcoxon test (number of distributions k = 2) or the Friedman test (k > 2). Independent variables were compared with the Mann–Whitney test (k = 2) or the Kruskal–Wallis test (k > 2). Binary and categorical variables, reported as counts and percentages, were studied with the Chi-square test (with Yates’ correction for 2 × 2) or with Fisher’s exact test. Interobserver variability was computed via Fleiss’ kappa. Open-source software (http://www.openepi.com and http://www.vassarstats.net) and StatPlus:mac Pro (Analyst Soft Inc. Walnut, CA, USA) were used.
Results
Patient characteristics and gadoxetate disodium dosage
At the time of the examination, all of them met the inclusion criteria of the recent ESGAR consensus statement [2]. The average duration of apnea was 16.9 ± 1.9 (range of 11–20) s. The gadoxetate disodium dosage was 8.3 ± 1.5 (range of 4–12 mL).
Evaluation of clinical parameters and motion artifacts
In Fig. 1, the values of HR and SatO2 were represented with box plots. The average basal SatO2 was 97.3 ± 2.0 (range of 89–100), and the average basal HR was 69.6 ± 13.5 beats per minute (bpm) (range of 47–116 bpm), with 8 patients (3.2%) suffering from diagnosed tachycardia (HR ≥ 100 bpm). After MR examination, the average values of HR and SatO2 were similar to the basal values: SatO2 = 97.5 ± 1.7 (range of 73–100) and HR = 69.0 ± 12.6 bpm (range of 47–113 bpm). The average values of HR and SatO2 during saline “arterial” phase and gadoxetate disodium arterial phase were also similar to the basal values (Fig. 1), with no statistical difference between saline and contrast agent (Table 1).
The calculation of the Fleiss’ kappa coefficient resulted in k = 0.82, which indicates an “almost perfect” agreement between readers. Motion artifacts (Table 1) had a low incidence during acquisition of the first gadoxetate disodium arterial phase and saline “arterial” phase (2.9 and 1%, respectively, p = 0.28). The incidence of motion artifacts increased slightly in the second gadoxetate disodium arterial phase, while it remained stable in that acquired after saline injection (4 and 1.2%, respectively); however, the difference between saline and contrast agent was not statistically significant (p = 0.09). On the other hand, during the acquisition of the third phase we observed a significantly higher incidence of motion artifacts in the gadoxetate disodium arterial phase than saline “arterial” phase (19.5 and 3%, respectively, p < 0.0001). In total, 236 patients (94.4%) were able to complete the MR examination, whereas 14 patients were unable to hold their breath till the end of the third arterial phase acquisition.
The assessment of breath-hold with PACE system showed a breath-hold failure (PACE+) in 16/250 patients (6.4%). The clinical parameters and incidence of motion artifacts in PACE+ and PACE− patients were compared (Table 2 and Fig. 2): We found that incidence of motion artifacts in the gadoxetate disodium triple arterial phase was significantly higher in PACE+ patients than in PACE− patients in the third phase only (64 and 16%, respectively, p < 0.0001).
Table 3 reports relevant data (age, sex, breath-hold ability prior examination, gadoxetate disodium dose, and occurrence of motion artifacts) and results of the questionnaire for the 16 PACE+ patients. Ten PACE+ patients did not report any difficulty in the questionnaire, although motion artifacts occurred in most of them (8 of 10, 80%). These 10 PACE+ patients with negative questionnaire had a shorter duration of their apnea in basal conditions in comparison with patients with PACE+ and positive questionnaire (15.5 ± 1.3 vs 19.5 ± 0.84 s; p < 0.0001). For these patients, the radiologists reported either “chronic cough,” “poor collaboration,” “poor breath-hold,” or “irregular breath-hold.”
Among patients having both breath-hold failure and self-reported respiratory problems (6 of 250), 2 patients (2 of 6, 33%) experienced transient dyspnea and 3 patients (3 of 6, 50%) had motion artifacts. It is noteworthy that transient dyspnea led to motion artifacts only in 1 of 2 patients (50%) experiencing dyspnea.
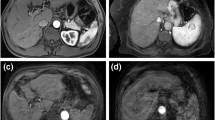
Figure 3 shows triple arterial phase imaging, with and without artifacts.
Discussion
In this observational prospective interindividual study, we evaluated the incidence of acute transient dyspnea, breath-hold failure, variation of clinical parameters (SatO2 and HR), and motion artifacts in patients undergoing gadoxetate disodium liver MR with multiarterial phase (CAIPIRINHA algorithm) technique. We also evaluated whether the procedure itself can affect the incidence of acute transient dyspnea and severe motion-related artifacts by comparing the gadoxetate disodium arterial phase with a saline “arterial” phase; the acquisition of the saline “arterial” phase allows to reproduce the same sensation than contrast administration in terms of cooling/warming, noise during the real-time bolus display method, and duration of apnea during acquisition. Although several studies have already investigated the association of gadoxetate disodium with motion artifacts and dyspnea, in the present work we hypothesized that the use of an optimized protocol (including a multiple parallel acquisition technique) in conjunction with a systematic comparison between data obtained after gadoxetate disodium administration and those observed after saline injection, could further elucidate the cause and rate of this phenomenon.
CAIPIRINHA is a multiple parallel acquisition technique enabling shortened acquisition times while still maintaining adequate spatial resolution [18, 19]. This technique has been previously used to acquire three subsequent arterial phases and to reduce motion artifacts in dynamic liver MR [6]. In our study this technique allowed the acquisition of at least one arterial phase not compromised by motion artifacts in all patients. Moreover, this technique enabled us to deeply analyze the incidence of artifacts after gadoxetate disodium administration, in comparison with saline administration. We found a similar rate of artifacts in the first (p = 0.28) and second (p = 0.09) arterial phase, whereas a significant difference between contrast agent and saline was found in the last arterial phase (p < 0.0001), as some patients were unable to hold their breath for 16–18 s after gadoxetate disodium administration. These observations support the recent findings of Yoo et al. [7], who demonstrated that short breath-hold MR technique could help to prevent degraded gadoxetate disodium arterial phase. Our results are also consistent with findings of Motosugi et al. [11] in Japanese patients.
No statistical differences in SatO2 and HR values were observed after gadoxetate disodium injection in comparison with saline administration (p = 0.30 and p = 0.48, respectively). We did not observe any variation of SatO2 and HR in patients with breath-hold failure and/or self-reported respiratory problems either, confirming previous findings [11, 17, 20, 21]. Therefore, these clinical parameters do not seem to be related to acute transient dyspnea and/or to severe motion artifacts.
Respiratory monitoring with PACE system demonstrated that imaging artifacts were associated with breath-hold failure (p < 0.0001) in the third arterial phase only. In addition, artifacts were associated with breath-hold failure only in 64% of patients. Data from self-evaluation questionnaire showed an association between motion artifacts, self-reported respiratory problems, and acute transient dyspnea in only 50% of patients. Dyspnea was reported only in 2 (0.8%) patients, who had also breath-hold failure. Therefore, acute transient dyspnea seems to be associated with breath-hold failure, but it does not necessarily lead to imaging artifacts.
In previous studies [5, 7, 8, 11, 13, 22] severe motion artifacts were found even in 17% of gadoxetate disodium arterial phase imaging, while breath-hold failure and/or acute transient dyspnea was observed even in 39% of gadoxetate disodium-enhanced MR examinations. Recently, Motosugi and colleagues conducted a prospective study at two sites (USA and Japan) to investigate the cause of these artifacts [11]. The prevalence of self-reported dyspnea, breath-hold failure, and substantial artifacts in gadoxetate disodium arterial phase imaging were reported to be 6, 34, and 22%, respectively, in the US site; 1.5, 16.2, and 17.7%, respectively, in the Japan site. Data also suggested that severe motion-related artifacts were associated with breath-hold failure but not with subjective feelings of dyspnea. Moreover, subjective feelings of dyspnea were not necessarily associated with imaging artifacts. Furthermore, one placebo-controlled trial reported that 80% (35 of 44) of subjects had reduced maximal hepatic arterial phase breath-holding duration when administered gadoxetate disodium compared with both saline and gadoterate meglumine, and 27% (12 of 44) was unable to hold their breath for at least 20 s after gadoxetate disodium administration [17].
Our results showed lower rates of breath-hold failure (6.4%) and acute transient dyspnea (0.8%) during gadoxetate disodium MR examinations. A possible explanation of these discrepancies could be the use of a lower dose of gadoxetate disodium in comparison with most of studies conducted in the USA [5, 9, 22], where gadoxetate disodium is often administered at a higher, off-label dose. It is noteworthy that our findings are similar to results obtained by Motosugi and colleagues in Japanese patients [11]. To the best of our knowledge, this is the first study conducted in Europe to evaluate dyspnea and motion artifacts related to gadoxetate disodium administration. A possible explication is that body mass index (BMI) values of our patients and Japanese patients are similar, while they are lower than American population. In fact, the maximum dose administered in our study did not exceed 10 ml. However, further studies in Europe are needed to confirm our findings.
Our study has some limitations. First, it was limited to a single center. Furthermore, we could not evaluate the impact of our protocol on gadoxetate disodium arterial phase in comparison with other contrast agents.
In conclusion, by using CAIPIRINHA multiarterial technique, the incidence of motion-related artifacts increased during the third arterial phase only, thus allowing the acquisition of at least one arterial phase not compromised by motion artifacts and adequate for acquisition timing, in all patients. Based on this and prior publications, an optimized MR protocol including the bolus tracking technique, on-label contrast agent dosage (0.025 mmoL/kg), lower injection rate (1 mL/s), saline flushing (30 mL), and multiarterial phase (CAIPIRINHA algorithm), can be useful to minimize the motion-related arterial phase artifacts.
References
Bruix J, Reig M, Sherman M (2016) Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 150(4):835–853
Neri E, Bali MA, Ba-Ssalamah A et al (2016) ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur Radiol 26(4):921–931
Kazmierczak PM, Theisen D, Thierfelder KM et al (2015) Improved detection of hypervascular liver lesions with CAIPIRINHA-Dixon-TWIST-volume-interpolated breath-hold examination. Invest Radiol 50(3):153–160
Frydrychowicz A, Lubner MG, Brown JJ et al (2012) Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging JMRI 35(3):492–511
Davenport MS, Viglianti BL, Al-Hawary MM et al (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266(2):452–461
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271(2):426–434
Yoo JL, Lee CH, Park YS et al (2016) The short breath-hold technique, controlled aliasing in parallel imaging results in higher acceleration, can be the first step to overcoming a degraded hepatic arterial phase in liver magnetic resonance imaging: a prospective randomized control study. Invest Radiol 51(7):440–446
Kim SY, Park SH, Wu EH et al (2015) Transient respiratory motion artifact during arterial phase MRI with gadoxetate disodium: risk factor analyses. AJR Am J Roentgenol 204(6):1220–1227
Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK (2014) Dose–toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol 203(4):796–802
Bashir MR, Castelli P, Davenport MS et al (2015) Respiratory motion artifact affecting hepatic arterial phase MR imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology 274(1):141–148
Motosugi U, Bannas P, Bookwalter CA, Sano K, Reeder SB (2016) An investigation of transient severe motion related to gadoxetic acid-enhanced MR imaging. Radiology 279(1):93–102
Haradome H, Grazioli L, Tsunoo M et al (2010) Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging JMRI 32(2):334–340
Luetkens JA, Kupczyk PA, Doerner J et al (2015) Respiratory motion artefacts in dynamic liver MRI: a comparison using gadoxetate disodium and gadobutrol. Eur Radiol 25(11):3207–3213
Gutzeit A, Matoori S, Froehlich J, Koh D (2016) Reduction in respiratory motion artifacts on gadoxetate acid-enhanced MR images after training technicians. Radiology 279(3):981–982
Kim YK, Lin WC, Sung K et al (2017) Reducing artifacts during arterial phase of gadoxetate disodium-enhanced MR imaging: dilution method versus reduced injection rate. Radiology 283(2):429–437
Kim YK, Lin WC, Sung K et al (2017) Reducing artifacts during arterial phase of gadoxetate disodium-enhanced MR imaging: dilution method versus reduced injection rate. Radiology 283(2):429–437
McClellan TR, Motosugi U, Middleton MS et al (2017) Intravenous gadoxetate disodium administration reduces breath-holding capacity in the hepatic arterial phase: a multi-center randomized placebo-controlled trial. Radiology 282(2):361–368
Ogawa M, Kawai T, Kan H et al (2015) Shortened breath-hold contrast-enhanced MRI of the liver using a new parallel imaging technique, CAIPIRINHA (controlled aliasing in parallel imaging results in higher acceleration): a comparison with conventional GRAPPA technique. Abdom Imaging 40(8):3091–3098
Albrecht MH, Bodelle B, Varga-Szemes A et al (2017) Intra-individual comparison of CAIPIRINHA VIBE technique with conventional VIBE sequences in contrast-enhanced MRI of focal liver lesions. Eur J Radiol 86:20–25
Hayashi T, Saitoh S, Tsuji Y et al (2015) Influence of gadoxetate disodium on oxygen saturation and heart rate during dynamic contrast-enhanced MR imaging. Radiology 276(3):756–765
Motosugi U (2015) Gadoxetic acid-induced acute transient dyspnea: the perspective of Japanese radiologists. Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med 14(2):163–164
Davenport MS, Caoili EM, Kaza RK, Hussain HK (2014) Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology 272(1):123–131
Acknowledgements
Authors would like to thank Dr Sara Cortinovis for her invaluable scientific advice and organizational support and Dr Daniela Cigognini for her medical writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Grazioli, L., Faletti, R., Frittoli, B. et al. Evaluation of incidence of acute transient dyspnea and related artifacts after administration of gadoxetate disodium: a prospective observational study. Radiol med 123, 910–917 (2018). https://doi.org/10.1007/s11547-018-0927-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0927-y